Abstract
SARS-CoV-2 may lead to a large spectrum of respiratory manifestations, including pulmonary sequelae. We conducted a single-center longitudinal study of survivors from severe COVID-19 cases who underwent a chest CT during hospitalization (CTH). Three months after being discharged, these patients were evaluated by a clinical examination, pulmonary function tests and a chest-CT scan (CTFU). Sixty-two patients were enrolled. At follow-up, 27% complained of exertional dyspnoea and 12% of cough. Dyspnoeic patients had a lower forced expiratory flow (FEF)25–75 (p = 0.015), while a CT scan (p = 0.016 showed that patients with cough had a higher extent of bronchiectasis. Lung volumes and diffusion of carbon monoxide (DLCO) at follow-up were lower in patients who had been invasively ventilated, which correlated inversely with the length of hospitalization and ground-glass extension at CTH. At follow-up, 14.5% of patients had a complete radiological resolution, while 85.5% presented persistence of ground-glass opacities, and 46.7% showed fibrotic-like alterations. Residual ground-glass at CTFU was related to the length of hospitalization (r = 0.48; p = 0.0002) and to the need for mechanical ventilation or high flow oxygen (p = 0.01) during the acute phase. In conclusion, although patients at three months from discharge showed functional impairment and radiological abnormalities, which correlated with a prolonged hospital stay and need for mechanical ventilation, the persistence of respiratory symptoms was related not to parenchymal but rather to airway sequelae.
1. Introduction
Coronavirus disease 2019 (COVID-19) caused by the new coronavirus 2 (SARS-CoV-2), began to spread in China in late-2019, and subsequently all over the world in 2020. SARS-CoV-2 may lead to a large spectrum of respiratory manifestations, from a flu-like syndrome to bilateral pneumonia with acute respiratory failure. Typical CT findings of COVID-19 pneumonia during the acute phase are ground-glass opacities, consolidations, and crazy paving, mainly displaying lower lobe and peripheral distribution [1]. Wei et al. [2] described for the first time (May 2020, PubMed) the presence of fibrotic sequelae in CT-scans of 59 patients hospitalized for COVID-19 one month after discharge. Later that year in August, Zaho et al. reported functional impairment and persistence of ground-glass opacities, crazy paving, and interstitial thickening in 55 COVID-19 survivors three months after discharge [3]. In 2002, radiological abnormalities (reticular fibrotic-like sequelae) and functional impairment were described in 36% of severe acute respiratory syndrome (SARS) patients [4,5], and in 33% of those with Middle East respiratory syndrome (MERS) also [6]. From the pandemic outbreak, several studies worldwide investigated the functional and radiological sequelae of COVID-19. However, the methodological approaches were dissimilar, and the results were not always consistent; thus, it was difficult to draw exhaustive conclusions. Moreover, only few studies longitudinally examined the evolution of radiological abnormalities by comparing CT scan imaging between the acute phase and follow-up.
From March 2020 to April 2021, the pulmonology intermediate care unit of Ca’ Foncello Hospital (Treviso) in North-east of Italy, treated more than 500 patients affected by severe respiratory failure secondary to COVID-19.
The aims of the study were to assess the presence of respiratory sequelae in a clinical-functional and radiological short-term follow-up of severe COVID-19 survivors —in particular, the evolution of CT abnormalities—, to explore possible predictors of radiological–functional evolution and to investigate potential correlations between clinical presentation and radiological–functional features at follow-up.
2. Materials and Methods
2.1. Study Design
We performed a prospective study on a three-month clinical, functional and radiological follow-up of patients admitted to the respiratory intermediate care unit at Ca’ Foncello Hospital (Treviso, Italy) for acute respiratory failure secondary to COVID-19, from March 2020 to April 2021. The study was approved by our referral ethics committee (No. 793 CE/Marca date 9 April 2020). Radiological and functional examinations at baseline and at follow-up both had been performed for a primary clinical purpose accordingly to clinical indications.
Inclusion criteria were (1) a diagnosis of acute respiratory failure (PaO2 < 60 mmHg in room air at ABG) secondary to bilateral pneumonia, (2) confirmed SARS-CoV-2 infection by real-time reverse transcription–polymerase chain reaction from a nasopharyngeal swab, (3) appropriate clinical indications for a chest CT scan during hospitalization, and (4) survival to hospitalization.
Exclusion criteria were (1) previous known chronic respiratory comorbidities, (2) low CT scan quality or presence of massive pneumothorax/pleural effusion, (3) death after discharge before follow-up visit, (4) poor clinical conditions after discharge that precluded a visit, (5) refusal to participate in baseline or follow-up examinations, and (6) unreachable after discharge (i.e., tourists).
Past medical history and clinical data at hospital admission were collected by a respiratory physician who analyzed the electronic patients’ registry. Demographics (smoking, comorbidities, laboratory tests, lymphocyte and neutrophil counts, c-reactive protein (CRP), lactate, lactate dehydrogenase, D-dimer and PaO2/fraction of inspired oxygen ratio (P/F) at COVID-area arrival), complications, medical treatment, respiratory support, length of hospitalization and length of invasive ventilation were recorded. All patients included in the study received pharmacological treatment and respiratory support based on updated national guidelines at the time of hospitalization [7,8]. Non-invasive and invasive mechanical ventilation parameters were similar for all patients according to national guidelines [8].
We performed a clinical, functional and radiological follow-up after three months from discharge (mean: 110 days). An expert respiratory physician (MB or PP) interviewed patients about general and respiratory symptoms. Exertional dyspnoea was quantified on the mMRC dyspnoea scale. Patients were considered symptomatic if they reported symptoms persisting after discharge and having a negative impact on daily activity or on night rest. At follow-up, patients underwent spirometry, diffusion lung CO and peripheral blood oxygen saturation (SpO2) measurement. Pulmonary function tests were performed following ERS/ATS guidelines [9].
2.2. CT Acquisitions and Image Analysis
CTs were performed at two centers depending on the phase of the study. During hospitalization (CTh), it was the radiology department of the Ca’ Foncello Hospital, which used an OPTIMA 64O (GE Healthcare, Chicago, IL, USA)—tube voltage 120 kV; tube current modulation (range 100–350 mA); slice thickness 1.25 mm; and the reconstruction filter Bone Plus—and a SOMATOM FLASH (Siemens, Ergangen, Germany): tube voltage 120 kV;, tube current modulation (reference 150 mA); slice thickness 1 mm; reconstruction kernels L70F; and very sharp ASA.
Follow up CT (CTFU) were performed at the Ca’ Foncello Hospital with the same scanners and parameters or at the Oderzo City Hospital (Oderzo, Italy) on a scan system CT Revolution EVO (GE, Milwaukee, WI, USA); tube voltage 120 kV, tube current modulation (range 100–350 mA range); slice thickness 0.625 mm; and reconstruction filter Type Lung. All high-resolution computed tomography (HRCT) was acquired in full inspiration; no contrast media was administrated.
At the beginning, a general radiologist with 30 years of experience (GM), excluded HRCT images of low quality caused by movement/respiratory artefacts, massive pneumothorax or pleural effusion, which, as a compressive atelectasis, could have affected lung evaluation. Then, a chest radiologist with eight years of experience (NL) visually assessed the extent of lung alterations of both the baseline and follow-up CT and adopted the following semi-quantitative score [10]: each lobe was scored from 0 to 5 based on involvement percentage (0, no involvement; 1, ≤5%; 2, 6–25%; 3, 26–50%, 4, 51–75%; and 5, ≥75%). The total score was the sum of all lobe scores. Both acute lung alterations (ALAs) and fibrotic-like abnormalities (FIBs) were scored in all CTs, and the finding of each one was considered to be part of an ALA based on the most common CT findings described in the literature [1]. In addition, the following were independently assessed: ground glass opacities (GG), crazy paving (CP) pattern and consolidation (CON). The following CT findings were considered as FIBs: parenchymal bands, subpleural lines or reticulation with bronchiectasis (BRN) or architectural distortions, and honeycombing. BRN was separately assessed as being in the severe range of the Brody scoring system [11], customized for cystic fibrosis and based on the extent and severity in each lobe. All alterations were defined according to the glossary of terms for thoracic imaging of the Fleischner Society [12]. All CTs were anonymized and randomized so that, the reader would be unaware of the acquisition date and clinical information. The CTs were evaluated using a standard lung window. One month later 1/3 of the HRCT scans, randomly selected, were reassessed to compute the intrareader agreement, which was scored by a thoracic radiologist with 15 years of experience (NR).
2.3. Statistical Analysis
Variables were presented with frequencies and percentages for categorical variables, as median (1st–3rd quartile) or mean ± standard deviation for continuous variables. The difference in explanatory variables was assessed using a Chi-squared or Fisher test for dichotomous and categorical variables, a Student’s t-test for normally distributed continuous variables and a Mann–Whitney U or Kruskal–Wallis test for non-normal distributed continuous variables. Correlations between continuous variables was assessed by a Spearman’s test. Intra- and interobserver agreement was evaluated by Cohen’s kappa test: p < 0.05 was considered significant. Statistical analyses were performed with SPSS (IBM SPSS Statistics version 23) (IBM, Armonk, NY, USA).
3. Results
Of the 589 patients admitted between March 2020 and April 2021 for acute respiratory failure due to COVID-19, a total of 175 (29.7%) met the inclusion criteria. Of these, 113 (64.5%) were excluded for previously reported criteria. Specifically, 82 did not attend the follow-up visit; 16 had previous respiratory comorbidities—COPD (n = 5), lung cancer (n = 3), bronchiectasis (n = 3), pulmonary fibrosis (n = 2), severe asthma (n = 1), previous lung transplant (n = 1), and severe obesity–hypoventilation syndrome (n = 1)—and 15 were excluded for radiological criteria. Thus, 62 patients (10.5%) were enrolled.
3.1. Clinical-Radiological Characteristics at Hospital Admission and Hospitalization
All demographic and clinical characteristics of the 62 patients at baseline are reported in Table 1. Most were males (45; 72.5%) with a median age of 71 years. The majority of subjects never smoked (58.1%), while only two were current smokers. The most common comorbidity was hypertension (58%). At hospital admission, patients presented a median P/F ratio of 213, median levels of CRP (9.3 (4.3–14.3) mg/L), LDH (369 (312–451) U/L) and D-dimer (813 (476–1288) ng/dL) were higher than normal, while median blood lymphocytes (830 (646–1080) cell/μL) were reduced. Median lactate (1.3 (0.9–1.9) mmol/mol) and blood neutrophil counts (6540 (4310–8852) cell/μL) were in the normal range. Two-thirds of patients needed mechanical ventilation during hospitalization (40.3% non-invasively, and 27.5% invasively), while the remaining 1/3 were on oxygen support only; 17.7% by conventional oxygen support, and 14.5% by high flow nasal cannulas. During hospitalization, 48.3% of patients had no complications; among the residual 51.7%, the most common complication was hyperglycemia (19.3%), followed by superimposed bacterial respiratory tract infection (9.6%), and pulmonary embolism, bacterial sepsis and arrhythmias (8% for all three). Fifty-one patients (83.6%) underwent intravenous steroid treatment: 76.4% with methylprednisolone and 23.6% with dexamethasone. Only 6 patients (9.6%) underwent treatment with tocilizumab, while 24 (38.7%) were given antivirals. The median length of hospitalization was 15 days.

Table 1.
Demographics, anamnestic data, laboratory tests, respiratory support and pharmacological treatment during hospitalization.
As reported in Table 2, CTH provided the following scores: ALA (13.6 ± 5.5), GG (10.4 ± 4.6), CP (3.7 ± 3.6) and CON (5.9 ± 3.9). FIB (2.7 ± 2.4) and BRN (1.2 ± 2.1. Intra- and inter-reader agreement varied accordingly with the score considered (Cohen’s kappa range 0.71–0.99).

Table 2.
CT scan abnormalities during hospitalization (CTH) and at follow-up (CTFU).
3.2. Clinical-Functional-Radiological Characteristics at Follow-Up
Clinical and functional characteristics of patients at follow-up are reported in Table 3. Almost half (56.5%) did not report any symptoms. Among symptomatic patients, 17 (27.4%) reported persistent exertional dyspnoea, 8 (12.9%) reported cough and 6 (9.6%) reported fatigue. Symptoms at follow up were not significantly related to CT parenchymal abnormalities (both CTH and CTFU), or to lung volumes or DLCO. However, as shown in Figure 1A, patients who reported dyspnoea at follow-up showed a reduced FEF25–75 (70.7 ± 30.2 vs. 102.1 ± 26.4% pred.; p = 0.015). Moreover, patients who reported cough showed a higher bronchiectasis score at follow-up (2.8 ± 3.3 vs. 1.1 ± 1.5; p = 0.016; Figure 1B). No patient had residual chronic respiratory failure. Fifty patients out of 62 (78.1%) underwent pulmonary function tests. Only 11 (24%) reported a functional respiratory disease: 7 patients had a restrictive disease while 4 had an obstructive one. The lung volume means were at the limit of normality. On the other hand, 40% of patients had a mild impairment of alveolar–capillary diffusion (mean 70 ± 14.3%). No associations were observed between radiological and functional impairment at follow-up. As reported in Table 2, CTFU provided the following scores: ALA (7.1 ± 5.7), GG (6.9 ± 5.4), CP (0.9 ± 2.2) and CON (0.3 ± 1.1). FIB (4.2 ± 3.6) and BRN (1.4 ± 2.0) had already been recorded during the acute phase. Intra- and inter-reader agreement varied accordingly with the score (Cohen’s kappa range 0.81–0.99).

Table 3.
Clinical findings and respiratory function at follow-up visit.
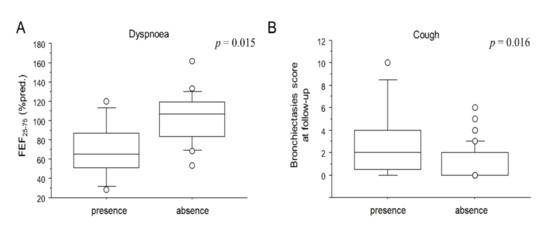
Figure 1.
Box plots comparison between the FEF25-75 and dyspnoea (A) and between BRN and cough (B) at follow-up visit. Solid line represents the median; bottom and top of the boxes are the 25th and 75th percentiles; brackets correspond to the 10th and the 90th percentiles.
3.3. Longitudinal Functional-Radiological Evolution from Hospitalization to Follow-Up
3.3.1. Evolution of Acute CT Abnormalities
A complete resolution of all acute abnormalities was observed in only 9 patients (14.5%). In the remaining cases, 53 acute abnormalities (85.5%) were still present at CTFU. However, a significant radiological decrease of mean ALA scores was overall observed from CTH and CTFU (−6.4 ± 6.3; p < 0.0001). More specifically, a significant reduction was observed for all three acute abnormality scores: GG (−3.5 ± 5.9; p = 0.0002), CP (−2.2 ± 3.5; p < 0.0001) and CON (−5.6 ± 3.9; p < 0.0001). Of note, while CP and CON were still present in only a minority of patients (n = 15 and n = 7, respectively), with a wide extent reduction in percentage between CTH and CTFU (−74 ± 74% and −94 ± 32%, respectively), GG was still present in 85.5% of patients (n = 53), and its reduction in percentage was lower in comparison to the other two scores (−31 ± 56%).
As shown in Figure 2A–C, the length of hospitalization moderately correlated with all three acute abnormalities (r = 0.48; p < 0.0001 for GG; r = 0.46; p = 0.0003 for CP; r = 0.45; p = 0.005 for CON) as well as to the residual ALA score at CTFU (r = 0.48; p = 0.0002). Residual GG at CTFU, but not CON or CP, was significantly lower in patients who required only conventional O2T support during hospitalization (2.9 ± 3.7 for conventional O2T vs. 8.8 ± 6.7 for IV, 7.0 ± 4.4 for NIV and 8.0 ± 5.1 for HFNC vs.; p = 0.01 Figure 3A). No associations were observed between the persistence of acute abnormalities at follow-up with gender, age, comorbidities, laboratory tests, complications, length of invasive ventilation, and pharmacological treatment during hospitalization.
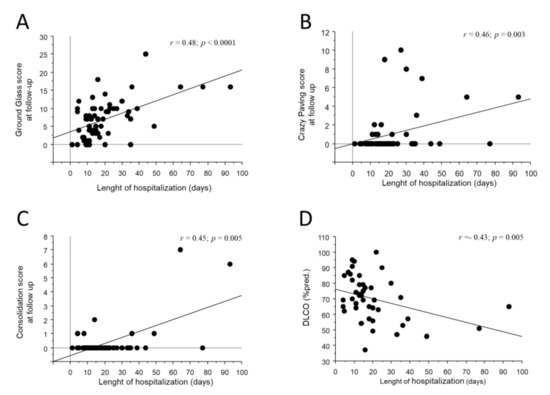
Figure 2.
Bivariate scatterplots reporting the relationship between the length of hospitalization (x axes) and GG (A), CP (B), CON (C) at CTFU and DLCO (D) at follow-up visit. Regression line is represented.
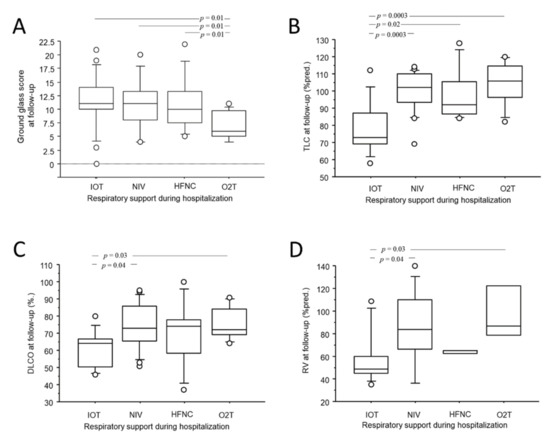
Figure 3.
Box-plot comparison of GG at CTFU (A), the TLC (B), the DLCO (C), the RV (D) at follow-up with respiratory support needed during hospitalization. Solid line represents the median; bottom and top of the boxes are the 25th and 75th percentiles; brackets correspond to the 10th and the 90th percentiles.
3.3.2. Development of Chronic CT Abnormalities
Fibrotic-like alterations and bronchiectasis were already observed during hospitalization. At follow-up, fibrotic-like alterations were observed in 29 patients (46.7%), although the extent was modest in comparison to the residual acute alterations (CTFU: FIB 4.2 ± 3.6 vs. TAP 7.1 ± 5.7; p = 0.0002). Despite this, a significant increase in FIB was observed from CTH to CTFU (+1.6 ± 3.3; p = 0.001). Conversely, BRN did not significantly increase from CTH to CTFU. FIB at CTFU was weakly but significantly correlated to the ALA score at CTH (r = 0.25; p = 0.04). No other acute CT abnormalities, nor gender, age, comorbidities, complications, pharmacological treatment, respiratory support, length of invasive ventilation, and laboratory tests during hospitalization, were predictive of FIB at CTFU.
3.3.3. Development of Functional Impairment
GG at CTH correlated inversely with total lung capacity, residual volume and DLCO at follow-up (r = −0.45; p = 0.003 for TLC; r = −0.42; p = 0.01 for RV; r = −0.48; p = 0.02 for DLCO). The length of hospitalization also correlated inversely with DLCO at follow-up (r = −0.43; p = 0.0005, Figure 2D).
As shown in Figure 3B–D, patients who had been invasively ventilated during hospitalization showed significantly reduced total lung capacity (78 ± 15.6%pred. for IV vs. 97 ± 15.6%pred. for HFNC and 99 ± 12%pred. for NIV and 89 ± 13.6%pred. for conventional O2T; p = 0.005), residual volume (58 ± 24.4%pred. for IV vs. 64 ± 1.7%pred. for HFNC and 86 ± 33%pred. for NIV and 99 ± 30%pred. for conventional O2T; p = 0.005) and DLCO (60 ± 10.8%pred. for IV vs. 69 ± 19.7%pred. for HFNC and 74 ± 13.1%pred. for NIV and 76 ± 10%pred. for conventional O2T; p = 0.03) at follow up. No associations were observed between functional impairment at follow-up and gender, age, comorbidities, complications, pharmacological treatment, length of invasive ventilation, and laboratory tests during hospitalization.
4. Discussion
The present study analyzed the clinical, functional and radiological evolution of severe COVID-19 three months from discharge in 62 patients. We demonstrated for the first time that the persistence of symptoms was not related to a residual parenchymal disease, but rather to an increased bronchiectasis extension and to a FEF25-75 impairment, as manifestation of airways abnormalities. At follow-up, the functional impairment and residual, acute radiologic alterations were related to the severity of acute COVID-19 pneumonia in terms of length of hospitalization and intensive respiratory support. Radiological acute abnormalities, especially ground-glass opacities, were still present in the majority of patients (85%) after the three months. Finally, the increase in fibrotic-like alterations was modest although present in 50% of patients.
About half of the patients complained of respiratory symptoms at follow-up, and unlike what was assumed, we observed a discrepancy between respiratory symptom with lung volumes/alveolar-capillary diffusion and residual radiological parenchymal impairment. These findings were consistent with previous studies that observed only a weak correlation between post-COVID respiratory symptoms and functional-radiological parenchymal impairment at follow-up [13,14,15]. Indeed, there is still a lack of evidence regarding the mechanisms of post-COVID respiratory syndrome.
In our cohort, dyspnoeic patients showed impaired FEF25–75, which was a sign of small-airways disease, whereas patients who reported cough showed increased bronchiectasies extension in the CT scan. The first result was consistent with the findings of other authors, who demonstrated on a paired inspiratory/expiratory CT scan that patients with persistent respiratory symptoms after acute COVID-19 showed air trapping [16,17]. Bronchiectasis in COVID-19 patients had already been described both during hospitalization and at follow up and seemed to be associated with a more severe infection [14,18]. Indeed, all these results suggested that the respiratory symptoms in patients with “long COVID-19” might be due to airway abnormalities rather than, or not exclusively to, lung parenchymal sequelae. These data deserve to be further investigated in larger cohorts since they may play an important role in patient management during follow-up.
Several studies have analysed CT parenchymal imaging of patients hospitalized for COVID-19 in a short- and medium-term follow-ups [2,3,13,14,15,19,20,21,22]. The methodological differences of these previous studies make a direct comparison among data difficult. However, the persistence of residual radiological lung abnormalities after discharge was reported by all authors with wide variable prevalence, ranging from 20 [15] to 97.7% [19]. Our results (85.5%) ranked among the higher values probably because a more severe disease occurred in our cohort: indeed, 2/3 of patients required mechanical ventilation support during hospitalization. Thus, we observed a moderate correlation between the severity of COVID-19 during the acute phase -in terms of length of hospitalization or need for mechanical ventilation- and the parenchymal sequelae at follow-up -both functional and radiological- confirming what was previously reported by other authors [13,14,15]. Similar to other papers, ground-glass persistence at follow-up was the most frequently observed CT abnormality [2,3,13,14,15,19,20,21,22], while consolidations recovered almost completely [14,15,19,21]. Concerning fibrotic-like alterations, our results were also in accordance with previous reports, which showed it being present in fewer than 50% of patients [2,14,15,19]. However, unlike the others, we did not observe any correlation at follow-up between the severity of acute phase and fibrotic-like extension [13,15]. In our population, while acute abnormalities showed a decreasing trend from acute phase to follow-up, there was a significant, although modest, increase of fibrotic-like alterations. Whether these findings represent early signs of pulmonary fibrosis is under debate [23,24,25]. Concerning our results, fibrotic-like alterations were also observed in the acute phase; therefore, it was difficult to determine whether these patients actually developed pulmonary fibrosis. We speculated that these lesions were likely not present before developing COVID-19 as the majority of our patients were less than 70 years old, non-smokers, and without previous chronic respiratory disease.
Our study had some limitations. First of all, the results were obtained for a relatively small number of patients and need to be confirmed by a larger cohort. Second, the study was conducted while facing the pandemic, and the rapid evolution of national guidelines on COVID-19 treatment did not allow the same protocols of pharmacological treatment for the whole cohort. Therefore, it was impossible to assess the possible influence of drug treatment in the acute phase on follow-up residual impairment/recovery. Finally, we did not use questionnaires for interviewing patients’ symptoms at follow-up because symptom assessment was not the focus of our study; however, we are confident that considering only symptoms that were persistent and had a tangible impact on patients’ lives provided an accurate assessment.
In conclusion, we performed a multidimensional assessment of severe COVID-19 survivors three months form hospital discharge. We reported for the first time that the persistence of respiratory symptoms were related to airway sequelae rather than to parenchymal alterations. This finding might deserve to be investigated during a longer follow-up, to evaluate whether it might be a target for a pharmacological approach.
Lung parenchymal abnormalities persisted at short follow-ups both on the functional and radiological side and were related to the severity of the acute disease for length of hospitalization and need for mechanical ventilation. Finally, the clinical and pathological meaning of fibrotic-like alterations remains under debate, and further investigations are needed to clarify their role.
Author Contributions
M.B., N.L., P.P., A.F., M.R. (Marcello Rattazzi)., M.P., S.B., M.F., M.S. (Marina Saetta), G.M. and M.R. (Micaela Romagnoli).: conception of the study design, drafting and revision of the manuscript. M.B., N.L., P.P., A.F., C.C., N.M., F.S., N.R., M.T., G.Z., F.Z. (Fabiola Zeraij) and F.Z. (Francesca Zampieri), M.S. (Mauro Salasnich), M.C.: acquisition and analysis of data. All authors have read and agreed to the published version of the manuscript.
Funding
Publication fees were funded by CONSORZIO FUTURO IN RICERCA (Via Saragat 1-44122-Ferrara, Italy). Ref. No. ROMA\01; 7 July 2022.
Institutional Review Board Statement
This study protocol was reviewed and approved by Azienda Unità Locale Socio-Sanitaria 2 Ethics Committee, approval number 793 CE/Marca date 9 April 2020.
Informed Consent Statement
Written informed consent was not required due to the primary clinical purpose of investigations under the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, M.B., upon reasonable request.
Conflicts of Interest
M.S. (Marina Saetta), has received unrestricted research grants from Takeda Ltd., Chiesi Farmaceutici, Laboratori Guidotti Spa, outside this specific study. M.R. (Micaela Romagnoli) has received unrestricted grants from AlfaSigma, AstraZeneca, Boehringer-Ingelheim, Chiesi, GSK, Guidotti, Menarini, Novartis, Olympus Roche outside this specific study. M.S. (Mauro Salasnich) has received fees from GSK and Zambon outside this specific study. All others have no conflicts to declare. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| ABG | Arterial blood gas analysis |
| ARDS | Acute respiratory distress syndrome |
| CON | Consolidation |
| CP | Crazy paving |
| CRP | C-reactive protein |
| DLCO | Diffusion of carbon monoxide |
| FIB | Fibrotic-like alterations |
| GG | Ground glass |
| HFNC | Heated humidified high-flow oxygen therapy |
| CT | Chest computed tomography |
| ICU | Intensive care unit |
| IQR | Interquartile range |
| IV | Invasive ventilation |
| FEV1 | Forced expired volume in the first second of expiration |
| FiO2 | Fraction of inspired oxygen |
| FVC | Forced vital capacity |
| K–W | Kruskal–Wallis test |
| LDH | Lactate dehydrogenase |
| mMRC | Modified medical research council |
| PaO2 | Arterial partial pressure of oxygen |
| P/F | PaO2/FiO2 ratio |
| NIV | Non-invasive ventilation |
| NV | Normal values |
| O2T | Oxygen therapy |
| RV | Residual volume |
| SD | Standard deviation |
| ALA | Acute lung alterations |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| TLC | Total lung capacity |
References
- Garg, M.; Gupta, P.; Maralakunte, M.; Praveen, K.-M.; Sinha, A.; Kang, M.; Sandhu, M.S. Diagnostic accuracy of CT and radiographic findings for novel coronavirus 2019 pneumonia: Systematic review and meta-analysis. Clin. Imaging 2021, 72, 75–82. [Google Scholar] [PubMed]
- Wei, J.; Yang, H.; Lei, P.; Fan, B.; Qiu, Y.; Zeng, B.; Yu, P.; Lv, J.; Jian, Y.; Wan, C. Analysis of thin-section CT in patients with coronavirus disease (COVID-19) after hospital discharge. J. X-ray Sci. Technol. 2020, 28, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Shang, Y.M.; Song, W.B.; Li, Q.Q.; Xie, H.; Xu, Q.F.; Jia, J.L.; Li, L.M.; Mao, H.L.; Zhou, X.M.; et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 2020, 25, 100463. [Google Scholar] [PubMed]
- Hui, D.S.; Joynt, G.M.; Wong, K.T.; Gomersall, C.D.; Li, T.S.; Antonio, G.; Ko, F.W.; Chan, M.C.; Chan, D.P.; Tong, M.W.; et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax 2005, 60, 401–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, D.S.; Wong, K.T.; Ko, F.W.; Tam, L.S.; Chan, D.P.; Woo, J.; Sung, J.J. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest 2005, 128, 2247–2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, K.M.; Lee, E.Y.; Singh, R.; Enani, M.A.; Al Dossari, K.; Van Gorkom, K.; Larsson, S.G.; Langer, R.D. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J. Radiol. Imaging 2017, 27, 342–349. [Google Scholar] [CrossRef]
- Available online: https://www.aifa.gov.it/aggiornamento-sui-farmaci-utilizzabili-per-il-trattamento-della-malattia-covid19 (accessed on 22 May 2022).
- Available online: https://www.flipsnack.com/SIAARTI/siaarti_raccomandazioni_per_la_gestione_del_paziente_criti/full-view.html (accessed on 22 May 2022).
- Stanojevic, S.; Kaminsky, D.A.; Miller, M.; Thompson, B.; Aliverti, A.; Barjaktarevic, I.; Cooper, B.G.; Culver, B.; Derom, E.; Hall, G.L.; et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur. Respir. J. 2021, 23, 2101499. [Google Scholar] [CrossRef]
- Salahshour, F.; Mehrabinejad, M.M.; Nassiri Toosi, M.; Gity, M.; Ghanaati, H.; Shakiba, M.; Kolahi, S. Clinical and chest CT features as a predictive tool for COVID-19 clinical progress: Introducing a novel semi-quantitative scoring system. Eur. Radiol. 2021, 31, 5178–5188. [Google Scholar] [CrossRef]
- Brody, A.S.; Klein, J.S.; Molina, P.L.; Quan, J.; Bean, J.A.; Wilmott, R.W. High-resolution computed tomography in young patients with cystic fibrosis: Distribution of abnormalities and correlation with pulmonary function tests. J. Pediatr. 2004, 145, 32–38. [Google Scholar] [CrossRef]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef] [Green Version]
- Froidure, A.; Mahsouli, A.; Liistro, G.; De Greef, J.; Belkhir, L.; Gérard, L.; Bertrand, A.; Koenig, S.; Pothen, L.; Yildiz, H.; et al. Integrative respiratory follow-up of severe COVID-19 reveals common functional and lung imaging sequelae. Respir. Med. 2021, 181, 106383. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, X.; Zhou, Y.; Yu, H.; Li, R.; Zhan, Q.; Ni, F.; Fang, S.; Lu, Y.; Ding, X.; et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: A prospective study. Lancet Respir. Med. 2021, 9, 747–754. [Google Scholar] [CrossRef]
- Cocconcelli, E.; Bernardinello, N.; Giraudo, C.; Castelli, G.; Giorgino, A.; Leoni, D.; Petrarulo, S.; Ferrari, A.; Saetta, M.; Cattelan, A.; et al. Characteristics and Prognostic Factors of Pulmonary Fibrosis after COVID-19 Pneumonia. Front. Med. 2022, 8, 823600. [Google Scholar] [CrossRef]
- Franquet, T.; Giménez, A.; Ketai, L.; Mazzini, S.; Rial, A.; Pomar, V.; Domingo, P. Air trapping in COVID-19 patients following hospital discharge: Retrospective evaluation with paired inspiratory/expiratory thin-section CT. Eur. Radiol. 2022, 32, 4427–4436. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhu, J.; Zhou, J.; Shang, Y.; Lin, X.; Gong, S.; Gu, L.; Dai, H.; Li, Y. Inspiratory and Expiratory Chest High-resolution CT: Small-airway Disease Evaluation in Patients with COVID-19. Curr. Med. Imaging 2021, 17, 1299–1307. [Google Scholar] [CrossRef]
- Choi, H.; Lee, H.; Lee, S.K.; Yang, B.; Chung, S.J.; Yeo, Y.; Park, T.S.; Park, D.W.; Moon, J.Y.; Kim, T.H.; et al. Impact of bronchiectasis on susceptibility to and severity of COVID-19: A nationwide cohort study. Adv. Respir. Dis. 2021, 15, 1753466621995043. [Google Scholar] [CrossRef]
- Baratella, E.; Ruaro, B.; Marrocchio, C.; Starvaggi, N.; Salton, F.; Giudici, F.; Quaia, E.; Confalonieri, M.; Cova, M.A. Interstitial Lung Disease at High Resolution CT after SARS-CoV-2-Related Acute Respiratory Distress Syndrome According to Pulmonary Segmental Anatomy. J. Clin. Med. 2021, 10, 3985. [Google Scholar] [CrossRef]
- Sonnweber, T.; Sahanic, S.; Pizzini, A.; Luger, A.; Schwabl, C.; Sonnweber, B.; Kurz, K.; Koppelstätter, S.; Haschka, D.; Petzer, V.; et al. Cardiopulmonary recovery after COVID-19: An observational prospective multicentre trial. Eur. Respir. J. 2021, 57, 2003481. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Guler, S.A.; Ebner, L.; Aubry-Beigelman, C.; Bridevaux, P.O.; Brutsche, M.; Clarenbach, C.; Garzoni, C.; Geiser, T.K.; Lenoir, A.; Mancinetti, M.; et al. Pulmonary function and radiological features 4 months after COVID-19: First results from the national prospective observational Swiss COVID-19 lung study. Eur. Respir. J. 2021, 57, 2003690. [Google Scholar] [CrossRef]
- Hama Amin, B.J.; Kakamad, F.H.; Ahmed, G.S.; Ahmed, S.F.; Abdulla, B.A.; Mohammed, S.H.; Mikael, T.M.; Salih, R.Q.; Ali, R.K.; Salh, A.M.; et al. Post COVID-19 pulmonary fibrosis; A meta-analysis study. Ann. Med. Surg. 2022, 77, 103590. [Google Scholar] [CrossRef] [PubMed]
- Kyung Soo, L.; Yu, M.W. Residual Lung Lesions at 1-year CT after COVID-19. Radiology 2022, 302, 720–721. [Google Scholar]
- Garg, M.; Maralakunte, M.; Dhooria, S. Sequelae of COVID-19 pneumonia: Is it correct to label everything as post-COVID lung fibrosis? J. Postgrad. Med. 2021, 67, 224–227. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).