Risk Stratification of Patients with Pulmonary Arterial Hypertension: The Role of Echocardiography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Variables of Interest
2.3. Outcome of Interest
2.4. Statistical Analysis
2.5. Risk Stratification
3. Results
3.1. Study Population and Characteristics
3.2. Outcomes
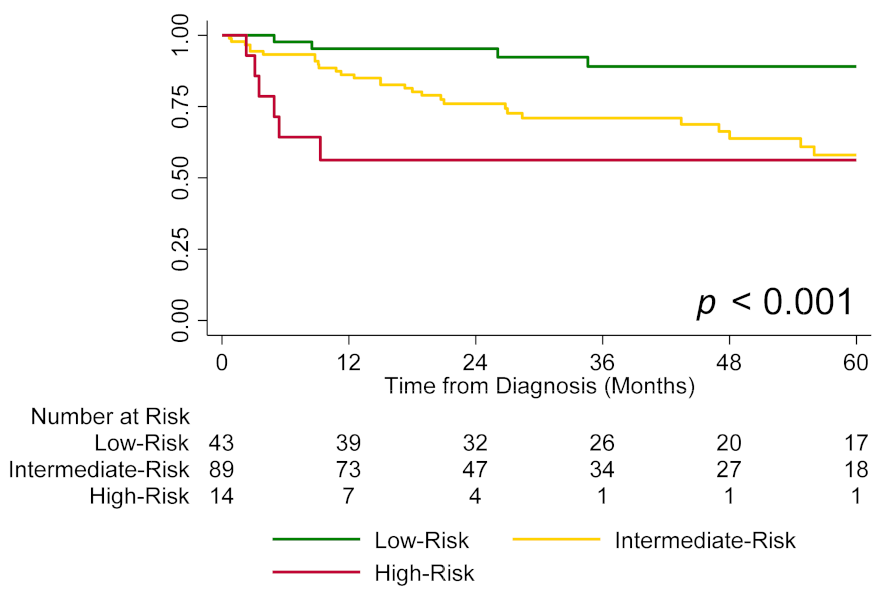
3.3. Echocardiography, Risk Groups, and Survival
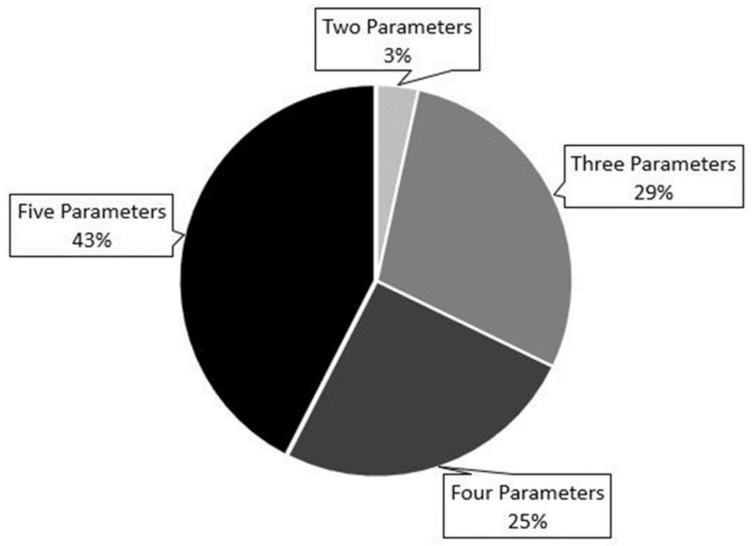
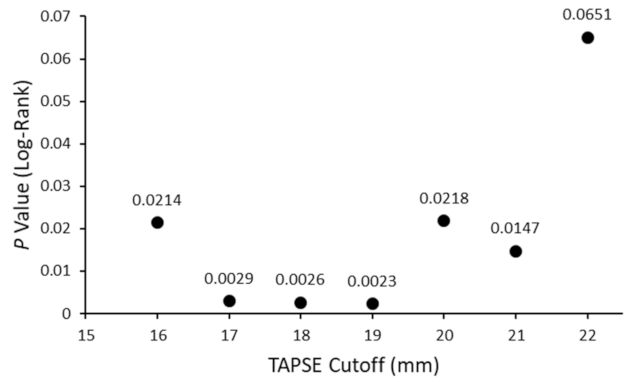
3.4. Use of Echocardiography for Risk Stratification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Parameter | Low Risk | Intermediate Risk | High Risk |
|---|---|---|---|
| WHO FC | I or II | III | IV |
| 6-MWD, m | >440 | 165–440 | <165 |
| NT-proBNP (BNP), ng/L | <300 (<50) | 300–1400 (50–300) | >1400 (>300) |
| RAP on RHC, mmHg | <8 | 8–14 | >14 |
| CI, L/min/m2 | ≥2.5 | 2–2.49 | <2 |
| Mean ± SD | |
|---|---|
| Heart Rate, b/min [n = 69] | 76 ± 14 |
| RAP, mmHg [n = 109] | 9 ± 4 |
| mPAP, mmHg [n = 109] | 46 ± 13 |
| PVR, Wood Units [n = 108] | 8.7 ± 1.8 |
| PCWP, mmHg [n = 75] | 10.6 ± 3.6 |
| CO, L/min [n = 75] | 5.0 ± 1.7 |
| CI, L/min/m2 [n = 105] | 2.6 ± 0.8 |
References
- Hassoun, P.M. Pulmonary Arterial Hypertension. N. Engl. J. Med. 2021, 385, 2361–2376. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, V.; Bianco, A.; Campi, G.; Cuomo, A.; Diab, N.; Mancini, A.; Parrella, P.; Petretta, M.; Hassoun, P.M.; Bonaduce, D. New Drugs, Therapeutic Strategies, and Future Direction for the Treatment of Pulmonary Arterial Hypertension. Curr. Med. Chem. 2019, 26, 2844–2864. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Channick, R.N.; Frantz, R.P.; Grünig, E.; Jing, Z.C.; Moiseeva, O.; Preston, I.R.; Pulido, T.; Safdar, Z.; Tamura, Y.; et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur. Respir. J. 2019, 53, 1801889. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiery, J.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [CrossRef]
- Boucly, A.; Weatherald, J.; Savale, L.; Jaïs, X.; Cottin, V.; Prevot, G.; Picard, F.; de Groote, P.; Jevnikar, M.; Bergot, E.; et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur. Respir. J. 2017, 50, 1700889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoeper, M.M.; Kramer, T.; Pan, Z.; Eichstaedt, C.A.; Spiesshoefer, J.; Benjamin, N.; Olsson, K.M.; Meyer, K.; Vizza, C.D.; Vonk-Noordegraaf, A.; et al. Mortality in pulmonary arterial hypertension: Prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur. Respir. J. 2017, 50, 1700740. [Google Scholar] [CrossRef] [Green Version]
- Kylhammar, D.; Kjellström, B.; Hjalmarsson, C.; Jansson, K.; Nisell, M.; Söderberg, S.; Wikström, G.; Rådegran, G. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur. Heart J. 2018, 39, 4175–4181. [Google Scholar] [CrossRef]
- Benza, R.L.; Gomberg-Maitland, M.; Miller, D.P.; Frost, A.; Frantz, R.P.; Foreman, A.J.; Badesch, D.B.; McGoon, M.D. The REVEAL Registry Risk Score Calculator in Patients Newly Diagnosed With Pulmonary Arterial Hypertension. Chest 2012, 141, 354–362. [Google Scholar] [CrossRef]
- Benza, R.L.; Kanwar, M.K.; Raina, A.; Scott, J.V.; Zhao, C.L.; Selej, M.; Elliott, C.G.; Farber, H.W. Development and Validation of an Abridged Version of the REVEAL 2.0 Risk Score Calculator, REVEAL Lite 2, for Use in Patients With Pulmonary Arterial Hypertension. Chest 2021, 159, 337–346. [Google Scholar] [CrossRef]
- Badagliacca, R.; D’Alto, M.; Ghio, S.; Argiento, P.; Bellomo, V.; Brunetti, N.D.; Casu, G.; Confalonieri, M.; Corda, M.; Correale, M.; et al. Risk Reduction and Hemodynamics with Initial Combination Therapy in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2021, 203, 484–492. [Google Scholar] [CrossRef]
- D’Alto, M.; Badagliacca, R.; Lo Giudice, F.; Argiento, P.; Casu, G.; Corda, M.; Correale, M.; Ghio, S.; Greco, A.; Lattanzio, M.; et al. Hemodynamics and risk assessment 2 years after the initiation of upfront ambrisentan—tadalafil in pulmonary arterial hypertension. J. Heart Lung Transplant. 2020, 39, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Weatherald, J.; Boucly, A.; Sitbon, O. Risk stratification in pulmonary arterial hypertension. Curr. Opin. Pulm. Med. 2018, 24, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Forfia, P.R.; Fisher, M.R.; Mathai, S.C.; Housten-Harris, T.; Hemnes, A.R.; Borlaug, B.A.; Chamera, E.; Corretti, M.C.; Champion, H.C.; Abraham, T.P.; et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2006, 174, 1034–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghio, S.; Klersy, C.; Magrini, G.; D’Armini, A.M.; Scelsi, L.; Raineri, C.; Pasotti, M.; Serio, A.; Campana, C.; Viganò, M. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int. J. Cardiol. 2010, 140, 272–278. [Google Scholar] [CrossRef]
- Mukherjee, M.; Mercurio, V.; Tedford, R.J.; Shah, A.A.; Hsu, S.; Mullin, C.J.; Sato, T.; Damico, R.; Kolb, T.M.; Mathai, S.C.; et al. Right ventricular longitudinal strain is diminished in systemic sclerosis compared with idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2017, 50, 1701436. [Google Scholar] [CrossRef]
- Badagliacca, R.; Pezzuto, B.; Papa, S.; Poscia, R.; Manzi, G.; Pascaretta, A.; Miotti, C.; Luongo, F.; Scoccia, G.; Ciciarello, F.; et al. Right Ventricular Strain Curve Morphology and Outcome in Idiopathic Pulmonary Arterial Hypertension. JACC Cardiovasc. Imaging 2021, 14, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Mathai, S.C.; Sibley, C.T.; Forfia, P.R.; Mudd, J.O.; Fisher, M.R.; Tedford, R.J.; Lechtzin, N.; Boyce, D.; Hummers, L.K.; Housten, T.; et al. Tricuspid annular plane systolic excursion is a robust outcome measure in systemic sclerosis-associated pulmonary arterial hypertension. J. Rheumatol. 2011, 38, 2410–2418. [Google Scholar] [CrossRef]
- Mazurek, J.A.; Vaidya, A.; Mathai, S.C.; Roberts, J.D.; Forfia, P.R. Follow-up tricuspid annular plane systolic excursion predicts survival in pulmonary arterial hypertension. Pulm. Circ. 2017, 7, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Hoeper, M.M.; Bogaard, H.J.; Condliffe, R.; Frantz, R.; Khanna, D.; Kurzyna, M.; Langleben, D.; Manes, A.; Satoh, T.; Torres, F.; et al. Definitions and diagnosis of pulmonary hypertension. Turk Kardiyol. Dern. Ars. 2014, 42 (Suppl. 1), 55–66. [Google Scholar] [CrossRef] [Green Version]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Newson, R.B. Comparing the Predictive Powers of Survival Models Using Harrell’s C or Somers’ D. Stata J. 2010, 10, 339–358. [Google Scholar] [CrossRef] [Green Version]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–688. [Google Scholar] [CrossRef]
- Ghio, S.; Mercurio, V.; Fortuni, F.; Forfia, P.R.; Gall, H.; Ghofrani, A.; Mathai, S.C.; Mazurek, J.A.; Mukherjee, M.; Richter, M.; et al. A comprehensive echocardiographic method for risk stratification in pulmonary arterial hypertension. Eur. Respir. J. 2020, 56, 2000513. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.; Sánchez-Quintana, D.; Bossone, E.; Bogaard, H.J.; Naeije, R. Anatomy, Function, and Dysfunction of the Right Ventricle: JACC State-of-the-Art Review. J. Am. Coll. Cardiology. 2019, 73, 1463–1482. [Google Scholar] [CrossRef] [PubMed]
- Badagliacca, R.; Papa, S.; Matsubara, H.; Lang, I.M.; Poscia, R.; Manzi, G.; Vizza, C.D. The importance of right ventricular evaluation in risk assessment and therapeutic strategies: Raising the bar in pulmonary arterial hypertension. Int. J. Cardiol. 2020, 301, 183–189. [Google Scholar] [CrossRef]
- Noordegraaf, A.V.; Chin, K.M.; Haddad, F.; Hassoun, P.M.; Hemnes, A.R.; Hopkins, S.R.; Kawut, S.M.; Langleben, D.; Lumens, J.; Naeije, R. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: An update. Eur. Respir. J. 2019, 53, 1801900. [Google Scholar] [CrossRef]
- Tello, K.; Axmann, J.; Ghofrani, H.A.; Naeije, R.; Narcin, N.; Rieth, A.; Seeger, W.; Gall, H.; Richter, M.J. Relevance of the TAPSE/PASP ratio in pulmonary arterial hypertension. Int. J. Cardiol. 2018, 266, 229–235. [Google Scholar] [CrossRef]
- Yogeswaran, A.; Richter, M.J.; Sommer, N.; Ghofrani, H.A.; Seeger, W.; Tello, K.; Gall, H. Advanced risk stratification of intermediate risk group in pulmonary arterial hypertension. Pulm. Circ. 2020, 10, 2045894020961739. [Google Scholar] [CrossRef]
- Sachdev, A.; Villarraga, H.R.; Frantz, R.P.; McGoon, M.D.; Hsiao, J.; Maalouf, J.F.; Ammash, N.M.; McCully, R.B.; Miller, F.A.; Pellikka, P.A.; et al. Right Ventricular Strain for Prediction of Survival in Patients With Pulmonary Arterial Hypertension. Chest 2011, 139, 1299–1309. [Google Scholar] [CrossRef] [Green Version]
- Boucly, A.; Weatherald, J.; Humbert, M.; Sitbon, O. Risk assessment in pulmonary arterial hypertension. Eur. Respir. J. 2018, 51, 1800279. [Google Scholar] [CrossRef]
- Coghlan, J.G.; Picken, C.; Clapp, L.H. Selexipag in the management of pulmonary arterial hypertension: An update. Drug Healthc. Patient Saf. 2019, 11, 55–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coghlan, J.G.; Galiè, N.; Barberà, J.A.; Frost, A.E.; Ghofrani, H.A.; Hoeper, M.M.; Kuwana, M.; McLaughlin, V.V.; Peacock, A.J.; Simonneau, G.; et al. AMBITION investigators. Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): Subgroup analysis from the AMBITION trial. Ann. Rheum. Dis. 2017, 76, 1219–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoeper, M.M.; Simonneau, G.; Corris, P.A.; Ghofrani, H.A.; Klinger, J.R.; Langleben, D.; Naeije, R.; Jansa, P.; Rosenkranz, S.; Scelsi, L.; et al. RESPITE: Switching to riociguat in pulmonary arterial hypertension patients with inadequate response to phosphodiesterase-5 inhibitors. Eur. Respir. J. 2017, 50, 1602425. [Google Scholar] [CrossRef] [Green Version]
- Toxvig, A.K.; Wehland, M.; Grimm, D.; Infanger, M.; Krüger, M. A focus on riociguat in the treatment of pulmonary arterial hypertension. Basic Clin Pharm. Toxicol 2019, 125, 202–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benza, R.; Corris, P.; Ghofrani, A.; Kanwar, M.K.; McLaughlin, V.V.; Raina, A.; Simonneau, G. EXPRESS: Switching to riociguat: A potential treatment strategy for the management of CTEPH and PAH. Pulm. Circ. 2019, 10, 2045894019837849. [Google Scholar] [CrossRef] [Green Version]
- Dardi, F.; Palazzini, M.; Gotti, E.; Zuffa, E.; De Lorenzis, A.; Pasca, F.; Guarino, D.; Magnani, I.; Rotunno, M.; Rinaldi, A.; et al. Abstract 9570: Prognostic Value of Stroke Volume Index in Patients With Pulmonary Arterial Hypertension at Intermediate Risk. Circulation 2019, 140, A9570. [Google Scholar]
- Hoeper, M.M.; Pausch, C.; Olsson, K.M.; Huscher, D.; Pittrow, D.; Grünig, E.; Staehler, G.; Vizza, C.D.; Gall, H.; Distler, O.; et al. COMPERA 2.0: A refined 4-strata risk assessment model for pulmonary arterial hypertension. Eur. Respir. J. 2021; 60, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Boucly, A.; Weatherald, J.; Savale, L.; de Groote, P.; Cottin, V.; Prévot, G.; Chaouat, A.; Picard, F.; Horeau-Langlard, D.; Bourdin, A.; et al. External validation of a refined 4-strata risk assessment score from the French pulmonary hypertension Registry. Eur. Respir. J. 2021, 59, 2102419. [Google Scholar] [CrossRef]
- Raphael, C.; Briscoe, C.; Davies, J.; Ian Whinnett, Z.; Manisty, C.; Sutton, R.; Mayet, J.; Francis, D.P. Limitations of the New York Heart Association functional classification system and self-reported walking distances in chronic heart failure. Heart 2007, 93, 476–482. [Google Scholar] [CrossRef]
- Avouac, J.; Kowal-Bielecka, O.; Pittrow, D.; Huscher, D.; Behrens, F.; Denton, C.P.; Foeldvari, I.; Humbert, M.; Matucci-Cerinic, M.; Nash, P.; et al. EPOSS Group. Validation of the 6 min walk test according to the OMERACT filter: A systematic literature review by the EPOSS-OMERACT group. Ann. Rheum. Dis. 2010, 69, 1360–1363. [Google Scholar] [CrossRef] [Green Version]
- Benza, R.L.; Gomberg-Maitland, M.; Elliott, C.G.; Farber, H.W.; Foreman, A.J.; Frost, A.E.; McGoon, M.D.; Pasta, D.J.; Selej, M.; Burger, C.D.; et al. Predicting Survival in Patients With Pulmonary Arterial Hypertension: The REVEAL Risk Score Calculator 2.0 and Comparison With ESC/ERS-Based Risk Assessment Strategies. Chest 2019, 156, 323–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicenzi, M.; Caravita, S.; Rota, I.; Casella, R.; Deboeck, G.; Beretta, L.; Lombi, A.; Vachiery, J.L. The added value of right ventricular function normalized for afterload to improve risk stratification of patients with pulmonary arterial hypertension. PLoS ONE 2022, 17, e0265059. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.R.; Forfia, P.R.; Chamera, E.; Housten-Harris, T.; Champion, H.C.; Girgis, R.E.; Corretti, M.C.; Hassoun, P.M. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2009, 179, 615–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Mean ± SD or n (%); n= 146 | ||
|---|---|---|
| PAH Diagnosis Age, Years | 56 ± 14 | |
| Female | 121 (82.9) | |
| Race | White | 114 (78.1) |
| African American | 24 (16.4) | |
| Other | 8 (5.5) | |
| PAH Subtype | IPAH | 68 (46.6) |
| CTD-PAH | 67 (45.9) | |
| Other | 11 (7.5) | |
| Treatment | CCB only | 8 (5.5) |
| PAH-specific therapy | 138 (94.5) | |
| Single/dual/triple * | 51 (36.9)/65 (47.1)/22 (16) | |
| PDE5-I/ERA/prostanoids * | 109 (79)/91 (65.9)/47 (34.1) |
| n (%) | ||
|---|---|---|
| WHO FC [n = 146] | I/II | 72 (49.3) |
| III | 49 (33.6) | |
| IV | 25 (17.1) | |
| 6-MWD, m [n = 133] | >440 m | 36 (27.1) |
| 165–440 m | 86 (64.7) | |
| <165 m | 11 (8.3) | |
| NT-proBNP/BNP, ng/L [n = 101] | <300/<50 | 28 (27.7) |
| 300–1400/50–300 | 41 (40.6) | |
| >1400/>300 | 32 (31.7) | |
| RAP, mmHg [n = 109] | <8 | 50 (45.9) |
| 8–14 | 46 (42.2) | |
| >14 | 13 (11.9) | |
| CI, L/min/m2 [n = 105] | ≥2.5 | 54 (51.4) |
| 2–2.4 | 27 (25.7) | |
| <2 | 24 (22.9) |
| Mean ± SD or n (%) | ||||
|---|---|---|---|---|
| Low | Intermediate | High | p-Value † | |
| RA Area, cm2 | 18.9 ± 6.2 [n = 36] | 21.3 ± 7.6 [n = 67] | 27.4 ± 7.4 [n = 8] | 0.011 |
| RV End-Systolic Area, cm2 | 18.9 ± 7.1 [n = 35] | 21.1 ± 8.4 [n = 63] | 29.0 ± 7.4 [n = 7] | 0.010 |
| RV End-Diastolic Area, cm2 | 26.5 ± 8.0 [n = 35] | 28.5 ± 9.1 [n = 63] | 35.7 ± 6.9 [n = 7] | 0.041 |
| Moderate/Severe RA Dilation * (vs. None/Mild) | 12 (33.3) [n = 36] | 26 (38.8) [n = 67] | 5 (62.5) [n = 8] | 0.353 |
| Moderate/Severe RV Dilation ° (vs. None/Mild) | 9 (25.7) [n = 35] | 20 (31.8) [n = 63] | 3 (42.9) [n = 7] | 0.587 |
| RV FAC, % | 30.1 ± 11.2[n = 42] | 27.8 ± 11.4 [n = 87] | 19.6 ± 9.9 [n = 14] | 0.011 |
| TAPSE, mm | 19.6 ± 4.0 [n = 39] | 19.2 ± 5.1[n = 85] | 13.4 ± 3.1 [n = 14] | <0.001 |
| PASP, mmHg | 47.5 ± 24.2 [n = 30] | 56.2 ± 24.9 [n = 60] | 52.8 ± 8.8 [n = 10] | 0.260 |
| TAPSE/PASP, mm/mmHg | 0.65 ± 0.53 [n = 27] | 0.42 ± 0.28 [n = 56] | 0.26 ± 0.05 [n = 10] | 0.005 |
| LVED Eccentricity Index | 1.04 ± 0.30 [n = 22] | 1.18 ± 0.35 [n = 54] | 1.27 ± 0.43 [n = 9] | 0.156 |
| LVES Eccentricity Index | 0.98 ± 0.31 [n = 24] | 1.36 ± 0.87 [n = 46] | 1.88 ± 2.05 [n = 8] | 0.056 |
| Moderate/Severe TR ” (vs. None/Trace/Mild) | 11 (25.6) [n = 43] | 27 (30.7) [n = 88] | 8 (61.5) [n = 13] | 0.044 |
| HR (95% CI) * | p-Value | |
|---|---|---|
| RA area, per 1 cm2 increase | 1.03 (0.98–1.07) | 0.239 |
| RV end-systolic area, per 1 cm2 increase | 1.04 (0.99–1.08) | 0.119 |
| RV end-diastolic area, per 1 cm2 increase | 1.04 (1.00–1.08) | 0.065 |
| Moderate/severe RA dilation (vs. none/mild) | 1.41 (0.64–3.12) | 0.393 |
| Moderate/severe RV dilation (vs. none/mild) | 1.27 (0.55–2.95) | 0.579 |
| RV FAC, per 5% decrease | 1.10 (0.96–1.28) | 0.177 |
| TAPSE, per 2 mm decrease | 1.24 (1.08–1.43) | 0.002 |
| PASP, per 5 mmHg | 1.04 (0.96–1.12) | 0.349 |
| TAPSE/PASP, per 1 mm/5 mmHg decrease | 1.5 (1.04–2.11) | 0.03 |
| LVED Eccentricity Index, per unit increase | 2.5 (0.84–7.36) | 0.100 |
| LVES Eccentricity Index, per unit increase | 1.21 (0.86–1.72) | 0.279 |
| Moderate/severe TR (vs. none/trace/mild) | 3.27 (1.72–6.23) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercurio, V.; Hassan, H.J.; Naranjo, M.; Cuomo, A.; Mazurek, J.A.; Forfia, P.R.; Balasubramanian, A.; Simpson, C.E.; Damico, R.L.; Kolb, T.M.; et al. Risk Stratification of Patients with Pulmonary Arterial Hypertension: The Role of Echocardiography. J. Clin. Med. 2022, 11, 4034. https://doi.org/10.3390/jcm11144034
Mercurio V, Hassan HJ, Naranjo M, Cuomo A, Mazurek JA, Forfia PR, Balasubramanian A, Simpson CE, Damico RL, Kolb TM, et al. Risk Stratification of Patients with Pulmonary Arterial Hypertension: The Role of Echocardiography. Journal of Clinical Medicine. 2022; 11(14):4034. https://doi.org/10.3390/jcm11144034
Chicago/Turabian StyleMercurio, Valentina, Hussein J. Hassan, Mario Naranjo, Alessandra Cuomo, Jeremy A. Mazurek, Paul R. Forfia, Aparna Balasubramanian, Catherine E. Simpson, Rachel L. Damico, Todd M. Kolb, and et al. 2022. "Risk Stratification of Patients with Pulmonary Arterial Hypertension: The Role of Echocardiography" Journal of Clinical Medicine 11, no. 14: 4034. https://doi.org/10.3390/jcm11144034
APA StyleMercurio, V., Hassan, H. J., Naranjo, M., Cuomo, A., Mazurek, J. A., Forfia, P. R., Balasubramanian, A., Simpson, C. E., Damico, R. L., Kolb, T. M., Mathai, S. C., Hsu, S., Mukherjee, M., & Hassoun, P. M. (2022). Risk Stratification of Patients with Pulmonary Arterial Hypertension: The Role of Echocardiography. Journal of Clinical Medicine, 11(14), 4034. https://doi.org/10.3390/jcm11144034







