Cell-Free Hemoglobin Concentration in Blood Prime Solution Is a Major Determinant of Cell-Free Hemoglobin Exposure during Cardiopulmonary Bypass Circulation in the Newborn
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Sampling Regimen
2.3. CPB Circuit Components and Flow
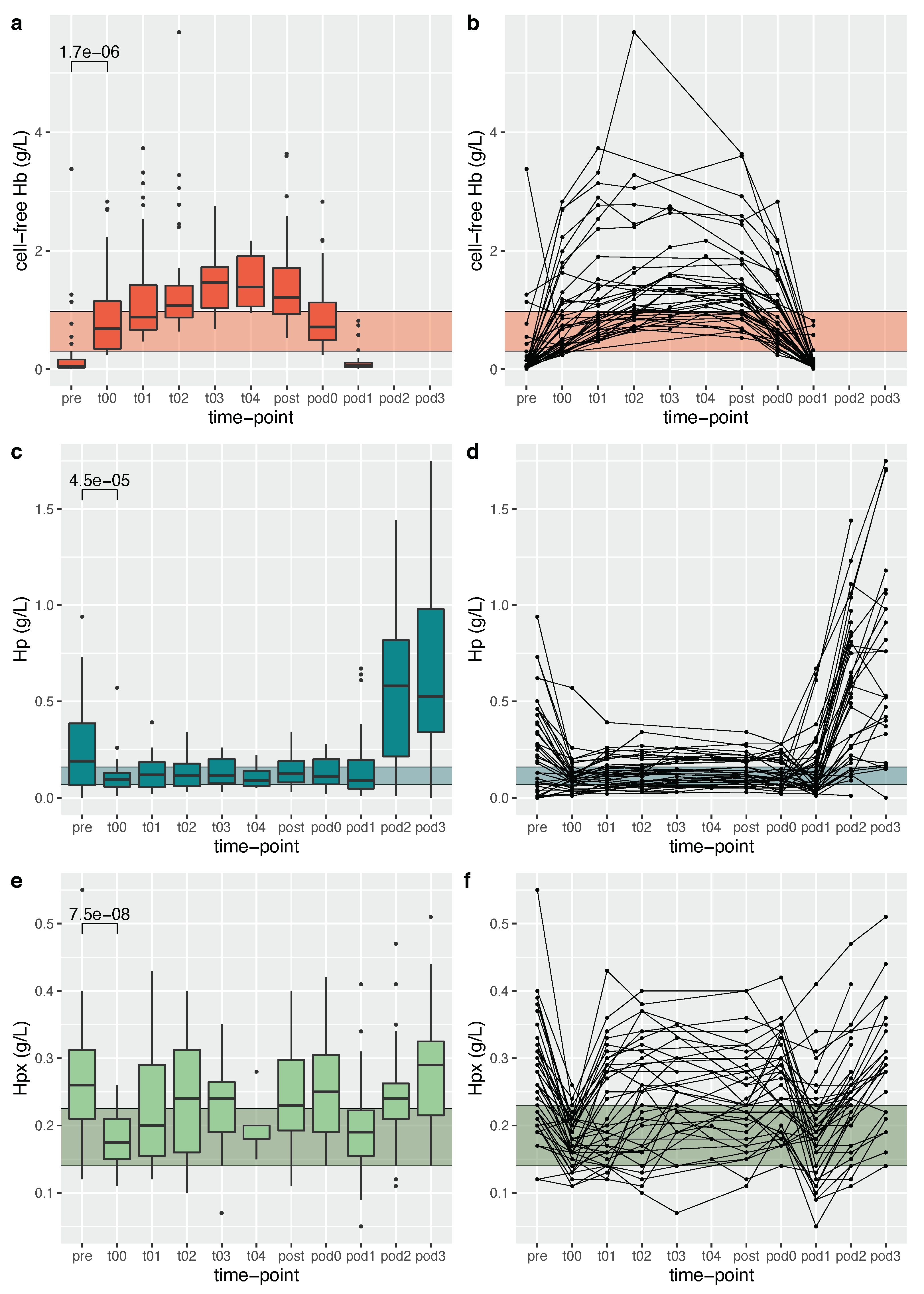
2.4. ELISA Analysis of Cell-Free Hemoglobin, Haptoglobin and Hemopexin
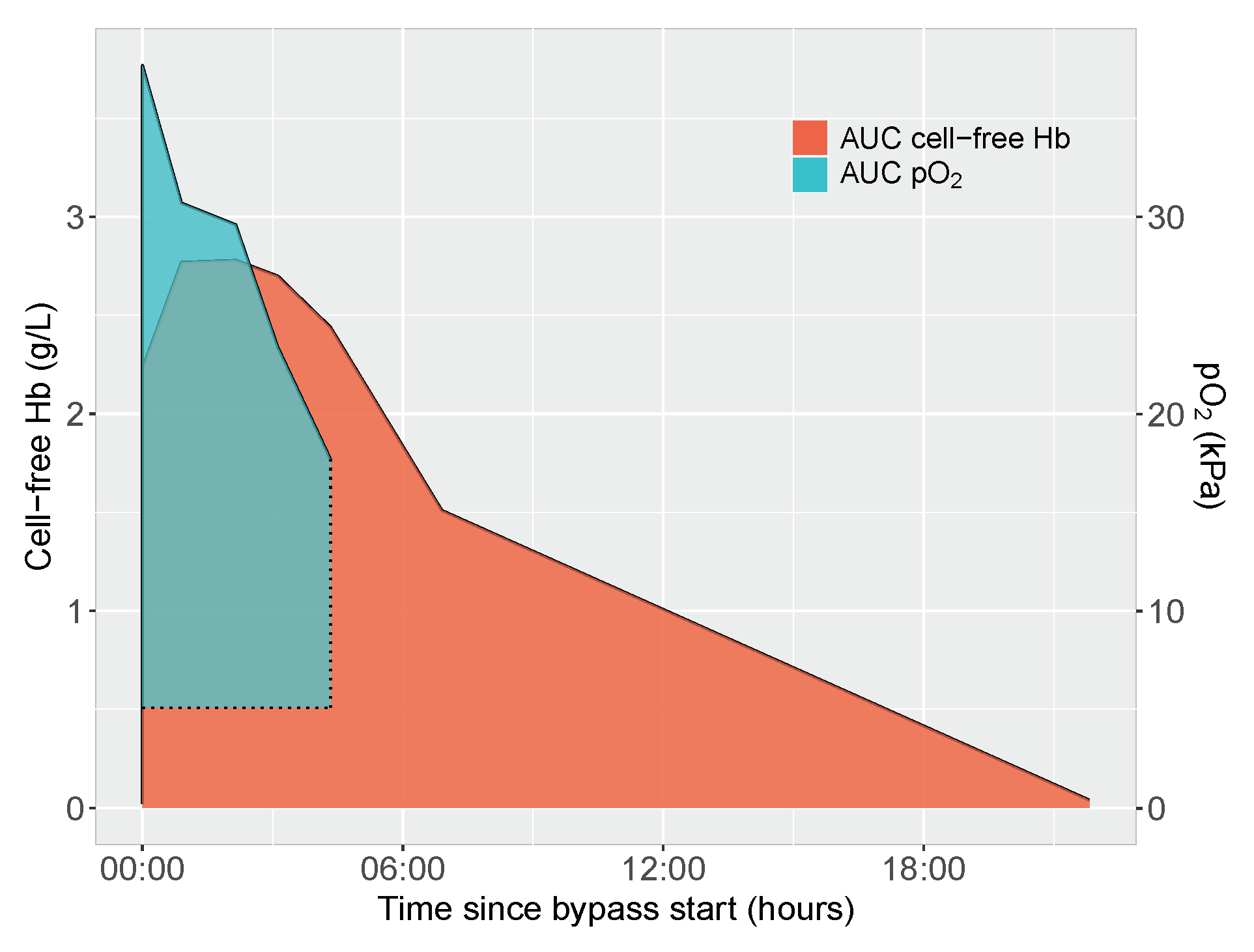
2.5. Area under Curve Calculations of Cell-Free Hemoglobin and Oxygen Exposure
2.6. Evaluated Clinical Determinants of Cell-Free Hemoglobin Exposure and Circulating Scavenger Protein Concentrations
2.7. Statistical Considerations
3. Results
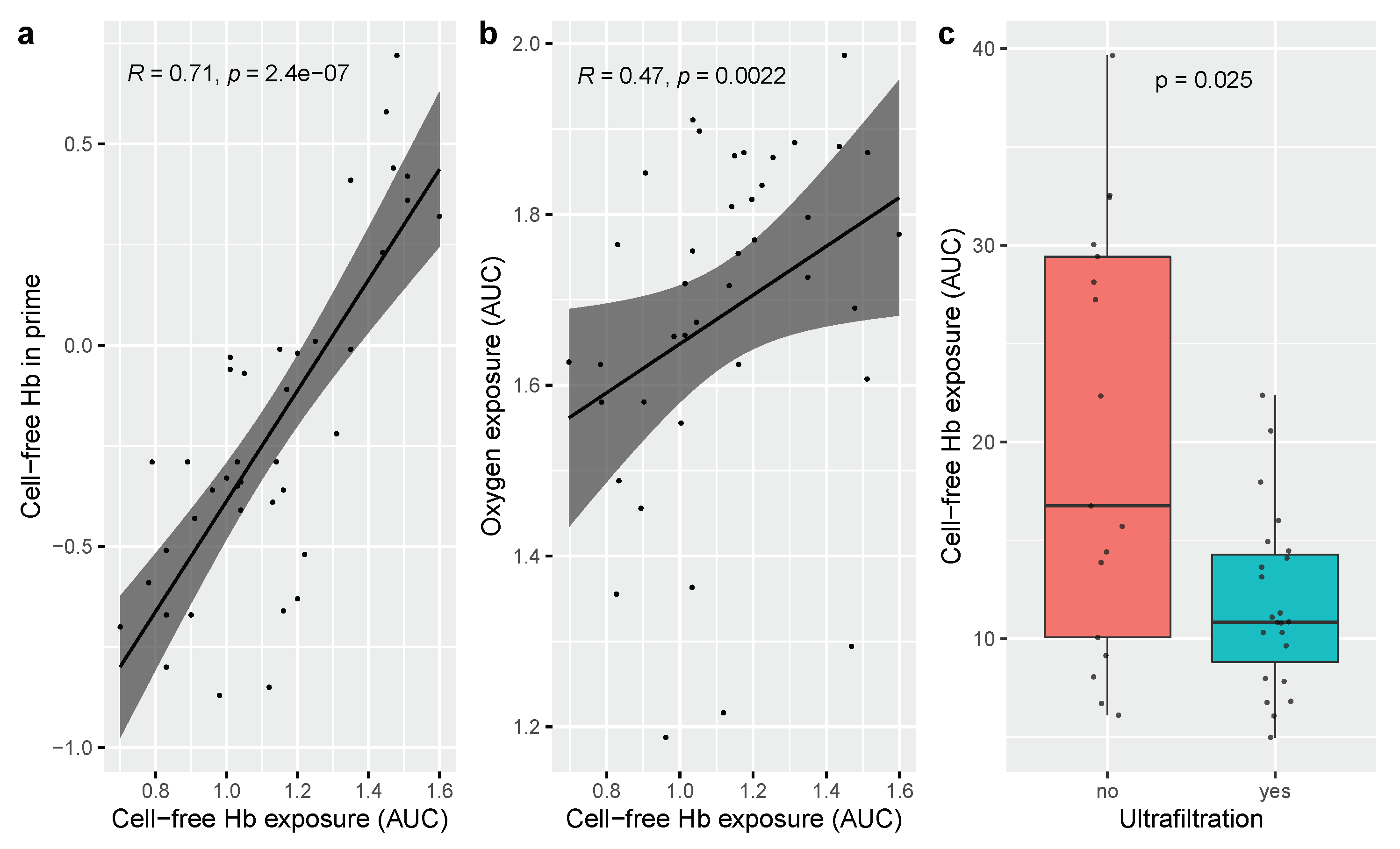
3.1. Determinants of Cell-Free Hemoglobin Exposure during Neonatal Cardiopulmonary Bypass Surgery
3.2. Determinants of Scavenger Protein Resources during Surgery
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under curve |
| cCHD | Critical congenital heart defect |
| CPB | Cardiopulmonary bypass |
| FFP | Fresh frozen plasma |
| Hb | Hemoglobin |
| PICU | Pediatric intensive care unit |
| pod | Postoperative day |
| RBC | Red blood cell |
| VAVD | Vacuum-assisted venous drainage |
References
- Buehler, P.W.; Humar, R.; Schaer, D.J. Haptoglobin Therapeutics and Compartmentalization of Cell-Free Hemoglobin Toxicity. Trends Mol. Med. 2020, 26, 683–697. [Google Scholar] [CrossRef] [Green Version]
- Schaer, D.J.; Alayash, A.I. Clearance and control mechanisms of hemoglobin from cradle to grave. Antioxid. Redox Signal. 2010, 12, 181–184. [Google Scholar] [CrossRef] [Green Version]
- Gladwin, M.T.; Kanias, T.; Kim-Shapiro, D.B. Hemolysis and cell-free hemoglobin drive an intrinsic mechanism for human disease. J. Clin. Investig. 2012, 122, 1205–1208. [Google Scholar] [CrossRef] [Green Version]
- Meegan, J.E.; Bastarache, J.A.; Ware, L.B. Toxic effects of cell-free hemoglobin on the microvascular endothelium: Implications for pulmonary and nonpulmonary organ dysfunction. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L429–L439. [Google Scholar] [CrossRef]
- Ricci, Z.; Pezzella, C.; Romagnoli, S.; Iodice, F.; Haiberger, R.; Carotti, A.; Cogo, P. High levels of free haemoglobin in neonates and infants undergoing surgery on cardiopulmonary bypass. Interact. Cardiovascular Thorac. Surg. 2014, 19, 183–187. [Google Scholar] [CrossRef]
- Cholette, J.M.; Pietropaoli, A.P.; Henrichs, K.F.; Alfieris, G.M.; Powers, K.S.; Gensini, F.; Rubenstein, J.S.; Sweeney, D.; Phipps, R.; Spinelli, S.L.; et al. Elevated free hemoglobin and decreased haptoglobin levels are associated with adverse clinical outcomes, unfavorable physiologic measures, and altered inflammatory markers in pediatric cardiac surgery patients. Transfusion 2018, 58, 1631–1639. [Google Scholar] [CrossRef]
- Mamikonian, L.S.; Mamo, L.B.; Smith, P.B.; Koo, J.; Lodge, A.J.; Turi, J.L. Cardiopulmonary bypass is associated with hemolysis and acute kidney injury in neonates, infants, and children. Pediatr. Crit. Care Med. 2014, 15, e111–e119. [Google Scholar] [CrossRef] [Green Version]
- Kim-Campbell, N.; Gretchen, C.; Callaway, C.; Felmet, K.; Kochanek, P.M.; Maul, T.; Wearden, P.; Sharma, M.; Viegas, M.; Munoz, R.; et al. Cell-Free Plasma Hemoglobin and Male Gender Are Risk Factors for Acute Kidney Injury in Low Risk Children Undergoing Cardiopulmonary Bypass. Crit. Care Med. 2017, 45, e1123–e1130. [Google Scholar] [CrossRef]
- Vercaemst, L. Hemolysis in cardiac surgery patients undergoing cardiopulmonary bypass: A review in search of a treatment algorithm. J. Extra-Corpor. Technol. 2008, 40, 257–267. [Google Scholar]
- Arensdorf, J.; Petitt, H.; Holt, D. Improving hemolysis levels associated with cardiotomy suction. Perfusion 2018, 33, 612–617. [Google Scholar] [CrossRef]
- Gretchen, C.; Bayir, H.; Kochanek, P.M.; Ruppert, K.; Viegas, M.; Palmer, D.; Kim-Campbell, N. Association between Hyperoxemia and Increased Cell-Free Plasma Hemoglobin during Cardiopulmonary Bypass in Infants and Children. Pediatr. Crit. Care Med. 2022, 23, E111–E119. [Google Scholar] [CrossRef]
- Fuchs, A.; Disma, N.; Virág, K.; Ulmer, F.; Habre, W.; de Graaff, J.C.; Riva, T. Peri-operative red blood cell transfusion in neonates and infants: NEonate and Children audiT of Anaesthesia pRactice IN Europe: A prospective European multicentre observational study. Eur. J. Anaesthesiol. 2022, 39, 252–2560. [Google Scholar] [CrossRef]
- Matto, F.; Kouretas, P.C.; Smith, R.; Ostrowsky, J.; Cina, A.J.; Hess, D.T.; Stamler, J.S.; Reynolds, J.D. S-Nitrosohemoglobin Levels and Patient Outcome after Transfusion during Pediatric Bypass Surgery. Clin. Transl. Sci. 2018, 11, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Wloch, A.; Boettcher, W.; Sinzobahamvya, N.; Cho, M.Y.; Redlin, M.; Dähnert, I.; Photiadis, J. Bloodless priming of the cardiopulmonary bypass circuit: Determinants of successful transfusion-free operation in neonates and infants with a maximum body weight of 7 kg. Cardiol. Young 2018, 28, 1141–1147. [Google Scholar] [CrossRef]
- Saylor, D.M.; Buehler, P.W.; Brown, R.P.; Malinauskas, R.A. Predicting plasma free hemoglobin levels in patients due to medical device-related hemolysis. ASAIO J. 2019, 65, 207–218. [Google Scholar] [CrossRef]
- Reiter, C.D.; Wang, X.; Tanus-Santos, J.E.; Hogg, N.; Cannon, R.O.; Schechter, A.N.; Gladwin, M.T. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat. Med. 2002, 8, 1383–1389. [Google Scholar] [CrossRef]
- Kanakoudi, F.; Drossou, V.; Tzimouli, V.; Diamanti, E.; Konstantinidis, T.; Germenis, A.; Kremenopoulos, G. Serum concentrations of 10 acute-phase proteins in healthy term and preterm infants from birth to age 6 months. Clin. Chem. 1995, 41, 605–608. [Google Scholar] [CrossRef]
- Haase, M.; Bellomo, R.; Haase-Fielitz, A. Novel Biomarkers, Oxidative Stress, and the Role of Labile Iron Toxicity in Cardiopulmonary Bypass-Associated Acute Kidney Injury. J. Am. Coll. Cardiol. 2010, 55, 2024–2033. [Google Scholar] [CrossRef] [Green Version]
- Rezoagli, E.; Ichinose, F.; Strelow, S.; Roy, N.; Shelton, K.; Matsumine, R.; Chen, L.; Bittner, E.A.; Bloch, D.B.; Zapol, W.M.; et al. Pulmonary and Systemic Vascular Resistances after Cardiopulmonary Bypass: Role of Hemolysis. J. Cardiothorac. Vasc. Anesth. 2017, 31, 505–515. [Google Scholar] [CrossRef]
- Vermeulen Windsant, I.C.; de Wit, N.C.J.; Sertorio, J.T.C.; van Bijnen, A.A.; Ganushchak, Y.M.; Heijmans, J.H.; Tanus-Santos, J.E.; Jacobs, M.J.; Maessen, J.G.; Buurman, W.A. Hemolysis during cardiac surgery is associated with increased intravascular nitric oxide consumption and perioperative kidney and intestinal tissue damage. Front. Physiol. 2014, 5, 340. [Google Scholar] [CrossRef] [Green Version]
- Miller, S.P.; McQuillen, P.S.; Hamrick, S.; Xu, D.; Glidden, D.V.; Charlton, N.; Karl, T.; Azakie, A.; Ferriero, D.M.; Barkovich, A.J.; et al. Abnormal brain development in newborns with congenital heart disease. N. Engl. J. Med. 2007, 357, 1928–1938. [Google Scholar] [CrossRef] [Green Version]
- McQuillen, P.S.; Miller, S.P. Congenital heart disease and brain development. Ann. N. Y. Acad. Sci. 2010, 1184, 68–86. [Google Scholar] [CrossRef]
- Volpe, J.J. Encephalopathy of congenital heart disease- destructive and developmental effects intertwined. J. Pediatr. 2014, 164, 962–965. [Google Scholar] [CrossRef]
- Back, S.A.; Rosenberg, P.A. Pathophysiology of glia in perinatal white matter injury. Glia 2014, 62, 1790–1815. [Google Scholar] [CrossRef]
- Schaer, C.A.; Deuel, J.W.; Schildknecht, D.; Mahmoudi, L.; Garcia-Rubio, I.; Owczarek, C.; Schauer, S.; Kissner, R.; Banerjee, U.; Palmer, A.F.; et al. Haptoglobin Preserves Vascular Nitric Oxide Signaling during Hemolysis. Am. J. Respir. Crit. Care Med. 2016, 193, 1111–1122. [Google Scholar] [CrossRef] [Green Version]
- Hugelshofer, M.; Buzzi, R.M.; Schaer, C.A.; Richter, H.; Akeret, K.; Anagnostakou, V.; Mahmoudi, L.; Vaccani, R.; Vallelian, F.; Deuel, J.W.; et al. Haptoglobin administration into the subarachnoid space prevents hemoglobin-induced cerebral vasospasm. J. Clin. Investig. 2019, 129, 5219–5235. [Google Scholar] [CrossRef] [Green Version]
- Romantsik, O.; Agyemang, A.A.; Sveinsdottir, S.; Rutardóttir, S.; Holmqvist, B.; Cinthio, M.; Mörgelin, M.; Gumus, G.; Karlsson, H.; Hansson, S.R.; et al. The heme and radical scavenger α1-microglobulin (A1M) confers early protection of the immature brain following preterm intraventricular hemorrhage. J. Neuroinflamm. 2019, 16, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Li, L. Efficient synthesis of molecularly imprinted polymers with bio-recognition sites for the selective separation of bovine hemoglobin. J. Sep. Sci. 2018, 41, 2479–2487. [Google Scholar] [CrossRef]
- Hulko, M.; Kunz, M.; Yildirim, M.; Homeyer, S.; Amon, O.; Krause, B. Cell-free plasma hemoglobin removal by dialyzers with various permeability profiles. Nat. Publ. Group 2015, 5, 16367. [Google Scholar] [CrossRef] [Green Version]
| Clinical Characteristics | |
|---|---|
| Sex (male/female) (n) | 25/15 |
| Birthweight (gm) (mean ± SD) | 3436 ± 326 |
| Gestational age at birth (wk) (mean (min–max)) | 39 + 4 (37 + 6–42 + 1) |
| Biventricular repair (n) | 32/40 |
| Palliative procedure | |
| with prior arch obstruction (n) | 3/40 |
| without prior arch obstruction (n) | 5/40 |
| RACHS 1 (median (min–max)) | 4 (3–6) |
| Postnatal age at surgery (d) (median (IQR)) | 5 (4–7) |
| Evaluated Predictor | |
|---|---|
| Age of blood in prime solution (d) (median (IQR)) | 3 (2–4) |
| RBC volume in prime solution (mL/kg) (median (IQR)) | 49 (43–56) |
| FFP volume in prime solution (mL/kg) (median (IQR)) | 12 (10–18) |
| Time on cardiopulmonary bypass (min) (median (IQR)) | 182 (142–208) |
| Ultrafiltration (n) | 23/40 |
| Vacuum-assisted venous drainage (n) | 11/40 |
| Oxygen exposure during bypass (AUC) (median(IQR)) | 52.8 (39.9–68.8) |
| Intraoperative transfusion RBC (mL/kg) (median (IQR)) | 52 (37–73) |
| Intraoperative transfusion FFP (mL/kg) (median (IQR)) | 34 (14–51) |
| Variable | Univariable Analysis (95% CI) | Multivariable Analysis (95% CI) |
|---|---|---|
| Storage length of RBC unit in prime (d) | −0.30 (−2.14–1.53) | |
| Cell-free Hb conc in prime (g/L) | 8.50 (6.68–10.31) | 8.11 (6.09–10.13) |
| RBC volume in prime (mL/kg) | 0.39 (0.12–0.66) | 0.01 (−0.04–0.06) |
| Time on bypass (min) | 0.02 (−0.03–0.06) | |
| Oxygen exposure during bypass (AUC) | 0.19 (0.09–0.29) | 0.07 (−0.003–0.14) |
| Ultrafiltration, yes = 1 | −8.30 (−13.15—3.44) | −0.44 (−3.53–2.65) |
| VAVD, yes = 1 | −2.91 (−8.96–3.13) | |
| Intraop RBC transfusion (mL/kg) | 0.08 (−0.02–0.18) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jungner, Å.; Vallius, S.; Gram, M.; Ley, D. Cell-Free Hemoglobin Concentration in Blood Prime Solution Is a Major Determinant of Cell-Free Hemoglobin Exposure during Cardiopulmonary Bypass Circulation in the Newborn. J. Clin. Med. 2022, 11, 4071. https://doi.org/10.3390/jcm11144071
Jungner Å, Vallius S, Gram M, Ley D. Cell-Free Hemoglobin Concentration in Blood Prime Solution Is a Major Determinant of Cell-Free Hemoglobin Exposure during Cardiopulmonary Bypass Circulation in the Newborn. Journal of Clinical Medicine. 2022; 11(14):4071. https://doi.org/10.3390/jcm11144071
Chicago/Turabian StyleJungner, Åsa, Suvi Vallius, Magnus Gram, and David Ley. 2022. "Cell-Free Hemoglobin Concentration in Blood Prime Solution Is a Major Determinant of Cell-Free Hemoglobin Exposure during Cardiopulmonary Bypass Circulation in the Newborn" Journal of Clinical Medicine 11, no. 14: 4071. https://doi.org/10.3390/jcm11144071
APA StyleJungner, Å., Vallius, S., Gram, M., & Ley, D. (2022). Cell-Free Hemoglobin Concentration in Blood Prime Solution Is a Major Determinant of Cell-Free Hemoglobin Exposure during Cardiopulmonary Bypass Circulation in the Newborn. Journal of Clinical Medicine, 11(14), 4071. https://doi.org/10.3390/jcm11144071







