Long-Term Outcomes after Catheter Ablation of Ventricular Tachycardia in Dilated vs. Ischemic Cardiomyopathy
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Electrophysiological Study
2.3. Complications
2.4. Definition of Endpoints and Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Patients and Periprocedural Characteristics
3.2. Procedure Associated Complications
3.3. Primary and Secondary Endpoints
4. Discussion
4.1. Major Findings
- VT recurrence after ablation was more often in patients with DCM such that 64% of the patients experienced at least one sustained VT in the follow-up period;
- Rehospitalization rate and MACE rate was also higher in patients with DCM, with leading readmissions because of VT recurrence;
- A significant difference in the overall mortality in the both cohorts during follow-up was not observed;
- Electrical storm at presentation attributes to higher risk of VT recurrence, while complete ablation success is a predictor for favorable outcome.
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldenberg, I.; Gillespie, J.; Moss, A.J.; Hall, W.J.; Klein, H.; McNitt, S.; Brown, M.W.; Cygankiewicz, I.; Zareba, W. Executive Committee of the Multicenter Automatic Defibrillator Implantation Trial II. Long-term benefit of primary prevention with an implantable cardioverter-defibrillator: An extended 8-year follow-up study of the Multicenter Automatic Defibrillator Implantation Trial II. Circulation 2010, 122, 1265–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardy, G.H.; Lee, K.L.; Mark, D.B.; Poole, J.E.; Packer, D.L.; Boineau, R.; Domanski, M.; Troutman, C.; Anderson, J.; Johnson, G.; et al. Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 2005, 352, 225–237, Erratum in: N. Engl. J. Med. 2005, 352, 2146. [Google Scholar] [CrossRef]
- Poole, J.E.; Johnson, G.W.; Hellkamp, A.S.; Anderson, J.; Callans, D.J.; Raitt, M.H.; Reddy, R.K.; Marchlinski, F.E.; Yee, R.; Guarnieri, T.; et al. Prognostic importance of defibrillator shocks in patients with heart failure. N. Engl. J. Med. 2008, 359, 1009–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohnloser, S.H.; Dorian, P.; Roberts, R.; Gent, M.; Israel, C.W.; Fain, E.; Champagne, J.; Connolly, S.J. Effect of amiodarone and sotalol on ventricular defibrillation threshold: The optimal pharmacological therapy in cardioverter defibrillator patients (OPTIC) trial. Circulation 2006, 114, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Tanner, H.; Hindricks, G.; Volkmer, M.; Furniss, S.; Kühlkamp, V.; Lacroix, D.; DEChillou, C.; Almendral, J.; Caponi, D.; Kuck, K.H.; et al. Catheter ablation of recurrent scar-related ventricular tachycardia using electroanatomical mapping and irrigated ablation technology: Results of the prospective multicenter Euro-VT-study. J. Cardiov. Electrophysiol. 2010, 21, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Pinto, Y.M.; Elliott, P.M.; Arbustini, E.; Adler, Y.; Anastasakis, A.; Böhm, M.; Duboc, D.; Gimeno, J.; de Groote, P.; Imazio, M.; et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: A position statement of the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 2016, 37, 1850–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halbfass, P.; Ludwig, D.; Sonne, K.; Nentwich, K.; Ene, E.; Berkovitz, A.; Foldyna, B.; Barth, S.; Müller, J.; Lehmkuhl, L.; et al. Acute and long-term outcomes of VT radiofrequency catheter ablation in patients with versus without an intramural septal substrate. Indian Pacing Electrophysiol. J. 2022, 22, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Sosa, E.; Scanavacca, M.; d’Avila, A.; Pilleggi, F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J. Cardiov. Electrophysi. 1996, 7, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Marchlinski, F.E.; Callans, D.J.; Gottlieb, C.D.; Zado, E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation 2000, 101, 1288–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, V.Y.; Reynolds, M.R.; Neuzil, P.; Richardson, A.W.; Taborsky, M.; Jongnarangsin, K.; Kralovec, S.; Sediva, L.; Ruskin, J.N.; Josephson, M.E. Prophylactic catheter ablation for the prevention of defibrillator therapy. N. Engl. J. Med. 2007, 357, 2657–2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuck, K.H.; Schaumann, A.; Eckardt, L.; Willems, S.; Ventura, R.; Delacrétaz, E.; Pitschner, H.F.; Kautzner, J.; Schumacher, B.; Hansen, P.S.; et al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): A multicentre randomised controlled trial. Lancet 2010, 375, 31–40. [Google Scholar] [CrossRef]
- Sapp, J.L.; Wells, G.A.; Parkash, R.; Stevenson, W.G.; Blier, L.; Sarrazin, J.F.; Thibault, B.; Rivard, L.; Gula, L.; Leong-Sit, P.; et al. Ventricular Tachycardia Ablation versus Escalation of Antiarrhythmic Drugs. N. Engl. J. Med. 2016, 375, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, S.M.; Daubert, J.P.; Anstrom, K.J.; Daoud, E.G.; Gonzalez, M.; Saba, S.; Jackson, K.P.; Reece, T.; Gu, J.; Pokorney, S.D.; et al. Catheter ablation for ventricular tachycardia in patients with an implantable cardioverter defibrillator (CALYPSO) pilot trial. J. Cardiov. Electrophysiol. 2015, 26, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Tung, R.; Xue, Y.; Chen, M.; Jiang, C.; Shatz, D.Y.; Besser, S.; Hu, H.; Chung, F.P.; Nakahara, S.; Kim, Y.H.; et al. First-Line Catheter Ablation of Monomorphic Ventricular Tachycardia in Cardiomyopathy Concurrent with Defibrillator Implantation: The PAUSE-SCD Randomized Trial. Circulation 2022, 145, 1839–1849. [Google Scholar] [CrossRef] [PubMed]
- Muser, D.; Santangeli, P.; Castro, S.A.; Pathak, R.K.; Liang, J.J.; Hayashi, T.; Magnani, S.; Garcia, F.C.; Hutchinson, M.D.; Supple, G.G.; et al. Long-Term Outcome After Catheter Ablation of Ventricular Tachycardia in Patients With Nonischemic Dilated Cardiomyopathy. Circ. Arrhythm. Electrophysiol. 2016, 9, e004328. [Google Scholar] [CrossRef] [PubMed]
- Dinov, B.; Fiedler, L.; Schönbauer, R.; Bollmann, A.; Rolf, S.; Piorkowski, C.; Hindricks, G.; Arya, A. Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy: Results from the Prospective Heart Centre of Leipzig VT (HELP-VT) Study. Circulation 2014, 129, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Ebert, M.; Wijnmaalen, A.P.; de Riva, M.; Trines, S.A.; Androulakis, A.F.A.; Glashan, C.A.; Schalij, M.J.; Peter van Tintelen, J.; Jongbloed, J.D.H.; Zeppenfeld, K. Prevalence and Prognostic Impact of Pathogenic Variants in Patients with Dilated Cardiomyopathy Referred for Ventricular Tachycardia Ablation. JACC Clin. Electrophysiol. 2020, 6, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Piers, S.R.; Leong, D.P.; van Huls van Taxis, C.F.; Tayyebi, M.; Trines, S.A.; Pijnappels, D.A.; Delgado, V.; Schalij, M.J.; Zeppenfeld, K. Outcome of ventricular tachycardia ablation in patients with nonischemic cardiomyopathy: The impact of noninducibility. Circ. Arrhythm. Electrophysiol. 2013, 6, 513–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristic | DCM (n = 69; 31%) | ICM (n = 156; 69%) | p-Value | ||
|---|---|---|---|---|---|
| Age, median (range) | 65 ± 9.2 | 68 ± 10.8 | 0.036 | ||
| Males, n (%) | 60 | (87) | 143 | (92) | 0.273 |
| Cardiovascular risk factors, n (%) | |||||
| Arterial hypertension | 55 | (80) | 146 | (94) | 0.001 |
| Diabetes mellitus | 17 | (25) | 52 | (34) | 0.182 |
| Hyperlipidemia | 41 | (59) | 129 | (83) | 0.001 |
| Smoking | 21 | (30) | 67 | (43) | 0.070 |
| Cardiac family history | 14 | (20) | 33 | (21) | 0.865 |
| Comorbidities, n (%) | |||||
| Atrial fibrillation | 36 | (52) | 65 | (42) | 0.144 |
| Stroke | 6 | (9) | 22 | (14) | 0.251 |
| Chronic kidney disease | 35 | (51) | 95 | (61) | 0.139 |
| Liver cirrhosis | 2 | (3) | 5 | (3) | 0.897 |
| COPD | 6 | (9) | 10 | (7) | 0.556 |
| Asthma | 0 | (0) | 3 | (2) | 0.245 |
| Medication at admission, n (%) | |||||
| Beta-blocker | 65 | (94) | 141 | (92) | 0.585 |
| Amiodarone | 34 | (49) | 44 | (29) | 0.003 |
| Other AAD | 2 | (3) | 1 | (1) | 0.182 |
| LVEF, % | 32 ± 12 | 33 ± 12 | 0.375 | ||
| Type of ICD, n (%) | |||||
| ICD | 36 | (57) | 95 | (74) | 0.005 |
| CRT-D | 27 | (43) | 27 | (22) | |
| s-ICD | 0 | (0) | 5 | (4) | |
| ICD indication, n (%) | |||||
| Primary prevention | 25 | (41) | 53 | (41) | 0.956 |
| Secondary prevention | 36 | (59) | 75 | (59) | |
| Characteristic | DCM (n = 69; 31%) | ICM (n = 156; 69%) | p-Value | ||
|---|---|---|---|---|---|
| Epicardial ablation, n (%) | 18 | (27) | 10 | (6) | 0.001 |
| Non-inducible with PES, n (%) | 15 | (22) | 32 | (21) | 0.835 |
| VTs inducible, n/patient | 1.8 ± 1.5 | 1.7 ± 1.5 | 0.618 | ||
| Clinical VT CL, ms | 357 ± 87 | 361 ± 88 | 0.788 | ||
| Procedural duration, min | 154 ± 51 | 134 ± 42 | 0.006 | ||
| Fluoroscopy duration, min | 15.2 ± 11.0 | 12.3 ± 8.6 | 0.050 | ||
| Ablation time, min | 29.1 ± 19.8 | 32.4 ± 46.7 | 0.473 | ||
| Clinical VT still inducible, n (%) | 3 | (4) | 9 | (6) | 0.130 |
| Any VT inducible, n (%) | 13 | (20) | 18 | (12) | 0.101 |
| Hemodynamic not tolerated VT, n (%) | 24 | (35) | 41 | (26) | 0.172 |
| Catecholamine, n (%) | 9 | (13) | 15 | (10) | 0.408 |
| Intubation, n (%) | 3 | (4) | 5 | (3) | 0.655 |
| Ablation of all VTs, n (%) | 48 | (69) | 123 | (79) | 0.580 |
| Betablocker at discharge, n (%) | 67 | (97) | 149 | (97) | 0.904 |
| Amiodaron at discharge, n (%) | 26 | (38) | 37 | (24) | 0.039 |
| Characteristic | DCM (n = 69; 31%) | ICM (n = 156; 69%) | p-Value | ||
|---|---|---|---|---|---|
| Major complications, n (%) | 12 | (16) | 12 | (8) | 0.030 |
| Vascular access related | 1 | (1) | 2 | (1) | 1.000 |
| Third degree AV block | 3 | (4) | 2 | (1) | 0.165 |
| Pneumonia | 2 | (3) | 1 | (1) | 0.223 |
| Cardiogenic shock | 1 | (1) | 3 | (2) | 1.000 |
| Pneumothorax | 1 | (1) | 2 | (1) | 1.000 |
| Stroke | 0 | (0) | 1 | (1) | 1.000 |
| In-hospital mortality, n (%) | 1 | (1) | 1 | (1) | 1.000 |
| Characteristic | DCM (n = 69; 31%) | ICM (n = 156; 69%) | p-Value | ||
|---|---|---|---|---|---|
| Primary endpoint, n (%) | |||||
| VT recurrence | 34 | (64) | 47 | (37) | 0.001 |
| Secondary endpoints, n (%) | |||||
| First rehospitalization, overall | 41 | (75) | 76 | (59) | 0.038 |
| VT | 33 | (59) | 42 | (32) | 0.001 |
| Acute heart failure | 4 | (7) | 30 | (23) | 0.010 |
| Acute myocardial infarction | 2 | (4) | 0 | (0) | 0.089 |
| Stroke | 1 | (2) | 2 | (1) | 1.000 |
| LVAD/HTX | 2 | (4) | 0 | (0) | 0.089 |
| MACE | 40 | (68) | 68 | (52) | 0.036 |
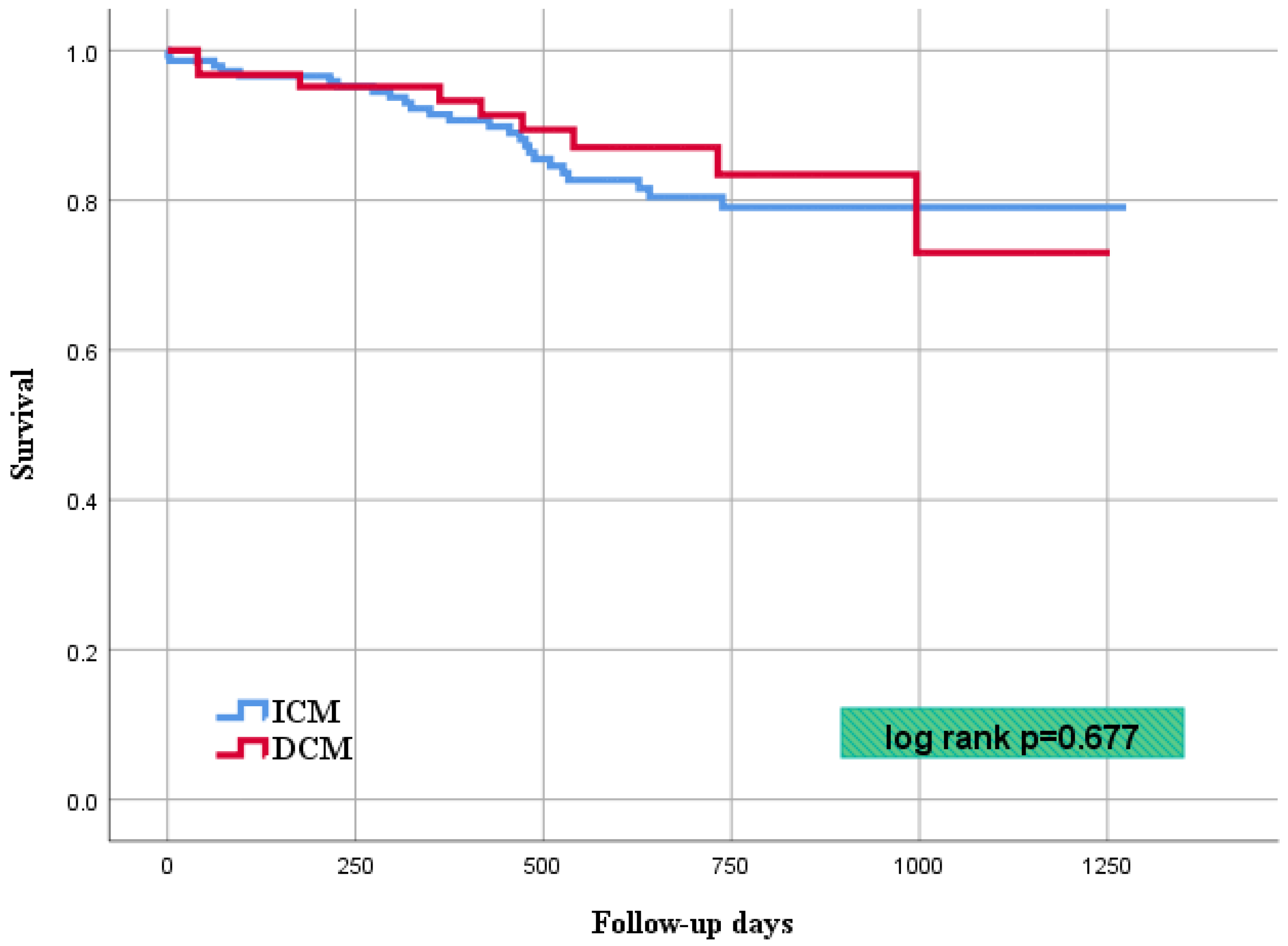
| Cardiovascular mortality | 9 | (15) | 22 | (16) | 0.677 |
| Univariable | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age | 1.003 (0.982–1.025) | 0.768 | - | - |
| Diabetes mellitus | 1.635 (1.039–2.572) | 0.032 | - | - |
| Chronic kidney disease | 1.235 (0.796–1.917) | 0.347 | - | - |
| Electrical storm | 2.118 (1.371–3.269) | 0.001 | 1.942 (1.237–3.050) | 0.004 |
| LVEF ≤ 35% | 1.224 (0.785–1.909) | 0.373 | - | - |
| Partial ablation success | 0.741 (0.322–1.705) | 0.499 | - | - |
| Complete ablation success | 0.374 (0.236–0.667) | 0.002 | 0.522 (0.307–0.885) | 0.016 |
| Epicardial ablation | 2.141 (1.222–3.754) | 0.008 | - | - |
| Amiodaron therapy | 1.946 (1.240–3.054) | 0.004 | - | - |
| Beta blockers therapy | 0.433 (0.158–1.183) | 0.103 | - | - |
| DCM | ICM | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age | 0.990(0.955–1.027) | 0.603 | - | - | 1.022 (0.992–1.053) | 0.154 | - | - |
| Diabetes mellitus | 1.375 (0.644–2.938) | 0.410 | - | - | 2.037 (1.142–3.633) | 0.016 | 2.032 (1.134–3.643) | 0.017 |
| Chronic kidney disease | 1.185 (0.610–2.301) | 0.617 | - | - | 1.411 (0.778–2.559) | 0.257 | - | - |
| LVEF ≤ 35% | 1.625 (0.800–3.302) | 0.180 | - | - | 1.042 (0.584–1.859) | 0.889 | - | - |
| Partial ablation success | 0.322 (0.095–1.089) | 0.068 | - | - | 1.002 (0.310–3.231) | 0.998 | - | - |
| Complete ablation success | 0.541 (0.258–1.173) | 0.108 | - | - | 0.342 (0.173–0.677) | 0.002 | 0.348 (0.176–0.689) | 0.002 |
| Epicardial ablation | 1.694 (0.829–3.465) | 0.148 | - | - | 1.542 (0.553–4.305) | 0.408 | - | - |
| Amiodaron therapy | 1.710 (0.873–3.348) | 0.118 | - | - | 1.827 (0.988–3.378) | 0.055 | - | - |
| Electrical Storm | 1.518 (0.779–2.955) | 0.220 | - | - | 2.287 (1.288–4.059) | 0.005 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakarov, I.; Mueller, J.; Ene, E.; Berkovitz, A.; Sonne, K.; Nentwich, K.; Schupp, T.; Behnes, M.; Deneke, T. Long-Term Outcomes after Catheter Ablation of Ventricular Tachycardia in Dilated vs. Ischemic Cardiomyopathy. J. Clin. Med. 2022, 11, 4000. https://doi.org/10.3390/jcm11144000
Chakarov I, Mueller J, Ene E, Berkovitz A, Sonne K, Nentwich K, Schupp T, Behnes M, Deneke T. Long-Term Outcomes after Catheter Ablation of Ventricular Tachycardia in Dilated vs. Ischemic Cardiomyopathy. Journal of Clinical Medicine. 2022; 11(14):4000. https://doi.org/10.3390/jcm11144000
Chicago/Turabian StyleChakarov, Ivaylo, Julian Mueller, Elena Ene, Arthur Berkovitz, Kai Sonne, Karin Nentwich, Tobias Schupp, Michael Behnes, and Thomas Deneke. 2022. "Long-Term Outcomes after Catheter Ablation of Ventricular Tachycardia in Dilated vs. Ischemic Cardiomyopathy" Journal of Clinical Medicine 11, no. 14: 4000. https://doi.org/10.3390/jcm11144000
APA StyleChakarov, I., Mueller, J., Ene, E., Berkovitz, A., Sonne, K., Nentwich, K., Schupp, T., Behnes, M., & Deneke, T. (2022). Long-Term Outcomes after Catheter Ablation of Ventricular Tachycardia in Dilated vs. Ischemic Cardiomyopathy. Journal of Clinical Medicine, 11(14), 4000. https://doi.org/10.3390/jcm11144000







