Advances in Percutaneous Patent Foramen Ovale Closure: From the Procedure to the Echocardiographic Guidance
Abstract
1. Introduction
2. Percutaneous Closure of PFO by Traditional Devices
2.1. Double Disc Devices
2.2. Procedural Steps
- -
- The device has well hugged the septum primum, particularly the septum secundum;
- -
- There is no interference with the atrioventricular valves or the other intracardiac structures
- -
- There are no patent accessory fenestrations
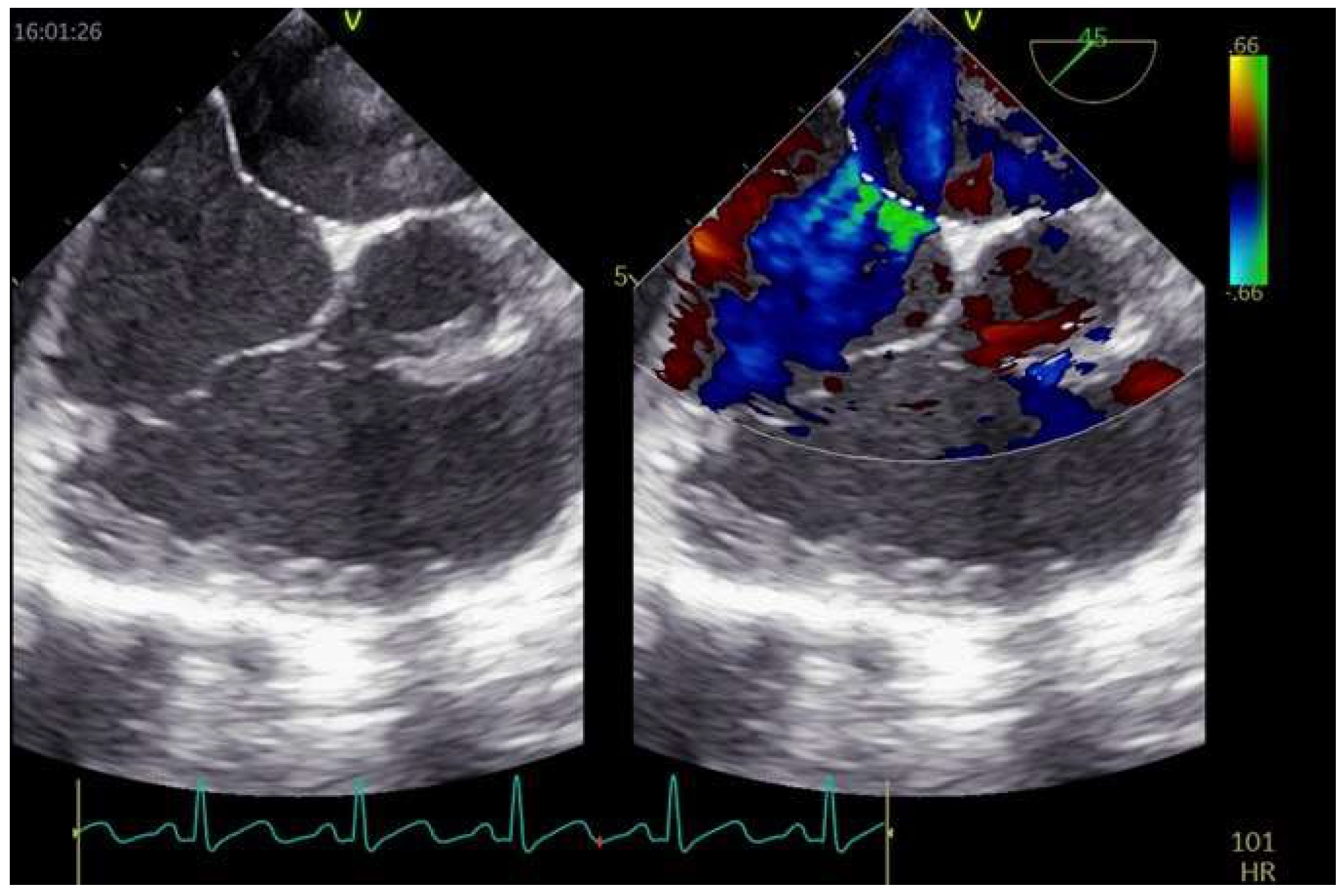
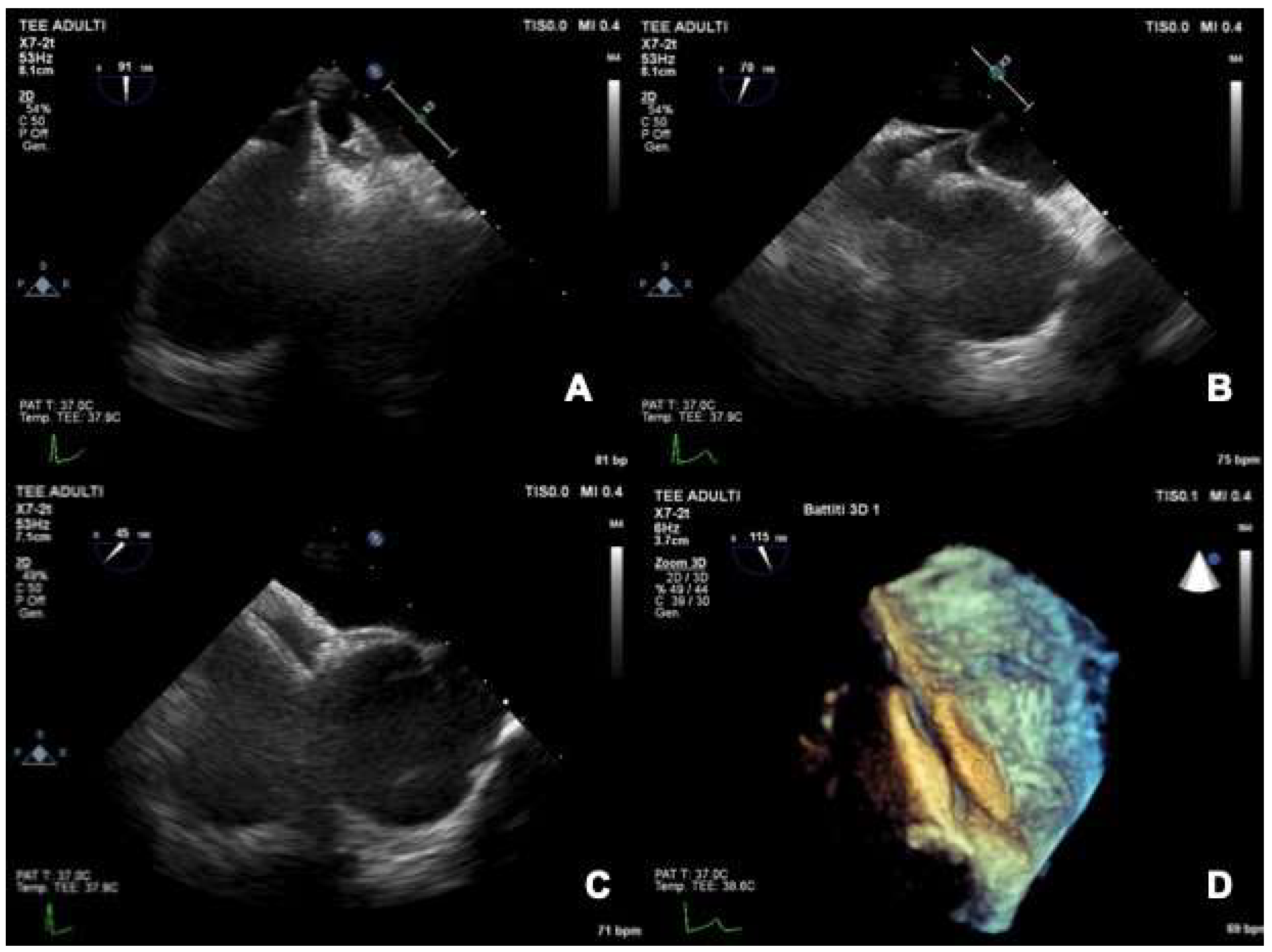
2.3. Role of TEE
2.3.1. TEE before PFO Closure: Characterization of the PFO and Decision Making
2.3.2. TEE during PFO Closure: Intra-Procedural Guidance
2.3.3. TEE after PFO Closure: Post-Procedural Follow-Up
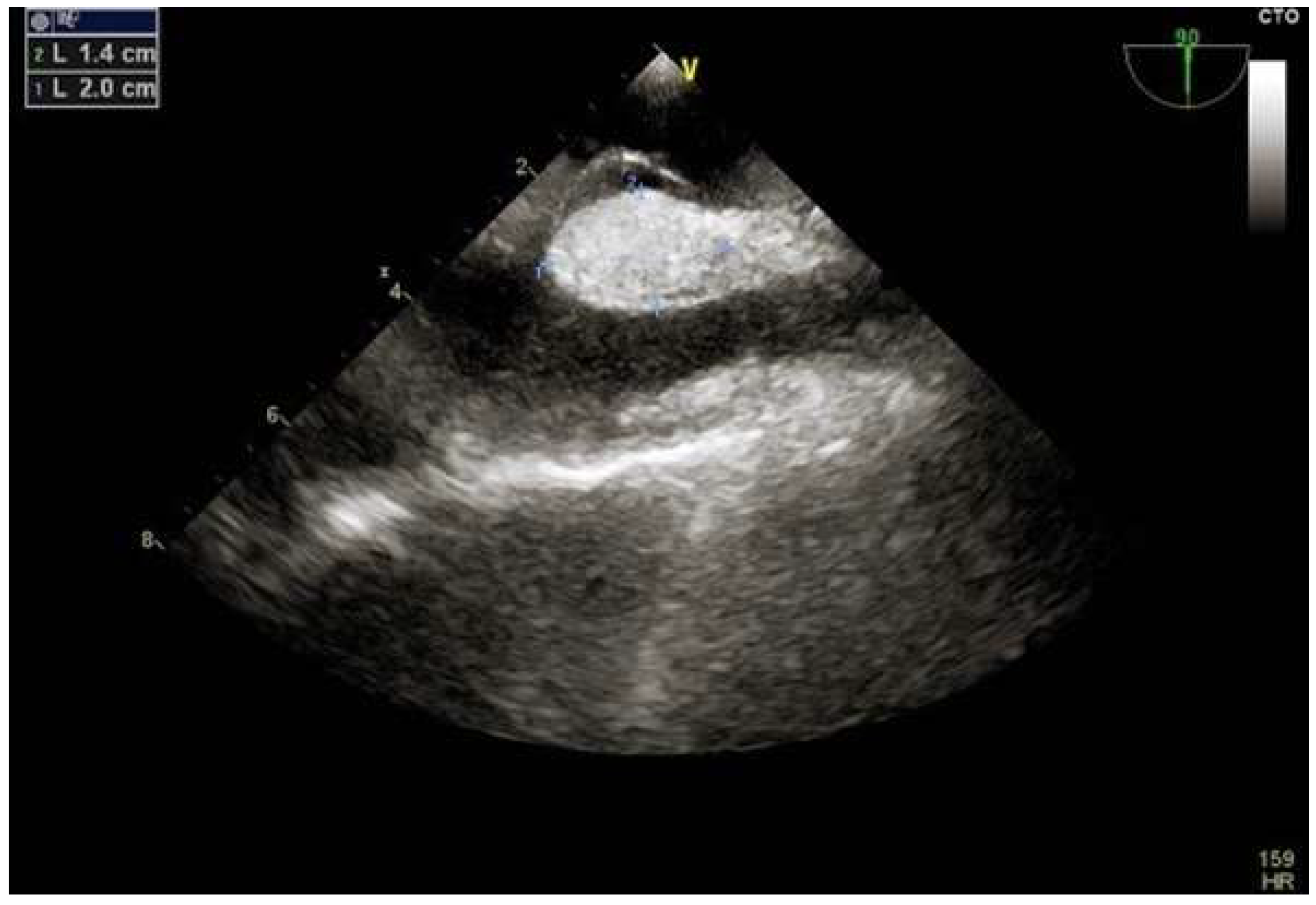
2.3.4. Intracardiac Echocardiography
3. Percutaneous Closure of PFO with Complex Anatomy
3.1. Aneurismatic Interatrial Septum
3.2. Multiple Accessory Fenestrations
3.3. Long and Stiff PFO Tunnel
3.4. Hypertrophy of the Septum Secundum
3.5. Redundant Eustachian Valve and Chiari Network
3.6. Malalignment of Interatrial Septum
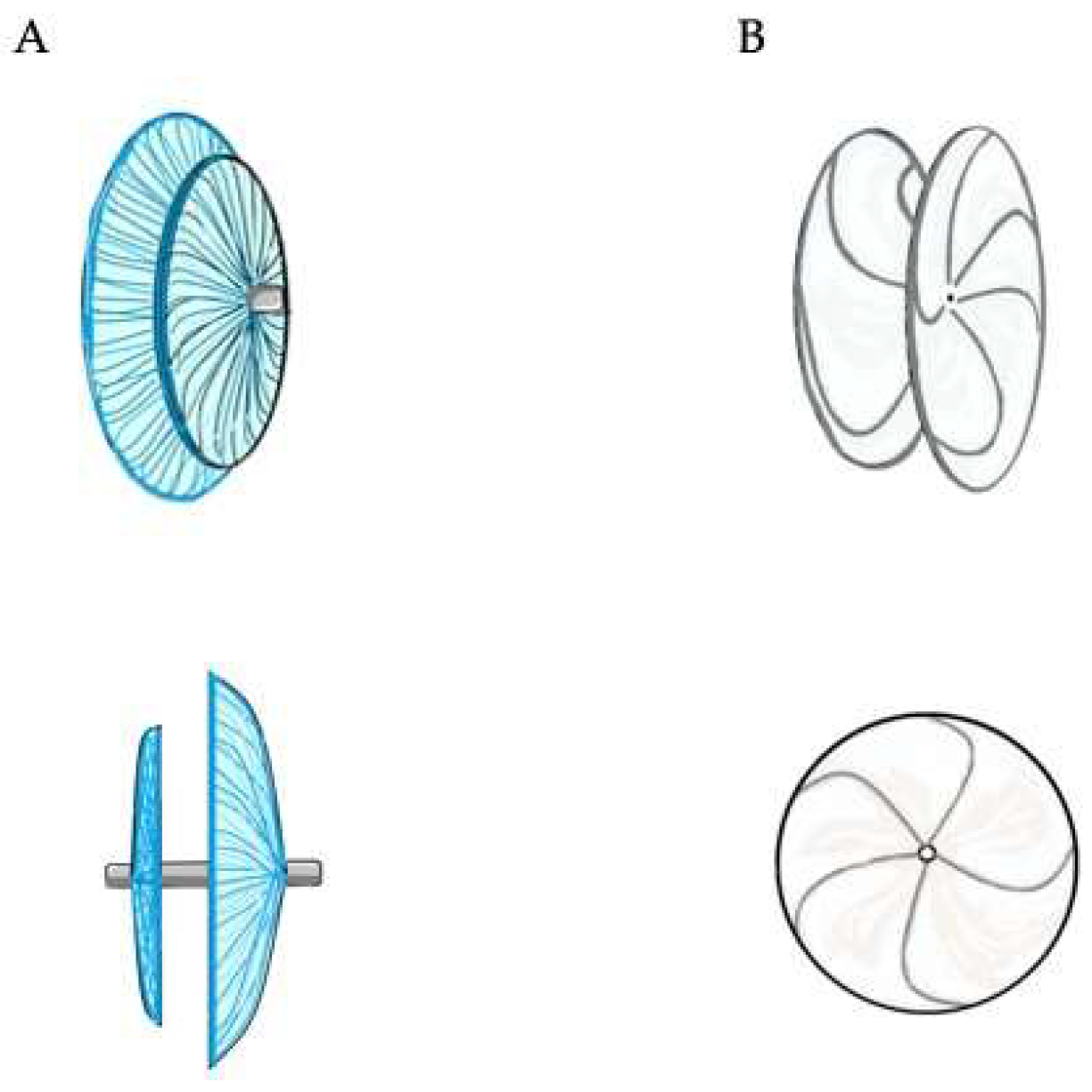
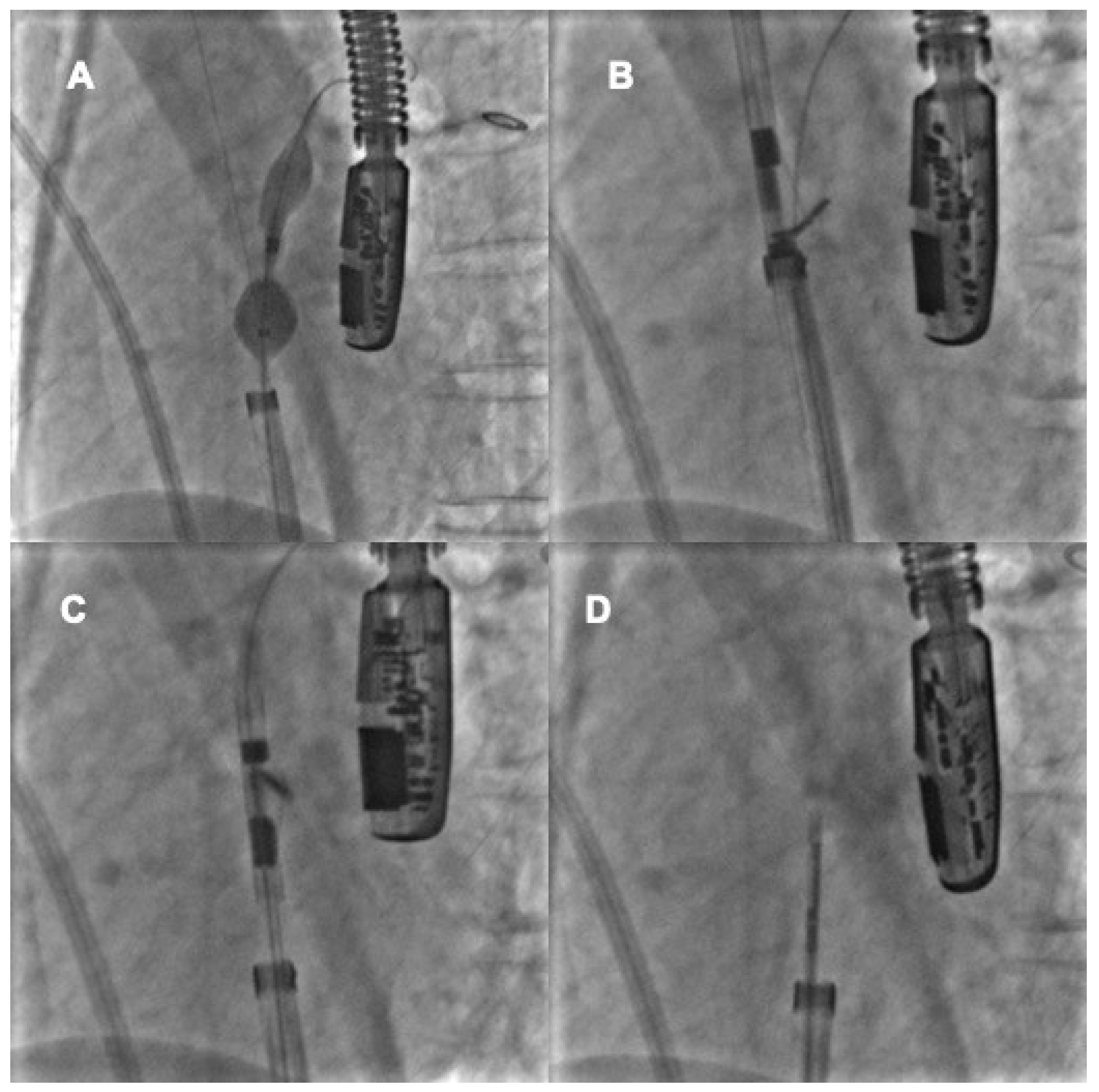
4. Percutaneous Closure of PFO by Novel Devices: The NobleStitch
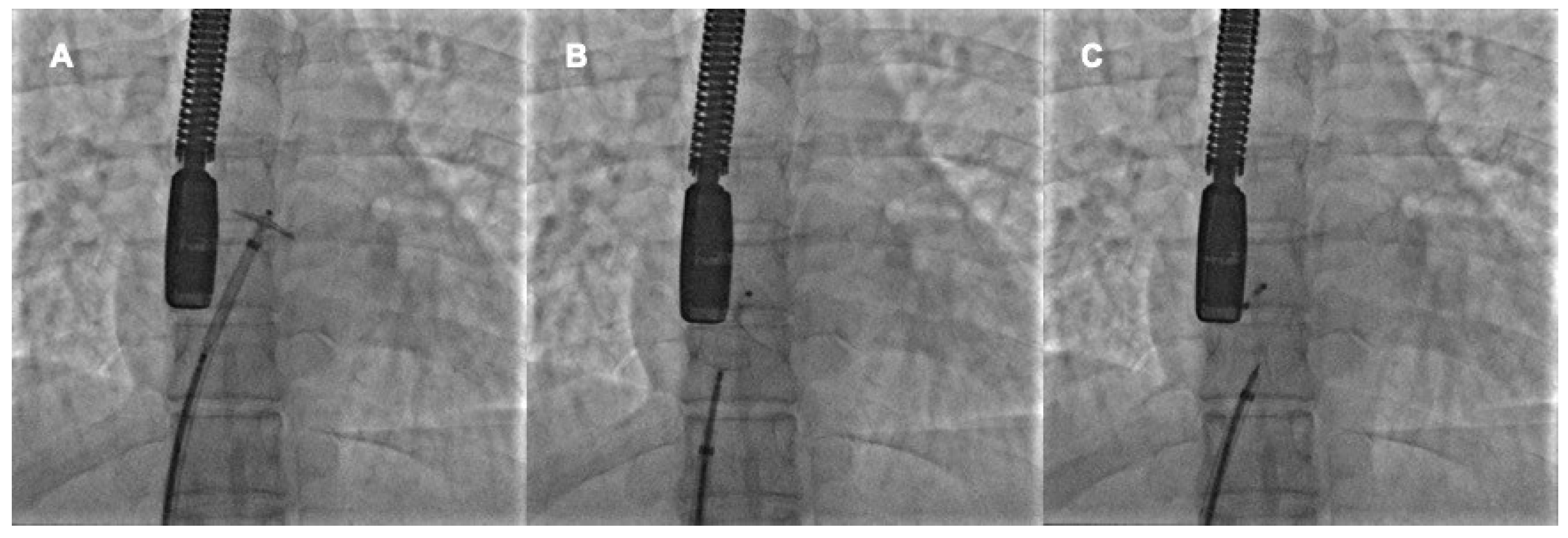
4.1. Procedural Steps
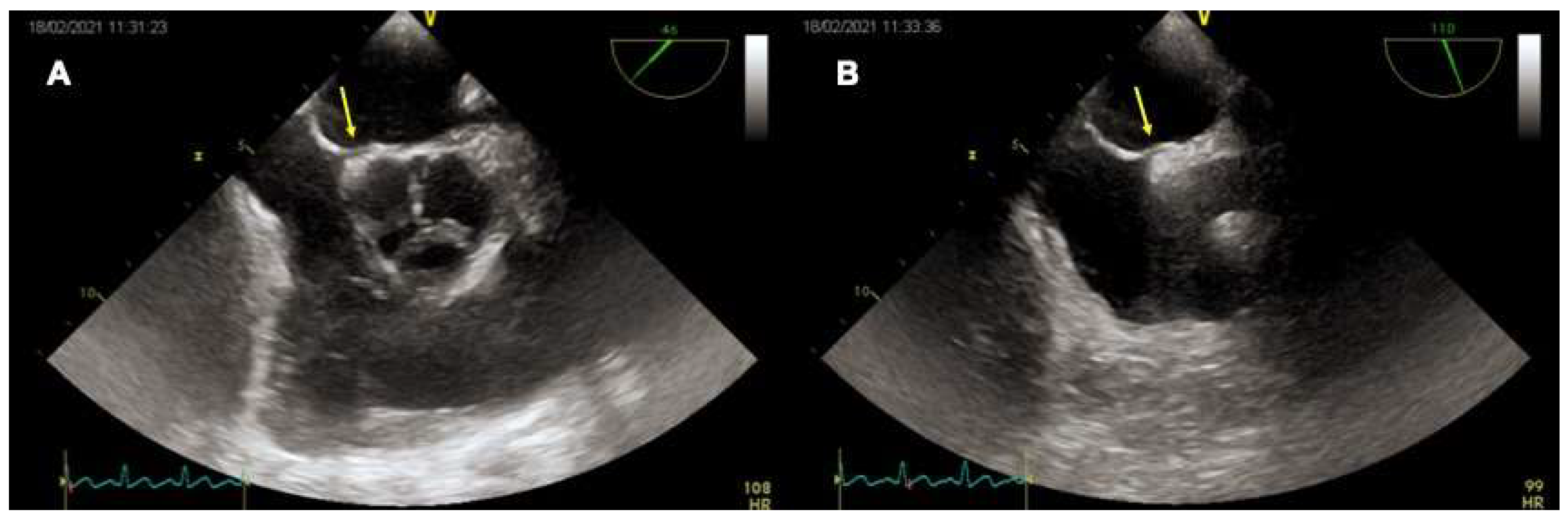
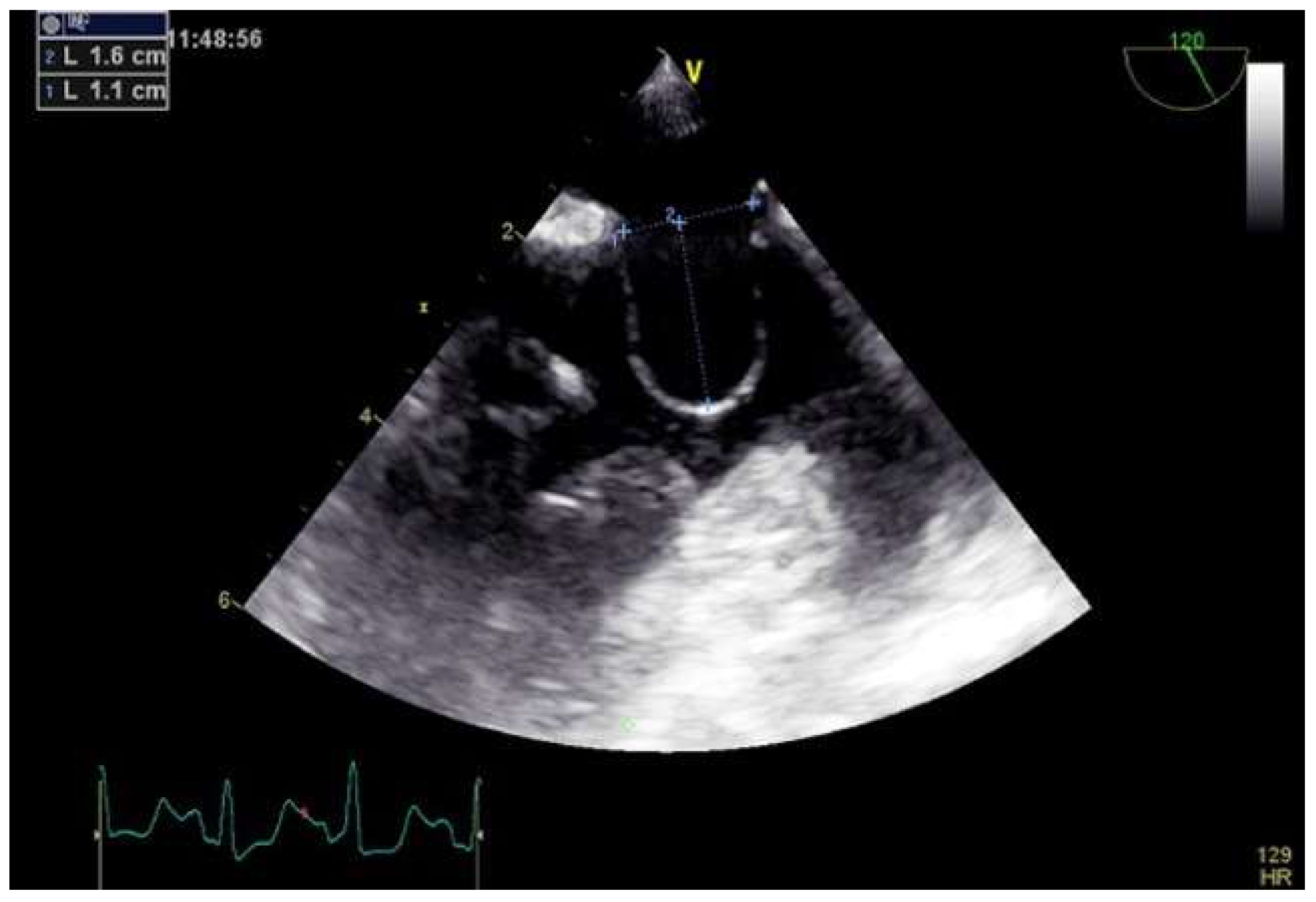
4.2. Role of TEE
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pristipino, C.; Germonpré, P.; Toni, D.; Sievert, H.; Meier, B.; D’Ascenzo, F.; Berti, S.; Onorato, E.M.; Bedogni, F.; Mas, J.L. European position paper on the management of patients with patent foramen ovale. Part II—Decompression sickness, migraine, arterial deoxygenation syndromes and select high-risk clinical conditions. EuroIntervention 2021, 17, e367–e375. [Google Scholar] [CrossRef]
- Pristipino, C.; Sievert, H.; D’Ascenzo, F.; Mas, J.L.; Meier, B.; Scacciatella, P.; Hildick-Smith, D.; Gaita, F.; Toni, D.; Kyrle, P.; et al. European position paper on the management of patients with patent foramen ovale. General approach and left circulation thromboembolism. Eur. Heart J. 2019, 40, 3182–3195. [Google Scholar] [CrossRef]
- Mas, J.-L.; Derumeaux, G.; Guillon, B.; Massardier, E.; Hosseini, H.; Mechtouff, L.; Arquizan, C.; Béjot, Y.; Vuillier, F.; Detante, O.; et al. Patent Foramen Ovale Closure or Anticoagulation vs. Antiplatelets after Stroke. N. Engl. J. Med. 2017, 377, 1011–1021. [Google Scholar] [CrossRef]
- Saver, J.L.; Carroll, J.D.; Thaler, D.E.; Smalling, R.W.; MacDonald, L.A.; Marks, D.S.; Tirschwell, D.L. Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after Stroke. N. Engl. J. Med. 2017, 377, 1022–1032. [Google Scholar]
- Søndergaard, L.; Kasner, S.E.; Rhodes, J.F.; Andersen, G.; Iversen, H.K.; Nielsen-Kudsk, J.E.; Settergren, M.; Sjöstrand, C.; Roine, R.O.; Hildick-Smith, D.; et al. Patent Foramen Ovale Closure or Antiplatelet Therapy for Cryptogenic Stroke. N. Engl. J. Med. 2017, 377, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Gaspardone, A.; De Marco, F.; Sgueglia, G.A.; De Santis, A.; Iamele, M.; D’Ascoli, E.; Tusa, M.; Corciu, A.; Mullen, M.; Nobles, A.; et al. Novel percutaneous suture-mediated patent foramen ovale closure technique: Early results of the NobleStitch EL Italian Registry. EuroIntervention 2018, 14, e272–e279. [Google Scholar] [CrossRef]
- King, T.D. Secundum atrial septal defect. Nonoperative closure during cardiac catheterization. JAMA 1976, 235, 2506–2509. [Google Scholar] [CrossRef]
- Bridges, N.D.; Hellenbrand, W.; Latson, L.; Filiano, J.; Newburger, J.W.; Lock, J.E. Transcatheter closure of patent foramen ovale after presumed paradoxical embolism. Circulation 1992, 86, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Sharafuddin, M.J.A.; Gu, X.; Titus, J.L.; Urness, M.; Cervera-Ceballos, J.J.; Amplatz, K. Transvenous Closure of Secundum Atrial Septal Defects. Circulation 1997, 95, 2162–2168. [Google Scholar] [CrossRef]
- Meier, B. Closure of patent foramen ovale: Technique, pitfalls, complications, and follow up. Heart 2005, 91, 444–448. [Google Scholar] [CrossRef][Green Version]
- Meier, B. Percutaneous Closure of Patent Foramen Ovale in Cryptogenic Embolism. N. Engl. J. Med. 2013, 368, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.D.; Saver, J.L.; Thaler, D.E.; Smalling, R.W.; Berry, S.; MacDonald, L.A.; Marks, D.S.; Tirschwell, D.L. Closure of Patent Foramen Ovale versus Medical Therapy after Cryptogenic Stroke. N. Engl. J. Med. 2013, 368, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Stortecky, S.; da Costa, B.R.; Mattle, H.P.; Carroll, J.; Hornung, M.; Sievert, H.; Trelle, S.; Windecker, S.; Meier, B.; Jüni, P. Percutaneous closure of patent foramen ovale in patients with cryptogenic embolism: A network meta-analysis. Eur. Heart J. 2015, 36, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Song, J.-K.; Kim, J.S.; Heo, R.; Lee, S.; Kim, D.-H.; Song, J.-M.; Kang, D.-H.; Kwon, S.U.; Kang, D.-W.; et al. Cryptogenic Stroke and High-Risk Patent Foramen Ovale. J. Am. Coll. Cardiol. 2018, 71, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Mattle, H.P.; Evers, S.; Hildick-Smith, D.; Becker, W.J.; Baumgartner, H.; Chataway, J.; Gawel, M.; Göbel, H.; Heinze, A.; Horlick, E.; et al. Percutaneous closure of patent foramen ovale in migraine with aura, a randomized controlled trial. Eur. Heart J. 2016, 37, 2029–2036. [Google Scholar] [CrossRef]
- Dowson, A.; Mullen, M.J.; Peatfield, R.; Muir, K.; Khan, A.A.; Wells, C.; Lipscombe, S.L.; Rees, T.; De Giovanni, J.V.; Morrison, W.L.; et al. Migraine Intervention With STARFlex Technology (MIST) trial: A prospective, multicenter, double-blind, sham-controlled trial to evaluate the effectiveness of patent foramen ovale closure with STARFlex septal repair implant to resolve refractory migraine headache. Circulation 2008, 117, 1397–1404. [Google Scholar]
- Furlan, A.J.; Reisman, M.; Massaro, J.; Mauri, L.; Adams, H.; Albers, G.W.; Felberg, R.; Herrmann, H.; Kar, S.; Landzberg, M.; et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N. Engl. J. Med. 2012, 366, 991–999. [Google Scholar] [CrossRef]
- Tobis, J.M.; Charles, A.; Silberstein, S.D.; Sorensen, S.; Maini, B.; Horwitz, P.A.; Gurley, J.C. Percutaneous Closure of Patent Foramen Ovale in Patients With Migraine: The PREMIUM Trial. J. Am. Coll Cardiol. 2017, 70, 2766–2774. [Google Scholar] [CrossRef]
- Slavin, L.; Tobis, J.M.; Rangarajan, K.; Dao, C.; Krivokapich, J.; Liebeskind, D.S. Five-year experience with percutaneous closure of patent foramen ovale. Am. J. Cardiol. 2007, 99, 1316–1320. [Google Scholar] [CrossRef]
- Harms, V.; Reisman, M.; Fuller, C.J.; Spencer, M.P.; Olsen, J.V.; Krabill, K.A.; Gray, W.A.; Jesurum, J.T. Outcomes after transcatheter closure of patent foramen ovale in patients with paradoxical embolism. Am. J. Cardiol. 2007, 99, 1312–1315. [Google Scholar] [CrossRef]
- Van den Branden, B.J.; Luermans, J.G.; Post, M.C.; Plokker, H.W.; Ten Berg, J.M.; Suttorp, M.J. The BioSTAR(r) device versus the CardioSEAL(r) device in patent foramen ovale closure: Comparison of mid-term efficacy and safety. EuroIntervention 2010, 6, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Baglini, R.; Baldari, D.; Amaducci, A.; D’Ancona, G. The new patent foramen ovale occluder FIGULLA in complex septal anatomy: A case series. Ther. Adv. Cardiovasc. Dis. 2013, 7, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Krizanic, F.; Sievert, H.; Pfeiffer, D.; Konorza, T.; Ferrari, M.; Figulla, H.R. Clinical evaluation of a novel occluder device (Occlutech) for percutaneous transcatheter closure of patent foramen ovale (PFO). Clin. Res. Cardiol. 2008, 97, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Ruygrok, P.N. The Coherex FlatStent: An advance in patent foramen ovale closure. Expert Rev. Med. Devices 2010, 7, 193–199. [Google Scholar] [CrossRef]
- Tobis, J.M. Patent foramen ovale: What cardiologists and neurologists need to know. Catheter. Cardiovasc. Interv. 2019, 93, 1085–1086. [Google Scholar] [CrossRef]
- Abaci, A.; Unlu, S.; Alsancak, Y.; Kaya, U.; Sezenoz, B. Short and long term complications of device closure of atrial septal defect and patent foramen ovale: Meta-analysis of 28,142 patients from 203 studies. Catheter. Cardiovasc. Interv. 2013, 82, 1123–1138. [Google Scholar] [CrossRef]
- Lattanzi, S.; Brigo, F.; Cagnetti, C.; Di Napoli, M.; Silvestrini, M. Patent Foramen Ovale and Cryptogenic Stroke or Transient Ischemic Attack: To Close or Not to Close? A Systematic Review and Meta-Analysis. Cerebrovasc. Dis. 2018, 45, 193–203. [Google Scholar] [CrossRef]
- Akobeng, A.K.; Abdelgadir, I.; Boudjemline, Y.; Hijazi, Z.M. Patent foramen ovale (PFO) closure versus medical therapy for prevention of recurrent stroke in patients with prior cryptogenic stroke: A systematic review and meta-analysis of randomized controlled trials. Catheter. Cardiovasc. Interv. 2018, 92, 165–173. [Google Scholar] [CrossRef]
- Windecker, S.; Wahl, A.; Chatterjee, T.; Garachemani, A.; Eberli, F.R.; Seiler, C.; Meier, B. Percutaneous closure of patent foramen ovale in patients with paradoxical embolism: Long-term risk of recurrent thromboembolic events. Circulation 2000, 101, 893–898. [Google Scholar] [CrossRef]
- Schwerzmann, M.; Windecker, S.; Wahl, A.; Nedeltchev, K.; Mattle, H.P.; Seiler, C.; Meier, B. Implantation of a second closure device in patients with residual shunt after percutaneous closure of patent foramen ovale. Catheter. Cardiovasc. Interv. 2004, 63, 490–495. [Google Scholar] [CrossRef]
- Susuri, N.; Obeid, S.; Ulmi, M.; Siontis, G.; Wahl, A.; Windecker, S.; Nietlispach, F.; Meier, B.; Praz, F.; Njomeza, S.; et al. Second transcatheter closure for residual shunt following percutaneous closure of patent foramen ovale. EuroIntervention 2017, 13, 858–866. [Google Scholar] [CrossRef]
- Elgendy, A.Y.; Elgendy, I.Y.; Mojadidi, M.K.; Mahmoud, A.N.; Barry, J.S.; Jneid, H.; Wayangankar, S.A.; Tobis, J.M.; Meier, B. New-onset atrial fibrillation following percutaneous patent foramen ovale closure: A systematic review and meta-analysis of randomised trials. EuroIntervention 2019, 14, 1788–1790. [Google Scholar] [CrossRef]
- Oliva, L.; Huszti, E.; Hall, R.; Abrahamyan, L.; Horlick, E. Incidence of new-onset atrial fibrillation after transcatheter patent foramen ovale closure using 15 years of Ontario administrative health data. Heart Rhythm 2022. [Google Scholar] [CrossRef]
- Hascoet, S.; Fraisse, A.; Elbaz, M. Successful percutaneous transcatheter patent foramen ovale closure through the right internal jugular vein using a steerable catheter. Catheter. Cardiovasc. Interv. 2013, 82, E598–E602. [Google Scholar] [CrossRef]
- Carter, L.I.; Cavendish, J.J. Percutaneous closure of a patent foramen ovale via left axillary vein approach with the Amplatzer Cribriform septal occluder. J. Interv. Cardiol. 2008, 21, 28–31. [Google Scholar] [CrossRef]
- Stehli, J.; Michail, M.; McGaw, D.; Harper, R. “Liver or Let Die”: Percutaneous PFO Closure Through Hepatic Vein Access. Heart Lung Circ. 2019, 28, e134–e136. [Google Scholar] [CrossRef]
- Alibegovic, J.; Bonvini, R.; Sigwart, U.; Dorsaz, P.; Camenzind, E.; Verin, V. The role of the sizing balloon in selection of the patent foramen ovale closure device size. Exp. Clin. Cardiol. 2008, 13, 42–46. [Google Scholar]
- Meier, B. Pacman sign during device closure of the patent foramen ovale. Catheter. Cardiovasc. Interv. 2003, 60, 221–223. [Google Scholar] [CrossRef]
- Mojadidi, M.K.; Bogush, N.; Caceres, J.D.; Msaouel, P.; Tobis, J.M. Diagnostic accuracy of transesophageal echocardiogram for the detection of patent foramen ovale: A meta-analysis. Echocardiography 2014, 31, 752–758. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Picard, M.H.; Carbone, A.; Arruda, A.L.; Flores, T.; Klohn, J.; Furtado, M.; Lira-Filho, E.B.; Cerri, G.G.; Andrade, J.L. Importance of adequately performed Valsalva maneuver to detect patent foramen ovale during transesophageal echocardiography. J. Am. Soc. Echocaardiogr. 2013, 26, 1337–1343. [Google Scholar] [CrossRef]
- Johansson, M.C.; Eriksson, P.; Guron, C.W.; Dellborg, M. Pitfalls in diagnosing PFO: Characteristics of false-negative contrast injections during transesophageal echocardiography in patients with patent foramen ovales. J. Am. Soc. Echocardiogr. 2010, 23, 1136–1142. [Google Scholar] [CrossRef]
- Rana, B.S.; Thomas, M.R.; Calvert, P.A.; Monaghan, M.J.; Hildick-Smith, D. Echocardiographic evaluation of patent foramen ovale prior to device closure. JACC Cardiovasc. Imaging 2010, 3, 749–760. [Google Scholar] [CrossRef]
- Davison, P.; Clift, P.F.; Steeds, R.P. The role of echocardiography in diagnosis, monitoring closure and post-procedural assessment of patent foramen ovale. Eur. J. Echocardiogr. 2010, 11, i27–i34. [Google Scholar] [CrossRef]
- Turc, G.; Calvet, D.; Guérin, P.; Sroussi, M.; Chatellier, G.; Mas, J.; The CLOSE Investigators. Closure, Anticoagulation, or Antiplatelet Therapy for Cryptogenic Stroke With Patent Foramen Ovale: Systematic Review of Randomized Trials, Sequential Meta-Analysis, and New Insights From the CLOSE Study. J. Am. Heart Assoc. 2018, 7, e008356. [Google Scholar] [CrossRef]
- Cabanes, L.; Coste, J.; Derumeaux, G.; Jeanrenaud, X.; Lamy, C.; Zuber, M.; Mas, J.-L. Interobserver and intraobserver variability in detection of patent foramen ovale and atrial septal aneurysm with transesophageal echocardiography. J. Am. Soc. Echocardiogr. 2002, 15, 441–446. [Google Scholar] [CrossRef]
- Mas, J.-L.; Arquizan, C.; Lamy, C.; Zuber, M.; Cabanes, L.; Derumeaux, G.; Coste, J. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N. Engl. J. Med. 2001, 345, 1740–1746. [Google Scholar] [CrossRef]
- Handke, M.; Harloff, A.; Olschewski, M.; Hetzel, A.; Geibel, A. Patent foramen ovale and cryptogenic stroke in older patients. N. Engl. J. Med. 2007, 357, 2262–2268. [Google Scholar] [CrossRef]
- Schuchlenz, H.W.; Saurer, G.; Weihs, W.; Rehak, P. Persisting eustachian valve in adults: Relation to patent foramen ovale and cerebrovascular events. J. Am. Soc. Echocardiogr. 2004, 17, 231–233. [Google Scholar] [CrossRef]
- Goel, S.S.; Tuzcu, E.M.; Shishehbor, M.H.; de Oliveira, E.I.; Borek, P.P.; Krasuski, R.A.; Rodriguez, L.L.; Kapadia, S.R. Morphology of the patent foramen ovale in asymptomatic versus symptomatic (stroke or transient ischemic attack) patients. Am. J. Cardiol. 2009, 103, 124–129. [Google Scholar] [CrossRef]
- Vitarelli, A. Patent Foramen Ovale: Pivotal Role of Transesophageal Echocardiography in the Indications for Closure, Assessment of Varying Anatomies and Post-procedure Follow-up. Ultrasound Med. Biol. 2019, 45, 1882–1895. [Google Scholar] [CrossRef]
- Schwerzmann, M.; Salehian, O. Hazards of percutaneous PFO closure. Eur. J. Echocardiogr. 2005, 6, 393–395. [Google Scholar] [CrossRef][Green Version]
- Yared, K.; Baggish, A.L.; Solis, J.; Durst, R.; Passeri, J.J.; Palacios, I.F.; Picard, M.H. Echocardiographic Assessment of Percutaneous Patent Foramen Ovale and Atrial Septal Defect Closure Complications. Circ. Cardiovasc. Imaging 2009, 2, 141–149. [Google Scholar] [CrossRef]
- Krantz, S.B.; Lawton, J.S. Subacute endocarditis of an atrial septal closure device in a patient with a patent foramen ovale. Ann. Thorac. Surg. 2014, 98, 1821–1823. [Google Scholar] [CrossRef] [PubMed]
- Bartel, T.; Konorza, T.; Arjumand, J.; Ebradlidze, T.; Eggebrecht, H.; Caspari, G.; Neudorf, U.; Erbel, R. Intracardiac echocardiography is superior to conventional monitoring for guiding device closure of interatrial communications. Circulation 2003, 107, 795–797. [Google Scholar] [CrossRef]
- Ren, J.-F.; Marchlinski, F.E.; Callans, D.J.; Herrmann, H.C. Clinical use of AcuNav diagnostic ultrasound catheter imaging during left heart radiofrequency ablation and transcatheter closure procedures. J. Am. Soc. Echocardiogr. 2002, 15 Pt 2, 1301–1308. [Google Scholar] [CrossRef]
- Han, K.-N.; Ma, X.-T.; Yang, S.-W.; Zhou, Y.-J. Intracardiac echocardiography in the diagnosis and closure of patent foramen ovale. J. Geriatr. Cardiol. 2021, 18, 697–701. [Google Scholar]
- Pearson, A.; Nagelhout, D.; Castello, R.; Gomez, C.R.; Labovitz, A.J. Atrial septal aneurysm and stroke: A transesophageal echocardiographic study. J. Am. Coll Cardiol. 1991, 18, 1223–1229. [Google Scholar] [CrossRef]
- Olivares-Reyes, A.; Chan, S.; Lazar, E.J.; Bandlamudi, K.; Narla, V.; Ong, K. Atrial septal aneurysm: A new classification in two hundred five adults. J. Am. Soc. Echocardiogr. 1997, 10, 644–656. [Google Scholar] [CrossRef]
- Vitarelli, A.; Mangieri, E.; Capotosto, L.; Tanzilli, G.; D’Angeli, I.; Toni, D.; Azzano, A.; Ricci, S.; Placanica, A.; Rinaldi, E.; et al. Echocardiographic findings in simple and complex patent foramen ovale before and after transcatheter closure. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1377–1385. [Google Scholar] [CrossRef]
- Giordano, M.; Gaio, G.; Santoro, G.; Palladino, M.T.; Sarubbi, B.; Golino, P.; Russo, M.G. Patent foramen ovale with complex anatomy: Comparison of two different devices (Amplatzer Septal Occluder device and Amplatzer PFO Occluder device 30/35). Int. J. Cardiol. 2019, 279, 47–50. [Google Scholar] [CrossRef]
- Matsumura, K.; Gevorgyan, R.; Mangels, D.; Masoomi, R.; Mojadidi, M.K.; Tobis, J. Comparison of residual shunt rates in five devices used to treat patent foramen ovale. Catheter. Cardiovasc. Interv. 2014, 84, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Awad, S.M.; Garay, F.F.; Cao, Q.-L.; Hijazi, Z.M. Multiple Amplatzer septal occluder devices for multiple atrial communications: Immediate and long-term follow-up results. Catheter. Cardiovasc. Interv. 2007, 70, 265–273. [Google Scholar] [CrossRef]
- Ewert, P.; Berger, F.; Vogel, M.; Dähnert, I.; Alexi-Meshkishvil, V.; Lange, P.E. Morphology of perforated atrial septal aneurysm suitable for closure by transcatheter device placement. Heart 2000, 84, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Farhaj, Z.; Hongxin, L.; Wenbin, G.; Zhang, W.; Liang, F.; Zhang, H.-Z.; Yuan, G.-D.; Zou, C.-W. Device closure of diverse layout of multi-hole secundum atrial septal defect: Different techniques and long-term follow-up. J. Cardiothorac. Surg. 2019, 14, 130. [Google Scholar] [CrossRef]
- Mahmoud, H.T.; Gaio, G.; Giordano, M.; Pizzuto, A.; Cuman, M.; Asklany, H.T.; Palladino, M.T.; Russo, M.G.; Santoro, G. Transcatheter closure of fenestrated atrial septal aneurysm: Feasibility and long-term results. J. Cardiovasc. Med. 2022, 23, 49–59. [Google Scholar] [CrossRef]
- Thompson, A.J.; Hagler, D.J.; Taggart, N.W. Transseptal puncture to facilitate device closure of “long-tunnel” patent foramen ovale. Catheter. Cardiovasc. Interv. 2015, 85, 1053–1057. [Google Scholar] [CrossRef]
- Chintala, K.; Turner, D.R.; Leaman, S.; Rodriguez-Cruz, E.; Wynne, J.; Greenbaum, A.; Forbes, T.J. Use of balloon pull-through technique to assist in CardioSEAL device closure of patent foramen ovale. Catheter. Cardiovasc. Interv. 2003, 60, 101–106. [Google Scholar] [CrossRef]
- Butera, G.; Piazza, L.; Heles, M. PFO “angioplasty”: The preparation of a very stiff and long tunnel for device closure. Catheter. Cardiovasc. Interv. 2017, 89, 480–483. [Google Scholar] [CrossRef]
- Lin, C.H.; Balzer, D.T.; Lasala, J.M. Defect closure in the lipomatous hypertrophied atrial septum with the Amplatzer muscular ventricular septal defect closure device: A case series. Catheter. Cardiovasc. Interv. 2011, 78, 102–107. [Google Scholar] [CrossRef]
- Butera, G.; Montinaro, A.; Carminati, M. The “pull-push” technique to deal with a redundant eustachian valve interfering with placement of a PFO occluder. Catheter. Cardiovasc. Interv. 2006, 68, 961–964. [Google Scholar] [CrossRef]
- Magraner, E.M.; Durante-López, A.; Domingo, E.B.; Pardeiro, C.A.; Sánchez-Recalde, Á.; Aguado, F.G.-L. Incomplete Cor Triatriatum Dexter: An Unsettling Guest in the Percutaneous Closure of Atrial Septal Defects. Rev. Esp. Cardiol. 2019, 72, 582–583. [Google Scholar] [CrossRef] [PubMed]
- Vettukattil, J.J.; Ahmed, Z.; Salmon, A.P.; Mohun, T.; Anderson, R.H. Defects in the oval fossa: Morphologic variations and impact on transcatheter closure. J. Am. Soc. Echocardiogr. 2013, 26, 192–199. [Google Scholar] [CrossRef]
- Meier, B. Closure of the patent foramen ovale, if only a stitch in time saved nine. EuroIntervention 2018, 14, e250–e251. [Google Scholar] [CrossRef] [PubMed]
- Musi, L.; Frigiola, A.; Giovannini, M.; Rosti, L. Tips and tricks: Come orientarsi tra i vari dispositivi per la chiusura del forame ovale pervio. G. Ital. Cardiol. 2019, 20, 9–16. [Google Scholar]
- Meucci, F.; Stolcova, M.; De Marco, F.; Mattesini, A.; Ristalli, F.; Chiriatti, N.; Squillantini, G.; Agostini, C.; Sarti, C.; Di Mario, C. Patent foramen ovale closure: How to choose the right device for the right patient. G. Ital. Cardiol. 2019, 20 (Suppl. S1), 9s–16s. [Google Scholar]

|
|
| Indication of use | PFO closure in patients with systemic thromboembolism and high probability of PFO causal role |
| Type of technology | Suture-mediated “deviceless” technology |
| Type of access | Percutaneous (right femoral vein preferentially) |
| Role of TEE |
|
| Advantages |
|
| Disadvantages | Non-feasible in some interatrial septum anatomies (e.g., PFOs atrial septal defects-like, PFOs with multifenestrated interatrial septum) |
| Success rate * | 96% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sperlongano, S.; Giordano, M.; Ciccarelli, G.; Bassi, G.; Malvezzi Caracciolo D’Aquino, M.; Del Giudice, C.; Gaio, G.; D’Andrea, A.; Postolache, A.; Cappelli Bigazzi, M.; et al. Advances in Percutaneous Patent Foramen Ovale Closure: From the Procedure to the Echocardiographic Guidance. J. Clin. Med. 2022, 11, 4001. https://doi.org/10.3390/jcm11144001
Sperlongano S, Giordano M, Ciccarelli G, Bassi G, Malvezzi Caracciolo D’Aquino M, Del Giudice C, Gaio G, D’Andrea A, Postolache A, Cappelli Bigazzi M, et al. Advances in Percutaneous Patent Foramen Ovale Closure: From the Procedure to the Echocardiographic Guidance. Journal of Clinical Medicine. 2022; 11(14):4001. https://doi.org/10.3390/jcm11144001
Chicago/Turabian StyleSperlongano, Simona, Mario Giordano, Giovanni Ciccarelli, Giuseppe Bassi, Marco Malvezzi Caracciolo D’Aquino, Carmen Del Giudice, Gianpiero Gaio, Antonello D’Andrea, Adriana Postolache, Maurizio Cappelli Bigazzi, and et al. 2022. "Advances in Percutaneous Patent Foramen Ovale Closure: From the Procedure to the Echocardiographic Guidance" Journal of Clinical Medicine 11, no. 14: 4001. https://doi.org/10.3390/jcm11144001
APA StyleSperlongano, S., Giordano, M., Ciccarelli, G., Bassi, G., Malvezzi Caracciolo D’Aquino, M., Del Giudice, C., Gaio, G., D’Andrea, A., Postolache, A., Cappelli Bigazzi, M., Scognamiglio, G., Sarubbi, B., Russo, M. G., Golino, P., & Lancellotti, P. (2022). Advances in Percutaneous Patent Foramen Ovale Closure: From the Procedure to the Echocardiographic Guidance. Journal of Clinical Medicine, 11(14), 4001. https://doi.org/10.3390/jcm11144001









