Postacute Laryngeal Injuries and Dysfunctions in COVID-19 Patients: A Scoping Review
Abstract
:1. Introduction
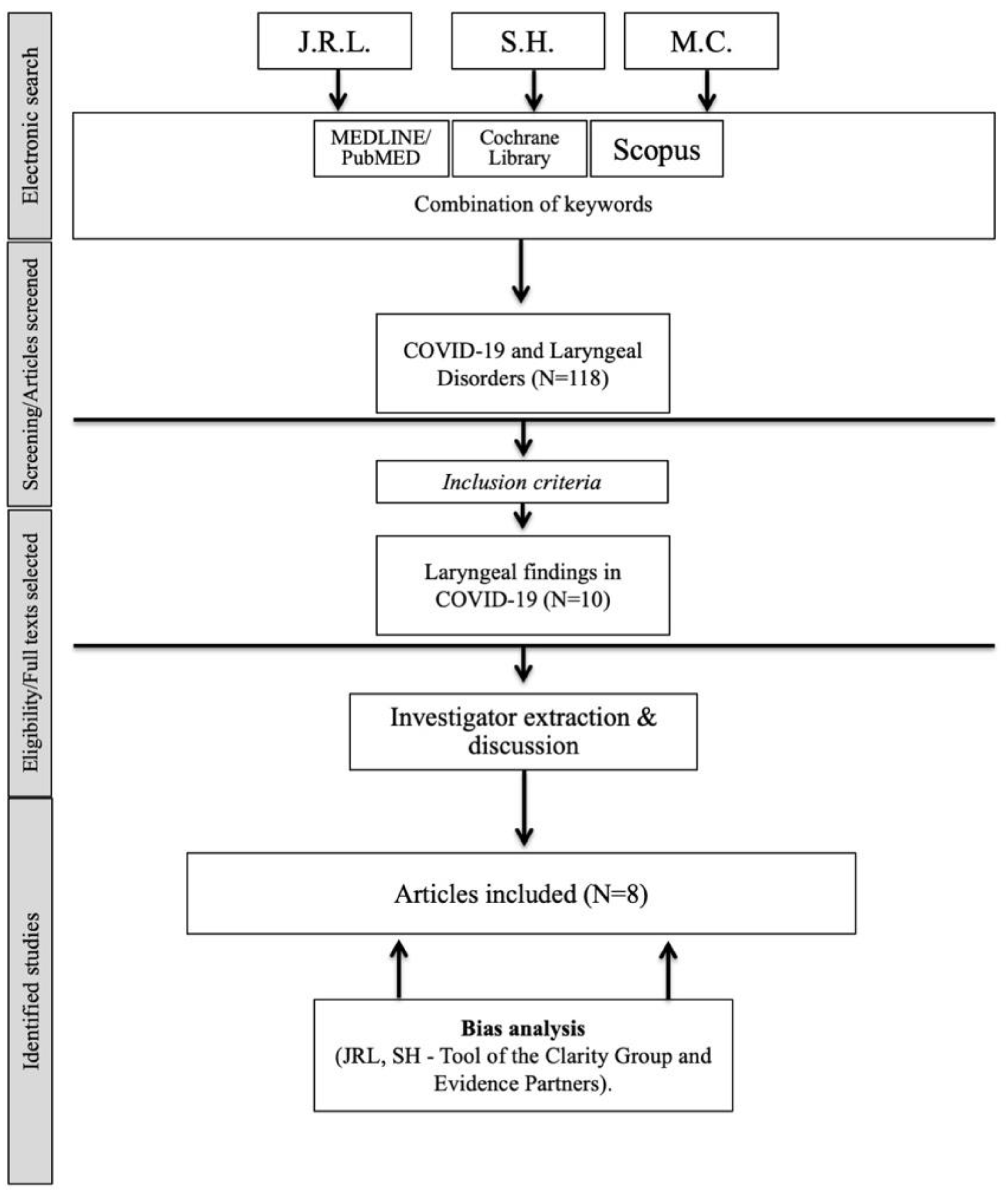
2. Materials and Methods
2.1. Patient Population
2.2. Intervention, Comparison, and Outcomes
2.3. Timing and Setting
2.4. Search Strategy
3. Results
3.1. Study Features
3.2. Laryngeal Abnormalities
3.3. Bias Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Confounding | Population | Int./Trach. | Postdischarge | ||
|---|---|---|---|---|---|
| Authors | Factors | Analysis | Delay | Details | Care |
| Naunheim [8] | Probably no | Probably yes | Probably yes | Yes | No |
| Scholfield [9] | No | Yes | Yes | Probably yes | No |
| Sandblom [10] | Probably no | Probably yes | Probably yes | Probably yes | No |
| Neevel [11] | Probably yes | Yes | Yes | Probably yes | No |
| Felix [12] | Probably yes | Probably yes | Probably yes | Probably yes | No |
| Azzam [17] | Probably yes | Yes | Probably yes | Yes | No |
| Allisan [18] | Probably yes | Probably yes | Probably yes | Probably yes | No |
| Hans [19] | Probably no | Probably yes | Probably yes | Probably yes | No |
References
- WHO Official Website. Report of 1 May 2022. Available online: https://covid19.who.int (accessed on 1 May 2022).
- Siemieniuk, R.A.; Bartoszko, J.J.; Ge, L.; Zeraatkar, D.; Izcovich, A.; Kum, E.; Pardo-Hernandez, H.; Qasim, A.; Martinez, J.P.D.; Rochwerg, B.; et al. Drug treatments for COVID-19: Living systematic review and network meta-analysis. BMJ 2020, 370, m2980. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.; Parikh, A.; Lopez-Ruiz, A.; Carrilo, M.; Goldberg, J.; Cearras, M.; Fernainy, K.; Andersen, S.; Mercado, L.; Guan, J.; et al. ICU outcomes and survival in patients with severe COVID-19 in the largest health care system in central Florida. PLoS ONE 2021, 16, e0249038. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipavath, S.; Wurfel, M.M.; Evans, L.; et al. COVID-19 in critically Ill Patients in the Seattle Region—Case Series. N. Engl. J. Med. 2020, 382, 2012–2022. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Malviya, A.; Ahirwar, A.K.; Chandra Tripathi, S.; Asia, P.; Gopal, N.; Kaim, K. COVID-19: A review on SARS-CoV-2 origin, epidemiology, virology, clinical manifestations and complications with special emphasis on adverse outcome in Bhopal Gas Tragedy survivor. Horm. Mol. Biol. Clin. Investig. 2021, 42, 63–68. [Google Scholar] [CrossRef]
- Halpin, S.J.; McIvor, C.; Whyatt, G.; Adams, A.; Harvey, O.; McLean, L.; Walshaw, C.; Kemp, S.; Corrado, J.; Singh, R.; et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J. Med. Virol. 2021, 93, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Naunheim, M.R.; Zhou, A.S.; Puka, E.; Franco, R.A., Jr.; Carroll, T.L.; Teng, S.E.; Mallur, P.S.; Song, P.C. Laryngeal complications of COVID-19. Laryngoscope Investig. Otolaryngol. 2020, 5, 1117–1124. [Google Scholar] [CrossRef]
- Scholfield, D.W.; Warner, E.; Ahmed, J.; Ghufoor, K. Subglottic and tracheal stenosis associated with coronavirus disease 2019. J. Laryngol. Otol. 2021, 135, 656–658. [Google Scholar] [CrossRef] [PubMed]
- Sandblom, H.; Dotevall, H.; Svennerholm, K.; Tuomi, L.; Finizia, C. Characterization of dysphagia and laryngeal findings in COVID-19 patients treated in the ICU-An observational clinical study. PLoS ONE 2021, 16, e0252347. [Google Scholar] [CrossRef]
- Neevel, A.J.; Smith, J.D.; Morrison, R.J.; Hogikyan, N.D.; Kupfer, R.A.; Stein, A.P. Postacute COVID-19 Laryngeal Injury and Dysfunction. OTO Open 2021, 5, 2473974X211041040. [Google Scholar] [CrossRef]
- Félix, L.; Tavares, T.L.; Almeida, V.P.B.; Tiago, R.S.L. Incidence of Laryngotracheal Lesions after Orotracheal Intubation in Coronavirus Disease Patients. Laryngoscope 2022, 132, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Tiwari, A.; Fu, R.; Moe, E.; Buckley, D.I. A Framework to Facilitate the Use of Systematic Reviews and Meta-Analyses in the Design of Primary Research Studies; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2012. Available online: http://www.ncbi.nlm.nih.gov/books/NBK83621/ (accessed on 22 February 2020).
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; Hooft, L.; et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Colditz, G.A. Overview of the epidemiology methods and applications: Strengths and limitations of observational study designs. Crit. Rev. Food Sci. Nutr. 2010, 50 (Suppl. 1), 10–12. [Google Scholar] [CrossRef]
- Viswanathan, M.; Berkman, N.D.; Dryden, D.M.; Hartling, L. Assessing Risk of Bias and Confounding in Observational Studies of Interventions or Exposures: Further Development of the RTI Item Bank; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2013. Available online: http://www.ncbi.nlm.nih.gov/books/NBK154461/ (accessed on 20 October 2019).
- Azzam, A.A.A.; Samy, A.; Sefein, I.; ElRouby, I. Vocal Disorders in patients with COVID 19 in Egypt. Indian J. Otolaryngol. Head Neck Surg. 2021, 1–7. [Google Scholar] [CrossRef]
- Allisan-Arrighi, A.E.; Rapoport, S.K.; Laitman, B.M.; Bahethi, R.; Mori, M.; Woo, P.; Genden, E.; Courey, M.; Kirke, D.N. Long-term upper aerodigestive sequelae as a result of infection with COVID-19. Laryngoscope Investig. Otolaryngol. 2022, 7, 476–485. [Google Scholar] [CrossRef]
- Hans, S.; Circiu, M.P.; Crevier-Buchman, L.; Annane, D.; Heming, N.; Lechien, J.R. Post-intubation laryngeal disorders in COVID-19 patients: A prospective study. Eur. Arch. Otorhinolaryngol. 2022. submitted. [Google Scholar]
- Lechien, J.R.; Chiesa-Estomba, C.M.; Hans, S.; Barillari, M.R.; Jouffe, L.; Saussez, S. Loss of smell and taste in 2013 European Patients with mild to moderate COVID-19. Ann. Intern. Med. 2020, 173, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Mat, Q.; Noël, A.; Loiselet, L.; Tainmont, S.; Chiesa-Estomba, C.M.; Lechien, J.R.; Duterme, J.P. Vestibular neuritis as clinical presentation Of COVID-19. Ear Nose Throat J. 2021, 145561321995021. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chetrit, A.; Chekkoury-Idrissi, Y.; Distinguin, L.; Circiu, M.; Saussez, S.; Berradja, N.; Edjlali, M.; Hans, S.; Carlier, R. Parotitis-like symptoms associated with COVID-19, France, March–April 2020. Emerg. Infect. Dis. 2020, 26, 2270–2271. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.A.; Silva, M.T.T.; Soares, C.N.; Coutinho, R.; Oliveira, H.S.; Afonso, L.; Espíndola, O.; Leite, A.C.; Araujo, A. Peripheral facial nerve palsy associated with COVID-19. J. Neurovirol. 2020, 26, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Circiu, M.P.; Crevier-Buchman, L.; Hans, S. Post-COVID-19 paradoxical vocal fold movement disorder. Eur. Arch. Otorhinolaryngol. 2021, 278, 845–846. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Chiesa-Estomba, C.M.; Cabaraux, P.; Mat, Q.; Huet, K.; Harmegnies, B.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; et al. Features of mild-to-moderate COVID-19 patients with dysphonia. J. Voice 2020, 36, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, M.B.; Levy, M.J.; Jedlanek, E.; Pandian, V.; Blackford, B.; Price, C.; Cole, G.; Hillel, A.T.; Best, S.R.; Akst, L.M. Laryngeal Injury and upper airway symptoms after oral endotracheal intubation with mechanical ventilation during critical care: A systematic review. Crit. Care Med. 2018, 46, 2010–2017. [Google Scholar] [CrossRef] [PubMed]
- Houtz, D.R.; Roy, N.; Merrill, R.M.; Smith, M.E. Differential diagnosis of muscle tension dysphonia and adductor spasmodic dysphonia using spectral moments of the long-term average spectrum. Laryngoscope 2010, 120, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Akst, L.M.; Hamdan, A.L.; Schindler, A.; Karkos, P.D.; Barillari, M.R.; Calvo-Henriquez, C.; Crevier-Buchman, L.; Finck, C.; Eun, Y.-G.; et al. Evaluation and Management of laryngopharyngeal reflux disease: State of the art review. Otolaryngol. Head Neck Surg. 2019, 160, 762–782. [Google Scholar] [CrossRef]
- Lina, I.; Tsai, H.W.; Ding, D.; Davis, R.; Motz, K.M.; Hillel, A.T. Characterization of Fibroblasts in Iatrogenic Laryngotracheal Stenosis and Type II Diabetes Mellitus. Laryngoscope 2021, 131, 1570–1577. [Google Scholar] [CrossRef]
| Authors | Study | EL | Population | ICU Outcomes | Voice/Laryngeal Outcomes | Results | Conclusion |
|---|---|---|---|---|---|---|---|
| Naunheim [8] | Prospective | III | Gr1 = 13 dysphonic ICU | Intubation (13): 22 d | Disorder prevalence | Gr1-2 N, % | 1.Most patients with dysphonia and history of COVID-19 had history of intubation. The occurrence of laryngeal lesions was found in post-intubated patients. |
| USA | Uncontrolled | Gr2 = 7 non-ICU patients | Tube size: 7.5 | Voice disorders | 3 (43)–9 (69) | ||
| Tracheostomy: 9 | Breathing disorders | 2 (28)–5 (38) | |||||
| Age = 59 yo | Duration: 16 d | Stroboscopy abnormalities | 17 (85) | ||||
| F/M = 5/15 | Proning: 9 | Vocal fold immobility | 8 (40) | ||||
| BMI = NP | Posterior glottic stenosis | 3 (15) | |||||
| Delay = NP | Subglottis stenosis | 2 (10) | |||||
| Posterior glottic diastasis | 2 (10) | 2. Nine patients required procedural interventions; 4 in operating room. | |||||
| Laryngopharyngeal reflux | 2 (10) | ||||||
| MTD | 1 (5) | ||||||
| Intervention need | 9 (45) | ||||||
| Scholfield [9] | Retrospective | IV | N = 3 post-intubated ICU | Intubation (3): 34 d | Prevalence of | 1. Subglottis stenosis may occur early in COVID-19 patients who were long-time intubated or tracheotomized. | |
| UK | Case-series | Age = 49 yo | Tube size: 8 | Subglottis stenosis | N = 3 | ||
| F/M = 1/2 | Tracheostomy: 3 | ||||||
| BMI = NP | Duration: 30 d | ||||||
| Delay = NP | Tube: 7–9 | ||||||
| Proning: N.P. | |||||||
| Sandblom [10] | Prospective | III | N = 25 post-intubated ICU | Intubation (25): 10 d | FEES penetration | 23 (96) | 1. Vocal fold movement disorders are prevalent in post-intubated patients. |
| Germany | Uncontrolled | Age = 63 yo | Tube size: NP | Vocal fold dysmotility | 19 (76) | ||
| F/M = 2/23 | Tracheostomy: 20 | Vocal fold immobility | 2 (8) | ||||
| BMI = 28 | Duration: 30 d | Granuloma | 2 (8) | 2. There was a positive correlation between ICU hospitalization duration and dysphagia severity. | |||
| Delay = NP | Tube: NP | Vocal fold hematoma | 1 (4) | ||||
| Proning: 12 | Vocal fold ulceration | 1 (4) | |||||
| Neevel [11] | Retrospective | IV | N = 18 dysphonic ICU | Intubation (18): 14 d | V-RQOL score (N = 14) | 73 | 1. Most patients had multiple chief voice complaints. |
| USA | Case-series | N = 2 non-ICU patients | Tube size: 8 | Intubated patients (N = 18): | |||
| Age = 50 yo | Tracheostomy: 10 | VF motion impairments | 9 (50) | 2. Intubated patients reported high prevalence of laryngeal injuries. | |||
| F/M = 12/12 | Duration: 18 d | VF edema/erosion | 7 (39) | ||||
| BMI = 29 | Tube: NP | Subglottis stenosis | 4 (22) | 3. Non-intubated patients reported tension muscle dysphonia (4), glottic edema (1), laryngitis (1), and unilateral VF paresis (1) | |||
| Delay= 107 d | Proning: 10 | Posterior glottic diastasis | 4 (22) | ||||
| Posterior glottic stenosis | 3 (17) | ||||||
| Unilateral VF immobility | 4 (22) | ||||||
| Unilateral VF hypomobility | 2 (11) | 4. Surgical/medical treatments were made in 10 and 4 patients. | |||||
| Bilateral VF hypomobility | 3 (17) | ||||||
| Felix [12] | Prospective | III | N = 95 post-intubated ICU | Intubation (95): 12 d | Laryngeal injuries | 38 (40) | 1. Laryngeal injuries were found in 40% of intubated patients. |
| Uncontrolled | Age = 59 yo | Tube size: 7–8 | Hyperemia | 6 (6) | |||
| F/M = 44/51 | Tracheostomy: 20 | Granuloma | 15 (16) | 2. Tube size and prone position were contributing factors of laryngeal injuries. | |||
| BMI = NP | Duration: NP | Posterior glottic stenosis | 16 (17) | ||||
| Delay = 100 d | Tube: NP | Unilateral VF immobility | 1 (1) | ||||
| Proning: 47 | |||||||
| Azzam [17] | Prospective | III | N = 106 non-intubated | - | Dysphonia | 84 (79) | 1. Dysphonia I in 79% of mild-to-moderate COVID-19 patients. |
| Egypt | Uncontrolled | Age = 42 yo | VF edema | 42 (40) | |||
| F/M = 78/28 | VF swelling | 18 (17) | |||||
| BMI = NP | Unilateral VF immobility | 14 (13) | 2. Various laryngeal findings were found in videostroboscopy. | ||||
| Delay = <30 d | Ventricular band edema | 20 (19) | |||||
| Allisan [18] | Retrospective | IV | Gr1 = 31 intubated | Intubation (31): 17 d | Disorders | Gr1-2, p-value | 1.COVID-19 may be associated with laryngeal injuries and disorders in intubated and not intubated patients. |
| USA | Case-series | Gr2 = 50 not intubated | Tube size: 8 | Dysphonia | 20, 38; NS | ||
| Age = 54 yo | Tracheostomy: 18 | MTD | 1-19; S | ||||
| F/M = 32/49 | Duration: 70 d | LPR | 1-18; S | ||||
| BMI = NP | Tube: 7–9 | VF paresis | 3-3, NS | 2.Granuloma, posterior glottis stenosis, VF paresis, and tracheal stenosis were the most prevalent diseases. | |||
| Delay = 122 d | Proning: NP | VF paralysis | 5-3, NS | ||||
| VF atrophy | 3-6, NS | ||||||
| VF polyp | 0-8, NS | ||||||
| Granuloma | 8-0, S | ||||||
| Glottis insufficiency | 4-3, NS | ||||||
| Arytenoid ankylosis | 1-5, NS | ||||||
| Posterior/subglottis stenosis | 5-0, NS | ||||||
| Tracheal stenosis | 5-0, NS | ||||||
| Hans [19] | Prospective | III | N = 43 intubated | Intubation (43): 10 d | Posterior glottic stenosis | 14 (33) | 1. Posterior glottis stenosis, laryngeal edema and granuloma were the most prevalent laryngeal findings. |
| France | Uncontrolled | Age = 52 yo | Tube size: NP | Laryngeal edema | 10 (23) | ||
| F/M = 10/33 | Tracheostomy: 8 | Granuloma | 8 (19) | ||||
| BMI = NP | Duration: NP | Laryngeal necrosis | 2 (5) | ||||
| Delay = 51 d | Tube: 7–9 | Posterior glottic diastasis | 2 (5) | 2. Prolonged intubation was associated with an increase of laryngeal injuries (posterior stenosis). | |||
| Proning: NP | VF atrophy | 2 (5) | |||||
| Subglottis stenosis | 1 (2) |
| Authors | Inclusion | Exclusion | Comorbidities |
|---|---|---|---|
| Naunheim [8] | Voice-related disorder | NP | Hypertension (11), Tobacco (9), |
| patients | Diabetes (8), Asthma (4), Obesity (3), | ||
| OSAS (2), COPD (1) | |||
| Scholfield [9] | - | - | Diabetes (2), Obesity (2), |
| Hypertension (2), OSAS (1), LPR (1) | |||
| Sandblom [10] | Post-intubated patients | NP | Hypertension (16), Diabetes (11), |
| with dysphonia | Obesity (8), OSAS (4), Stroke (2), | ||
| Coronary disease (6) | |||
| Neevel [11] | Dysphonic patients | Dysphonia before COVID-19 | Diabetes (9), Hypertension (9), |
| No confirmation of COVID-19 | Tobacco history (8), Asthma/COPB (5) | ||
| Coronary disease (2) | |||
| Felix [12] | Post-intubated patients | Dysphonia before COVID-19 | Hypertension (52) |
| with dysphonia | No confirmation of COVID-19 | Diabetes (41) | |
| Obesity (28) | |||
| Azzam [17] | Mild-to-moderate | Dysphonia before COVID-19 | NP |
| COVID-19 cases | No confirmation of COVID-19 | ||
| >1-month delay post-COVID-19 | |||
| Severe COVID-19 | |||
| Laryngeal lesion before COVID-19 | |||
| Chemo/radiotherapy, Head Neck | |||
| Trauma or cancer histories | |||
| Allisan [18] | Dysphonic patients | Laryngeal disorders before | Reflux (10), tobacco (9), Diabetes (9), |
| COVID-19 | Asthma (6), Anxiety (6), Obesity (4), | ||
| Hypertension (4), OSAS (2), COPD (3), | |||
| Depression (2) | |||
| Depression (2), Panic disorder (2) | |||
| Hans [19] | Post-intubated patients | NP | Hypertension (30), Diabetes (20), |
| with dysphonia | Tobacco (18), Dyslipidemia (12), | ||
| Obesity (10), Coronary disease (7), | |||
| COPD (4), OSAS (4) |
| Laryngeal Disorders | Number/Total | Prevalence | References |
|---|---|---|---|
| VF dysmotility | 28/43 | 65.1 | [10,11] |
| VF edema | 59/167 | 35.3 | [11,17,19] |
| MTD | 20/81 | 24.7 | [18] |
| Laryngopharyngeal reflux | 19/81 | 23.5 | [18] |
| Ventricular band edema | 20/106 | 18.9 | [17] |
| Bilateral VF hypo/immobility | 3/18 | 16.7 | [11] |
| Posterior glottic stenosis | 39/237 | 16.5 | [11,12,18,19] |
| Granuloma | 33/244 | 13.5 | [10,12,18,19] |
| Posterior glottic diastasis or atrophy | 17/142 | 12.0 | [11,18,19] |
| VF polyp | 8/81 | 9.9 | [18] |
| VF immobility | 29/325 | 8.9 | [10,11,12,17,18] |
| Subglottis stenosis | 13/145 | 8.9 | [9,11,18,19] |
| Glottis insuffisiency | 7/81 | 8.6 | [18] |
| VF hypomobility | 8/99 | 8.1 | [11,18] |
| VF ulceration or necrosis | 3/68 | 4.4 | [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lechien, J.R.; Hans, S. Postacute Laryngeal Injuries and Dysfunctions in COVID-19 Patients: A Scoping Review. J. Clin. Med. 2022, 11, 3989. https://doi.org/10.3390/jcm11143989
Lechien JR, Hans S. Postacute Laryngeal Injuries and Dysfunctions in COVID-19 Patients: A Scoping Review. Journal of Clinical Medicine. 2022; 11(14):3989. https://doi.org/10.3390/jcm11143989
Chicago/Turabian StyleLechien, Jérôme R., and Stéphane Hans. 2022. "Postacute Laryngeal Injuries and Dysfunctions in COVID-19 Patients: A Scoping Review" Journal of Clinical Medicine 11, no. 14: 3989. https://doi.org/10.3390/jcm11143989
APA StyleLechien, J. R., & Hans, S. (2022). Postacute Laryngeal Injuries and Dysfunctions in COVID-19 Patients: A Scoping Review. Journal of Clinical Medicine, 11(14), 3989. https://doi.org/10.3390/jcm11143989







