Fertility Counseling in BRCA1/2-Mutated Women with Breast Cancer and Healthy Individuals
Abstract
1. Introduction
2. BRCA1/2 Mutation Carriers–Special Considerations Regarding Cancer Risk, Prevention, and Treatment
2.1. Mutations in BRCA1/2 Gene and Cancer Risk
2.2. RRM and RRSO
2.3. PARP Inhibitors
2.4. Hormonal Contraception
3. Fertility Barriers for BRCA1/2 Mutation Carriers
Effects of Oncology Treatment
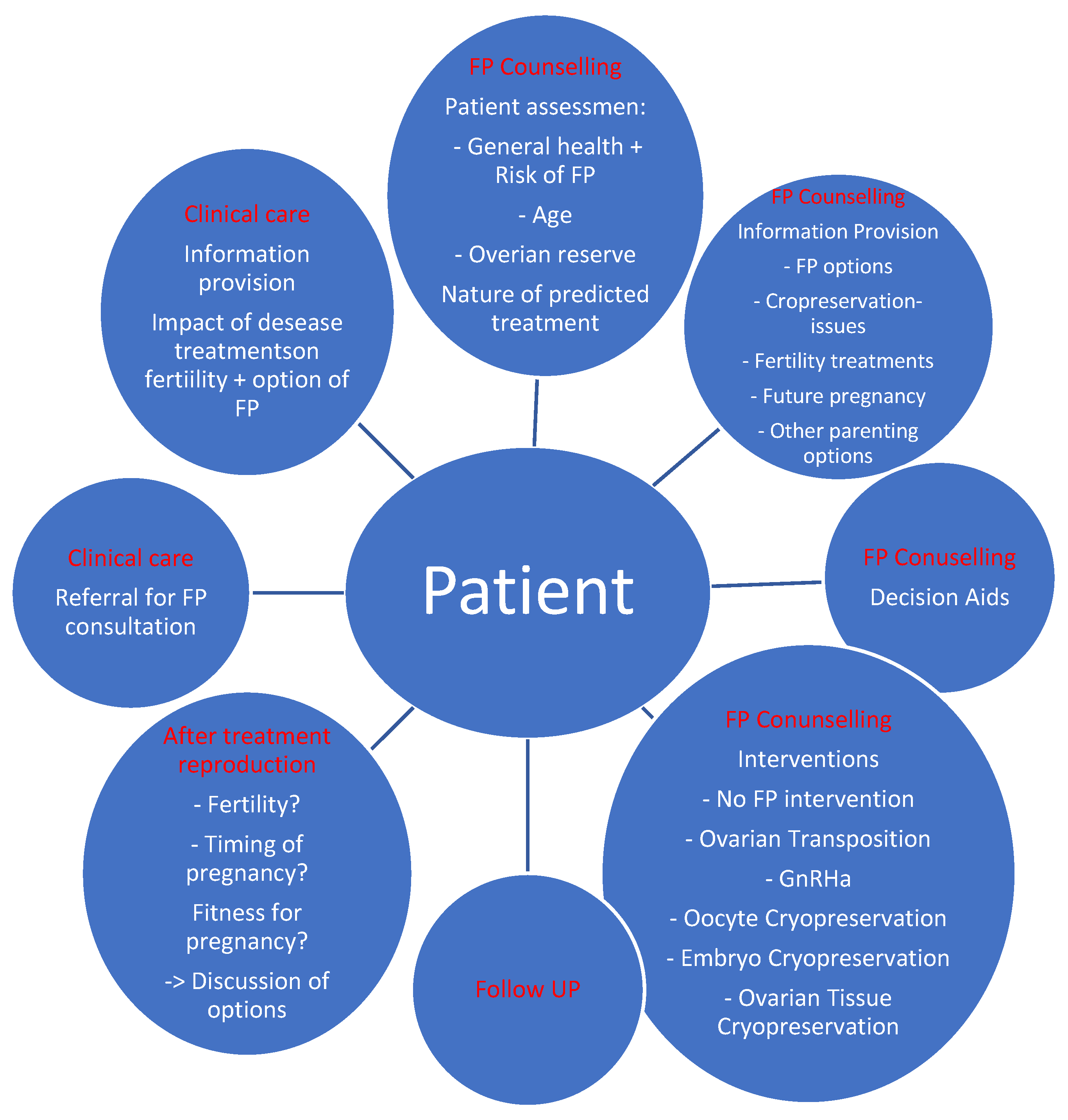
4. Fertility Counseling in BRCA1/2 Gene Mutation Carriers
4.1. Fertility Preservation Techniques
4.2. Preimplantation Genetic Testing–Types and Applications
5. Pregnancy in BRCA1/2 Mutation Carriers
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Mazzara, C.; Pagani, O. Diagnosis and Treatment of Breast Cancer in Young Women. Curr. Treat. Options Oncol. 2019, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, H.; Magnusson, K.; Lindström, L.S.; Garmo, H.; Fält, S.E.; Lindman, H.; Bergh, J.; Holmberg, L.; Pontén, F.; Frisell, J.; et al. Long-term outcome in young women with breast cancer: A population-based study. Breast Cancer Res. Treat. 2016, 160, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Malone, K.E.; Daling, J.R.; Neal, C.; Suter, N.M.; O’Brien, C.; Cushing-Haugen, K.; Jonasdottir, T.J.; Thompson, J.D.; Ostrander, E.A. Frequency of BRCA1/BRCA2 mutations in a population-based sample of young breast carcinoma cases. Cancer 2000, 88, 1393–1402. [Google Scholar] [CrossRef]
- Desreux, J.A.C. Breast cancer screening in young women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 230, 208–211. [Google Scholar] [CrossRef]
- Cancer Facts and Figures 2020. The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html (accessed on 21 March 2022).
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220, Erratum in Ann. Oncol. 2019, 30, 1674; Erratum in Ann. Oncol. 2021, 32, 284. [Google Scholar] [CrossRef]
- Roy, R.; Chun, J.; Powell, S.N. BRCA1 and BRCA2: Different roles in a common pathway of genome protection. Nat. Rev. Cancer 2011, 12, 68–78. [Google Scholar] [CrossRef]
- Sadeghi, F.; Asgari, M.; Matloubi, M.; Ranjbar, M.; Karkhaneh Yousefi, N.; Azari, T.; Zaki-Dizaji, M. Molecular contribution of BRCA1 and BRCA2 to genome instability in breast cancer patients: Review of radiosensitivity assays. Biol. Proced. Online 2020, 22, 23. [Google Scholar] [CrossRef]
- Chen, C.C.; Feng, W.; Lim, P.X.; Kass, E.M.; Jasin, M. Homology-Directed Repair and the Role of BRCA1, BRCA2, and Related Proteins in Genome Integrity and Cancer. Annu. Rev. Cancer Biol. 2018, 2, 313–336. [Google Scholar] [CrossRef]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef]
- Lakhani, S.R.; Van De Vijver, M.J.; Jacquemier, J.; Anderson, T.J.; Osin, P.P.; McGuffog, L.; Easton, D.F. The pathology of familial breast cancer: Predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J. Clin. Oncol. 2002, 20, 2310–2318. [Google Scholar] [CrossRef]
- Hartmann, L.C.; Schaid, D.J.; Woods, J.E.; Crotty, T.P.; Myers, J.L.; Arnold, P.G.; Petty, P.M.; Sellers, T.A.; Johnson, J.L.; McDonnell, S.K.; et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N. Engl. J. Med. 1999, 340, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, L.C.; Sellers, T.A.; Schaid, D.J.; Frank, T.S.; Soderberg, C.L.; Sitta, D.L.; Frost, M.H.; Grant, C.S.; Donohue, J.H.; Woods, J.E.; et al. Efficacy of bilateral prophylactic mastectomy in BRCA1 and BRCA2 gene mutation carriers. J. Natl. Cancer Inst. 2001, 93, 1633–1637. [Google Scholar] [CrossRef] [PubMed]
- Meijers-Heijboer, H.; van Geel, B.; van Putten, W.L.; Henzen-Logmans, S.C.; Seynaeve, C.; Menke-Pluymers, M.B.; Bartels, C.C.; Verhoog, L.C.; van den Ouweland, A.M.; Niermeijer, M.F.; et al. Breast cancer after prophylactic bilateral mastectomy in women with a BRCA1 or BRCA2 mutation. N. Engl. J. Med. 2001, 345, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Rebbeck, T.R.; Friebel, T.; Lynch, H.T.; Neuhausen, S.L.; van ‘t Veer, L.; Garber, J.E.; Evans, G.R.; Narod, S.A.; Isaacs, C.; Matloff, E.; et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: The PROSE Study Group. J. Clin. Oncol. 2004, 22, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Kiely, B.E.; Jenkins, M.A.; McKinley, J.M.; Friedlander, M.L.; Weideman, P.; Milne, R.L.; McLachlan, S.A.; Hopper, J.L.; Phillips, K.A. Contralateral risk-reducing mastectomy in BRCA1 and BRCA2 mutation carriers and other high-risk women in the Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (kConFab). Breast Cancer Res. Treat. 2010, 120, 715–723, Erratum in Breast Cancer Res. Treat. 2010, 120, 725–726. [Google Scholar] [CrossRef]
- Metcalfe, K.; Gershman, S.; Ghadirian, P.; Lynch, H.T.; Snyder, C.; Tung, N.; Kim-Sing, C.; Eisen, A.; Foulkes, W.D.; Rosen, B.; et al. Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: Retrospective analysis. BMJ 2014, 348, g226. [Google Scholar] [CrossRef]
- Rebbeck, T.R.; Kauff, N.D.; Domchek, S.M. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J. Natl. Cancer Inst. 2009, 101, 80–87. [Google Scholar] [CrossRef]
- Finch, A.P.; Lubinski, J.; Møller, P.; Singer, C.F.; Karlan, B.; Senter, L.; Rosen, B.; Maehle, L.; Ghadirian, P.; Cybulski, C.; et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J. Clin. Oncol. 2014, 32, 1547–1553. [Google Scholar] [CrossRef]
- De Felice, F.; Marchetti, C.; Boccia, S.M.; Romito, A.; Sassu, C.M.; Porpora, M.G.; Muzii, L.; Tombolini, V.; Benedetti Panici, P. Risk-reducing salpingo-oophorectomy in BRCA1 and BRCA2 mutated patients: An evidence-based approach on what women should know. Cancer Treat. Rev. 2017, 61, 1–5. [Google Scholar] [CrossRef]
- Eleje, G.U.; Eke, A.C.; Ezebialu, I.U.; Ikechebelu, J.I.; Ugwu, E.O.; Okonkwo, O.O. Risk-reducing bilateral salpingo-oophorectomy in women with BRCA1 or BRCA2 mutations. Cochrane Database Syst. Rev. 2018, 8, CD012464. [Google Scholar] [CrossRef]
- Daly, M.B.; Pal, T.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Goggins, M.; Hutton, M.L.; et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 77–102. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.J.; Ashworth, A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533, Erratum in N. Engl. J. Med. 2017, 377, 1700. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Gonçalves, A.; Lee, K.H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmaña, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef]
- Narod, S.A.; Risch, H.; Moslehi, R.; Dørum, A.; Neuhausen, S.; Olsson, H.; Provencher, D.; Radice, P.; Evans, G.; Bishop, S.; et al. Oral contraceptives and the risk of hereditary ovarian cancer. N. Engl. J. Med. 1998, 339, 424–428. [Google Scholar] [CrossRef]
- Iodice, S.; Barile, M.; Rotmensz, N.; Feroce, I.; Bonanni, B.; Radice, P.; Bernard, L.; Maisonneuve, P.; Gandini, S. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: A meta-analysis. Eur. J. Cancer 2010, 46, 2275–2284. [Google Scholar] [CrossRef]
- Cibula, D.; Gompel, A.; Mueck, A.O.; La Vecchia, C.; Hannaford, P.C.; Skouby, S.O.; Zikan, M.; Dusek, L. Hormonal contraception and risk of cancer. Hum. Reprod. Update 2010, 16, 631–650. [Google Scholar] [CrossRef]
- Cibula, D.; Zikan, M.; Dusek, L.; Majek, O. Oral contraceptives and risk of ovarian and breast cancers in BRCA mutation carriers: A meta-analysis. Expert Rev. Anticancer Ther. 2011, 11, 1197–1207. [Google Scholar] [CrossRef]
- Perri, T.; Lifshitz, D.; Sadetzki, S.; Oberman, B.; Meirow, D.; Ben-Baruch, G.; Friedman, E.; Korach, J. Fertility treatments and invasive epithelial ovarian cancer risk in Jewish Israeli BRCA1 or BRCA2 mutation carriers. Fertil. Steril. 2015, 103, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Moorman, P.G.; Havrilesky, L.J.; Gierisch, J.M.; Coeytaux, R.R.; Lowery, W.J.; Peragallo Urrutia, R.; Dinan, M.; McBroom, A.J.; Hasselblad, V.; Sander, G.D.; et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: A systematic review and meta-analysis. J. Clin. Oncol. 2013, 31, 4188–4198. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Hopper, J.L.; Win, A.K.; Dowty, J.G.; Sung, H.K.; Ahn, C.; Kim, S.W.; Lee, M.H.; Lee, J.; Lee, J.W.; et al. Reproductive factors as risk modifiers of breast cancer in BRCA mutation carriers and high-risk non-carriers. Oncotarget 2017, 8, 102110–102118. [Google Scholar] [CrossRef] [PubMed]
- Pasanisi, P.; Hédelin, G.; Berrino, J.; Chang-Claude, J.; Hermann, S.; Steel, M.; Haites, N.; Hart, J.; Peled, R.; Gafa, L.; et al. Oral contraceptive use and BRCA penetrance: A case-only study. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2107–2113. [Google Scholar] [CrossRef]
- Kotsopoulos, J.; Lubinski, J.; Moller, P.; Lynch, H.T.; Singer, C.F.; Eng, C.; Neuhausen, S.L.; Karlan, B.; Kim-Sing, C.; Huzarski, T. Timing of oral contraceptive use and the risk of breast cancer in BRCA1 mutation carriers. Breast Cancer Res. Treat. 2014, 143, 579–586. [Google Scholar] [CrossRef]
- Lambertini, M.; Goldrat, O.; Ferreira, A.R.; Dechene, J.; Azim, H.A.; Desirl, J.; Delbaere, A.; t’Kint de Roodenbeke, M.D.; de Azambuja, E.; Ignatiadis, M.; et al. Reproductive potential and performance of fertility preservation strategies in BRCA-mutated breast cancer patients. Ann. Oncol. 2018, 29, 237–243. [Google Scholar] [CrossRef]
- Lambertini, M.; Peccatori, F.A.; Demeestere, I.; Amant, F.; Wyns, C.; Stukenborg, J.B.; Paluch-Shimon, S.; Halaska, M.J.; Uzan, C.; Meissner, J.; et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 31, 1664–1678. [Google Scholar] [CrossRef]
- ESHRE Guideline Group on Female Fertility Preservation; Anderson, R.A.; Amant, F.; Braat, D.; D’Angelo, A.; Chuva de Sousa Lopes, S.M.; Demeestere, I.; Dwek, S.; Frith, L.; Lambertini, M.; et al. ESHRE guideline: Female fertility preservation. Hum. Reprod. Open. 2020, 2020, hoaa052. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, J.; Chen, K.; Li, S.; Wang, Y.; Yang, Y.; Deng, H.; Jia, W.; Rao, N.; Liu, Q.; et al. What lies behind chemotherapy-induced amenorrhea for breast cancer patients: A meta-analysis. Breast Cancer Res. Treat. 2014, 145, 113–128. [Google Scholar] [CrossRef]
- Lee, S.J.; Schover, L.R.; Partridge, A.H.; Patrizio, P.; Wallace, W.H.; Hagerty, K.; Beck, L.N.; Brennan, L.V.; Oktay, K. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J. Clin. Oncol. 2006, 24, 2917–2931. [Google Scholar] [CrossRef]
- Dezellus, A.; Barriere, P.; Campone, M.; Lemanski, C.; Vanlemmens, L.; Mignot, L.; Delozier, T.; Levy, C.; Bendavid, C.; Debled, M.; et al. Prospective evaluation of serum anti-Müllerian hormone dynamics in 250 women of reproductive age treated with chemotherapy for breast cancer. Eur. J. Cancer 2017, 79, 72–80. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Goldrat, O.; Toss, A.; Azim, H.A.; Peccatori, F.A.; Ignatiadis, M.; Del Mastro, L.; Demeestere, I. Fertility and pregnancy issues in BRCA-mutated breast cancer patients. Cancer Treat. Rev. 2017, 59, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Shenfield, F.; Pennings, G.; Devroey, P.; Sureau, C.; Tarlatzis, B.; Cohen, J.; ESHRE Ethics Task Force. Taskforce 5: Preimplantation genetic diagnosis. Hum. Reprod. 2003, 18, 649–651. [Google Scholar] [CrossRef]
- Jasper, M.J.; Liebelt, J.; Hussey, N.D. Preimplantation genetic diagnosis for BRCA1 exon 13 duplication mutation using linked polymorphic markers resulting in a live birth. Prenat. Diagn. 2008, 28, 292–298. [Google Scholar] [CrossRef]
- Derks-Smeets, I.A.; de Die-Smulders, C.E.; Mackens, S.; van Golde, R.; Paulussen, A.D.; Dreesen, J.; Tournaye, H.; Verdyck, P.; Tjan-Heijnen, V.C.; Meijer-Hoogeveen, M.; et al. Hereditary breast and ovarian cancer and reproduction: An observational study on the suitability of preimplantation genetic diagnosis for both asymptomatic carriers and breast cancer survivors. Breast Cancer Res. Treat. 2014, 145, 673–681. [Google Scholar] [CrossRef]
- Cobo, A.; García-Velasco, J.; Domingo, J.; Pellicer, A.; Remohí, J. Elective and Onco-fertility preservation: Factors related to IVF outcomes. Hum. Reprod. 2018, 33, 2222–2231. [Google Scholar] [CrossRef]
- Oktay, K.; Hourvitz, A.; Sahin, G.; Oktem, O.; Safro, B.; Cil, A.; Bang, H. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J. Clin. Endocrinol. Metab. 2006, 91, 3885–3890. [Google Scholar] [CrossRef]
- van den Belt-Dusebout, A.W.; Spaan, M.; Lambalk, C.B.; Kortman, M.; Laven, J.S.; van Santbrink, E.J.; van der Westerlaken, L.A.; Cohlen, B.J.; Braat, D.D.; Smeenk, J.M.; et al. Ovarian Stimulation for In Vitro Fertilization and Long-term Risk of Breast Cancer. JAMA 2016, 316, 300–312. [Google Scholar] [CrossRef]
- Domingo, J.; Guillén, V.; Ayllón, Y.; Martínez, M.; Muñoz, E.; Pellicer, A.; Garcia-Velasco, J.A. Ovarian response to controlled ovarian hyperstimulation in cancer patients is diminished even before oncological treatment. Fertil. Steril. 2012, 97, 930–934. [Google Scholar] [CrossRef]
- von Wolff, M.; Bruckner, T.; Strowitzki, T.; Germeyer, A. Fertility preservation: Ovarian response to freeze oocytes is not affected by different malignant diseases-an analysis of 992 stimulations. J. Assist. Reprod. Genet. 2018, 35, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; O’Neill, S.; Walsh, G.; Smith, I.E. Goserelin with chemotherapy to preserve ovarian function in pre-menopausal women with early breast cancer: Menstruation and pregnancy outcomes. Ann. Oncol. 2013, 24, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Gellert, S.E.; Pors, S.E.; Kristensen, S.G.; Bay-Bjørn, A.M.; Ernst, E.; Yding Andersen, C. Transplantation of frozen-thawed ovarian tissue: An update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J. Assist. Reprod. Genet. 2018, 35, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Blondeaux, E.; Bruzzone, M.; Perachino, M.; Anderson, R.A.; de Azambuja, E.; Poorvu, P.D.; Kim, H.J.; Villarreal-Garza, C.; Pistilli, B.; et al. Pregnancy After Breast Cancer: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2021, 39, 3293–3305. [Google Scholar] [CrossRef] [PubMed]
- Łukaszuk, K.; Kunicki, M.; Liss, J.; Bednarowska, A.; Jakiel, G. Probability of live birth in women with extremely low anti-Müllerian hormone concentrations. Reprod. Biomed. Online 2014, 28, 64–69. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oktay, K. Spontaneous conceptions and live birth after heterotopic ovarian transplantation: Is there a germline stem cell connection? Hum. Reprod. 2006, 21, 1345–1348. [Google Scholar] [CrossRef]
- Sonmezer, M.; Oktay, K. Orthotopic and heterotopic ovarian tissue transplantation. Best Pr. Res. Clin. Obs. Gynaecol. 2010, 24, 113–126. [Google Scholar] [CrossRef]
- Lambertini, M.; Richard, F.; Nguyen, B.; Viglietti, G.; Villarreal-Garza, C. Ovarian Function and Fertility Preservation in Breast Cancer: Should Gonadotropin-Releasing Hormone Agonist be administered to All Premenopausal Patients Receiving Chemotherapy? Clin. Med. Insights Reprod. Health 2019, 13, 1179558119828393. [Google Scholar] [CrossRef]
- Michaan, N.; Leshno, M.; Cohen, Y.; Safra, T.; Peleg-Hasson, S.; Laskov, I.; Grisaru, D. Preimplantation genetic testing for BRCA gene mutation carriers: A cost effectiveness analysis. Reprod. Biol. Endocrinol. 2021, 19, 153. [Google Scholar] [CrossRef]
- Parikh, F.R.; Athalye, A.S.; Naik, N.J.; Naik, D.J.; Sanap, R.R.; Madon, P.F. Preimplantation Genetic Testing: Its Evolution, Where Are We Today? J. Hum. Reprod. Sci. 2018, 11, 306–314. [Google Scholar] [CrossRef]
- Treff, N.R.; Eccles, J.; Lello, L.; Bechor, E.; Hsu, J.; Plunkett, K.; Zimmerman, R.; Rana, B.; Samoilenko, A.; Hsu, S.; et al. Utility and First Clinical Application of Screening Embryos for Polygenic Disease Risk Reduction. Front. Endocrinol. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Stensheim, H.; Cvancarova, M.; Møller, B.; Fosså, S. Pregnancy after adolescent and adult cancer: A population-based matched cohort study. Int. J. Cancer 2011, 129, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Ameye, L.; Hamy, A.S.; Zingarello, A.; Poorvu, P.D.; Carrasco, E.; Grinshpun, A.; Han, S.; Rousset-Jablonski, C.; Ferrari, A.; et al. Pregnancy After Breast Cancer in Patients with GermlineBRCAMutations. J. Clin. Oncol. 2020, 38, 3012–3023. [Google Scholar] [CrossRef]
- Narod, S.A.; Giannakeas, V. In Response to “Pregnancy After Breast Cancer in Patients with Germline BRCA Mutations”. J. Clin. Oncol. 2020, 38, 4352. [Google Scholar] [CrossRef] [PubMed]
- Azim, H.A.; Kroman, N.; Paesmans, M.; Gelber, S.; Rotmensz, N.; Ameye, L.; De Mattos-Arruda, L.; Pistilli, B.; Pinto, A.; Jensen, M.-B.; et al. Prognostic impact of pregnancy after breast cancer according to estrogen receptor status: A multicenter retrospective study. J. Clin. Oncol. 2013, 31, 73–79. [Google Scholar] [CrossRef]
- Lambertini, M.; Kroman, N.; Ameye, L.; Cordoba, O.; Pinto, A.; Benedetti, G.; Jensen, M.B.; Gelber, S.; Del Grande, M.; Ignatiadis, M.; et al. Long-term Safety of Pregnancy Following Breast Cancer According to Estrogen Receptor Status. J. Natl. Cancer Inst. 2018, 110, 426–429. [Google Scholar] [CrossRef]
- Llarena, N.C.; Estevez, S.L.; Tucker, S.L.; Jeruss, J.S. Impact of Fertility Concerns on Tamoxifen Initiation and Persistence. J. Natl. Cancer Inst. 2015, 107, djv202. [Google Scholar] [CrossRef]
- Partridge, A.H.; Niman, S.M.; Ruggeri, M.; Peccatori, F.A.; Azim, H.A., Jr.; Colleoni, M.; Saura, C.; Shimizu, C.; Sætersdal, A.B.; Kroep, J.R.; et al. Who are the women who enrolled in the POSITIVE trial: A global study to support young hormone receptor positive breast cancer survivors desiring pregnancy. Breast 2021, 59, 327–338. [Google Scholar] [CrossRef]

| Degree of Risk | Regiment | Comments |
|---|---|---|
| High risk (>80%) | 6 cycles of CMF, CEF, CAF/TAC in women of ≥40 years | Significant decline in AMH level after treatment |
| Intermediate risk (20–80%) | 6 cycles of CMF, CEF, CAF/TAC in women of 30–39 years 4 cycles of AC in women of ≥40 years 4 cycles of AC/EC → taxane 4 cycles od cc (F)EC → taxane | Significant decline in AMH level after treatment |
| Low risk (<20%) | 6 cycles of CMF, CEF, CAF/TAC in women of <30 years 4 cycles of AC in women of <40 years Antimetabolites (methotrexate, fluorouracil) Vinca alkaloids Bevacizumab | Significant decline in AMH level after treatment |
| Unknown risk | Platinum- and taxane-based ChT Most targeted therapies Immunotherapy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kufel-Grabowska, J.; Podolak, A.; Maliszewski, D.; Bartoszkiewicz, M.; Ramlau, R.; Lukaszuk, K. Fertility Counseling in BRCA1/2-Mutated Women with Breast Cancer and Healthy Individuals. J. Clin. Med. 2022, 11, 3996. https://doi.org/10.3390/jcm11143996
Kufel-Grabowska J, Podolak A, Maliszewski D, Bartoszkiewicz M, Ramlau R, Lukaszuk K. Fertility Counseling in BRCA1/2-Mutated Women with Breast Cancer and Healthy Individuals. Journal of Clinical Medicine. 2022; 11(14):3996. https://doi.org/10.3390/jcm11143996
Chicago/Turabian StyleKufel-Grabowska, Joanna, Amira Podolak, Daniel Maliszewski, Mikołaj Bartoszkiewicz, Rodryg Ramlau, and Krzysztof Lukaszuk. 2022. "Fertility Counseling in BRCA1/2-Mutated Women with Breast Cancer and Healthy Individuals" Journal of Clinical Medicine 11, no. 14: 3996. https://doi.org/10.3390/jcm11143996
APA StyleKufel-Grabowska, J., Podolak, A., Maliszewski, D., Bartoszkiewicz, M., Ramlau, R., & Lukaszuk, K. (2022). Fertility Counseling in BRCA1/2-Mutated Women with Breast Cancer and Healthy Individuals. Journal of Clinical Medicine, 11(14), 3996. https://doi.org/10.3390/jcm11143996






