Hypercholesterolemia Diagnosis, Treatment Patterns, and 12-Month Target Achievement in Clinical Practice in Germany in Patients with Familial Hypercholesterolemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Selection and Patient Selection
2.2. Sample Size
2.3. Data Collection
2.4. Objectives
2.5. Statistics
3. Results
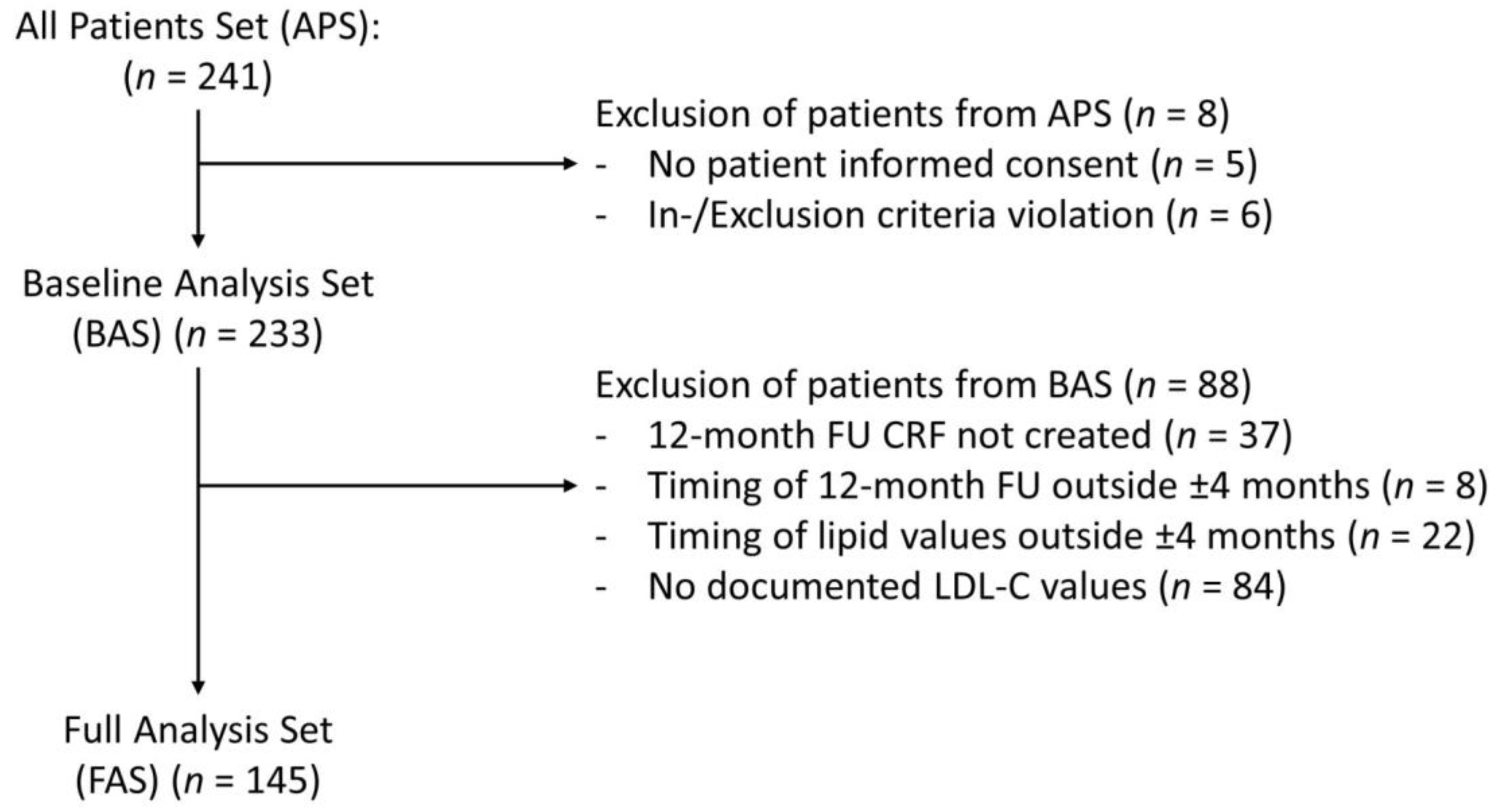
3.1. Cross-Sectional Analysis (BAS, n = 233)
3.2. Longitudinal Analysis at 12 Months (FAS, n = 145)
3.3. Outcomes Analysis
4. Discussion
4.1. Patient Characteristics
4.2. Genetic Testing
4.3. Treatment
4.4. Longitudinal Follow-Up
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akioyamen, L.E.; Genest, J.; Shan, S.D.; Reel, R.L.; Albaum, J.M.; Chu, A.; Tu, J.V. Estimating the prevalence of heterozygous familial hypercholesterolaemia: A systematic review and meta-analysis. BMJ Open 2017, 7, e016461. [Google Scholar] [CrossRef]
- Benn, M.; Watts, G.F.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. Familial hypercholesterolemia in the danish general population: Prevalence, coronary artery disease, and cholesterol-lowering medication. J. Clin. Endocrinol. Metab. 2012, 97, 3956–3964. [Google Scholar] [CrossRef]
- Perak, A.M.; Ning, H.; de Ferranti, S.D.; Gooding, H.C.; Wilkins, J.T.; Lloyd-Jones, D.M. Long-Term Risk of Atherosclerotic Cardiovascular Disease in US Adults With the Familial Hypercholesterolemia Phenotype. Circulation 2016, 134, 9–19. [Google Scholar] [CrossRef] [Green Version]
- De Luca, L.; Arca, M.; Temporelli, P.L.; Colivicchi, F.; Gonzini, L.; Lucci, D.; Bosco, B.; Callerame, M.; Lettica, G.V.; Di Lenarda, A.; et al. Prevalence and pharmacologic management of familial hypercholesterolemia in an unselected contemporary cohort of patients with stable coronary artery disease. Clin. Cardiol. 2018, 41, 1075–1083. [Google Scholar] [CrossRef] [Green Version]
- Teramoto, T.; Sawa, T.; Iimuro, S.; Inomata, H.; Koshimizu, T.; Sakakibara, I.; Hiramatsu, K. The Prevalence and Diagnostic Ratio of Familial Hypercholesterolemia (FH) and Proportion of Acute Coronary Syndrome in Japanese FH Patients in a Healthcare Record Database Study. Cardiovasc. Ther. 2020, 2020, 5936748. [Google Scholar] [CrossRef]
- Lui, D.T.W.; Lee, A.C.H.; Tan, K.C.B. Management of Familial Hypercholesterolemia: Current Status and Future Perspectives. J. Endocr. Soc. 2021, 5, bvaa122. [Google Scholar] [CrossRef]
- EAS Familial Hypercholesterolaemia Studies Collaboration; Vallejo-Vaz, A.J.; De Marco, M.; Stevens, C.A.T.; Akram, A.; Freiberger, T.; Hovingh, G.K.; Kastelein, J.J.P.; Mata, P.; Raal, F.J.; et al. Overview of the current status of familial hypercholesterolaemia care in over 60 countries—The EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Atherosclerosis 2018, 277, 234–255. [Google Scholar] [CrossRef] [Green Version]
- FHSC. Global perspective of familial hypercholesterolaemia: A cross-sectional study from the EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Lancet 2021, 398, 1713–1725. [Google Scholar] [CrossRef]
- Catapano, A.L.; Lautsch, D.; Tokgozoglu, L.; Ferrieres, J.; Horack, M.; Farnier, M.; Toth, P.P.; Brudi, P.; Tomassini, J.E.; Ambegaonkar, B.; et al. Prevalence of potential familial hypercholesteremia (FH) in 54,811 statin-treated patients in clinical practice. Atherosclerosis 2016, 252, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Perez-Calahorra, S.; Laclaustra, M.; Marco-Benedi, V.; Lamiquiz-Moneo, I.; Pedro-Botet, J.; Plana, N.; Sanchez-Hernandez, R.M.; Amor, A.J.; Almagro, F.; Fuentes, F.; et al. Effect of lipid-lowering treatment in cardiovascular disease prevalence in familial hypercholesterolemia. Atherosclerosis 2019, 284, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur. Heart J. 2016, 37, 2999–3058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Erasmo, L.; Commodari, D.; Di Costanzo, A.; Minicocci, I.; Polito, L.; Ceci, F.; Montali, A.; Maranghi, M.; Arca, M. Evolving trend in the management of heterozygous familial hypercholesterolemia in Italy: A retrospective, single center, observational study. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 2027–2035. [Google Scholar] [CrossRef] [PubMed]
- Petrov, I.S.; Postadzhiyan, A.S.; Tokmakova, M.P.; Kitova, L.G.; Tsonev, S.N.; Addison, J.; Petkova, R.T.; Lachev, V.I. Management of High and Very High-Risk Subjects with Familial Hypercholesterolemia: Results from an Observational Study in Bulgaria. Folia Med. 2018, 60, 389–396. [Google Scholar] [CrossRef]
- Lalic, K.; Rajkovic, N.; Popovic, L.; Lukac, S.S.; Stosic, L.; Rasulic, I.; Lalic, N.M. The effects of 3-year statin therapy and the achievement of LDL cholesterol target values in familial hypercholesterolemia patients: An experience from Serbia. Atherosclerosis 2018, 277, 298–303. [Google Scholar] [CrossRef]
- Katzmann, J.L.; Lehmann, M.; Tunnemann-Tarr, A.; An Haack, I.; Dressel, A.; Marz, W.; Laufs, U. Cutaneous manifestations in familial hypercholesterolaemia. Atherosclerosis 2021, 333, 116–123. [Google Scholar] [CrossRef]
- Schmidt, N.; Dressel, A.; Grammer, T.B.; Gouni-Berthold, I.; Julius, U.; Kassner, U.; Klose, G.; Konig, C.; Koenig, W.; Otte, B.; et al. Lipid-modifying therapy and low-density lipoprotein cholesterol goal attainment in patients with familial hypercholesterolemia in Germany: The CaReHigh Registry. Atherosclerosis 2018, 277, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, N.; Grammer, T.; Gouni-Berthold, I.; Julius, U.; Kassner, U.; Klose, G.; Konig, C.; Laufs, U.; Otte, B.; Steinhagen-Thiessen, E.; et al. CaRe high—Cascade screening and registry for high cholesterol in Germany. Atheroscler. Suppl. 2017, 30, 72–76. [Google Scholar] [CrossRef]
- Schmidt, N.; Schmidt, B.; Dressel, A.; Gergei, I.; Klotsche, J.; Pieper, L.; Scharnagl, H.; Kleber, M.E.; Marz, W.; Lehnert, H.; et al. Familial hypercholesterolemia in primary care in Germany. Diabetes and cardiovascular risk evaluation: Targets and Essential Data for Commitment of Treatment (DETECT) study. Atherosclerosis 2017, 266, 24–30. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Chapman, M.J.; Humphries, S.E.; Ginsberg, H.N.; Masana, L.; Descamps, O.S.; Wiklund, O.; Hegele, R.A.; Raal, F.J.; Defesche, J.C.; et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: Guidance for clinicians to prevent coronary heart disease: Consensus statement of the European Atherosclerosis Society. Eur. Heart J. 2013, 34, 3478–3490a. [Google Scholar] [CrossRef] [Green Version]
- Austin, M.A.; Hutter, C.M.; Zimmern, R.L.; Humphries, S.E. Genetic causes of monogenic heterozygous familial hypercholesterolemia: A HuGE prevalence review. Am. J. Epidemiol. 2004, 160, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Hovland, A.; Mundal, L.J.; Igland, J.; Veierod, M.B.; Holven, K.B.; Bogsrud, M.P.; Tell, G.S.; Leren, T.P.; Retterstol, K. Increased risk of heart failure and atrial fibrillation in heterozygous familial hypercholesterolemia. Atherosclerosis 2017, 266, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Vongpromek, R.; Bos, S.; Ten Kate, G.J.; Yahya, R.; Verhoeven, A.J.; de Feyter, P.J.; Kronenberg, F.; Roeters van Lennep, J.E.; Sijbrands, E.J.; Mulder, M.T. Lipoprotein(a) levels are associated with aortic valve calcification in asymptomatic patients with familial hypercholesterolaemia. J. Intern. Med. 2015, 278, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Fahed, A.C.; Shibbani, K.; Andary, R.R.; Arabi, M.T.; Habib, R.H.; Nguyen, D.D.; Haddad, F.F.; Moubarak, E.; Nemer, G.; Azar, S.T.; et al. Premature Valvular Heart Disease in Homozygous Familial Hypercholesterolemia. Cholesterol 2017, 2017, 3685265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ten Kate, G.R.; Bos, S.; Dedic, A.; Neefjes, L.A.; Kurata, A.; Langendonk, J.G.; Liem, A.; Moelker, A.; Krestin, G.P.; de Feyter, P.J.; et al. Increased Aortic Valve Calcification in Familial Hypercholesterolemia: Prevalence, Extent, and Associated Risk Factors. J. Am. Coll. Cardiol. 2015, 66, 2687–2695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffey, S.; Roberts-Thomson, R.; Brown, A.; Carapetis, J.; Chen, M.; Enriquez-Sarano, M.; Zuhlke, L.; Prendergast, B.D. Global epidemiology of valvular heart disease. Nat. Rev. Cardiol. 2021, 18, 853–864. [Google Scholar] [CrossRef]
- Grenkowitz, T.; Kassner, U.; Wuhle-Demuth, M.; Salewsky, B.; Rosada, A.; Zemojtel, T.; Hopfenmuller, W.; Isermann, B.; Borucki, K.; Heigl, F.; et al. Clinical characterization and mutation spectrum of German patients with familial hypercholesterolemia. Atherosclerosis 2016, 253, 88–93. [Google Scholar] [CrossRef]
- Rieck, L.; Bardey, F.; Grenkowitz, T.; Bertram, L.; Helmuth, J.; Mischung, C.; Spranger, J.; Steinhagen-Thiessen, E.; Bobbert, T.; Kassner, U.; et al. Mutation spectrum and polygenic score in German patients with familial hypercholesterolemia. Clin. Genet. 2020, 98, 457–467. [Google Scholar] [CrossRef]
- Raal, F.J.; Hovingh, G.K.; Catapano, A.L. Familial hypercholesterolemia treatments: Guidelines and new therapies. Atherosclerosis 2018, 277, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Nordoy, A. Statins and omega-3 fatty acids in the treatment of dyslipidemia and coronary heart disease. Minerva Med. 2002, 93, 357–363. [Google Scholar]
- Kokkinos, P.F.; Faselis, C.; Myers, J.; Panagiotakos, D.; Doumas, M. Interactive effects of fitness and statin treatment on mortality risk in veterans with dyslipidaemia: A cohort study. Lancet 2013, 381, 394–399. [Google Scholar] [CrossRef]
- Brunham, L.R.; Cermakova, L.; Lee, T.; Priecelova, I.; Alloul, K.; de Chantal, M.; Francis, G.A.; Frohlich, J. Contemporary Trends in the Management and Outcomes of Patients With Familial Hypercholesterolemia in Canada: A Prospective Observational Study. Can. J. Cardiol. 2017, 33, 385–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| BAS n = 233 | FAS n = 145 | |
|---|---|---|
| Age, years mean ± SD | 61.1 ± 14.0 (n = 233) | 60.6 ± 13.7 (n = 145) |
| Median (IQR) | 62 (53, 71) | 63 (55, 70) |
| Male gender, n (%) | 137/233 (58.8) | 82/145 (56.6) |
| Body mass index, kg/m2 mean ± SD | 28.5 ± 4.50 (n = 228) | 28.2 ± 4.3 (n = 145) |
| Median (IQR) | 28 (25, 31) | 28 (25, 31) |
| Phenotypic findings—no. of participants | ||
| Xanthelasma, n/N (%) | 48/227 (21.1) | 38/145 (26.2) |
| Xanthomas, n/N (%) | 28/228 (12.3) | 18/145 (12.4) |
| Arcus cornealis, n/N (%) | 20/227 (8.8) | 15/145 (10.3) |
| Funct. mutation in LDLR, apoB or PCSK9 gene, n/N (%) | 21/99 (21.2) | 15/57 (26.3) |
| Age at initial diagnosis, years, mean ± SD | 46.4 ± 10.5 (n = 20) | 48.1 ± 9.6 (n = 15) |
| Median (IQR) | 47 (41, 55) | 49 (43, 58) |
| Family history—no. of participants | (n = 228) | (n = 145) |
| Family history of elevated cholesterol levels, n (%) | 140 (60.1) | 97 (66.9) |
| Family history of coronary heart disease, n (%) | 125 (53.7) | 87 (60.0) |
| Family history of myocardial infarction, n (%) | 114 (48.9) | 81 (55.9) |
| Family history of cerebrovascular disease, n (%) | 53 (22.8) | 37 (25.5) |
| Family history of tendon xanthomas, n (%) | 19 (8.2) | 13 (9.0) |
| Family history of arcus cornealis, n (%) | 8 (3.4) | 3 (2.1) |
| Lipoprotein apheresis therapy, n (%) | 11 (4.8) * | 9 (6.2) |
| Cardiovascular history—no. of participants | (n = 228) | (n = 145) |
| Coronary heart disease, n (%) | 126 (55.3) | 89 (61.4) |
| Percutaneous coronary intervention, n (%) | 78 (34.2) | 57 (39.3) |
| Acute coronary syndrome, n (%) | 46 (20.2) | 32 (22.1) |
| Aortocoronary bypass, n (%) | 30 (13.2) | 22 (15.2) |
| Stroke, n (%) | 12 (5.3) ** | 9 (6.2) |
| Transitory ischemic attack, n (%) | 3 (1.3) | 1 (0.7) |
| Comorbidities—no. of participants | (n = 228) | (n = 145) |
| Heart failure, n (%) | 52 (22.8) | 38 (26.2) |
| Heart valve disease, n (%) | 37 (15.9) | 25 (17.2) |
| Renal insufficiency, n (%) | 24 (10.5) | 17 (11.7) |
| Stable angina pectoris, n (%) | 22 (9.6) | 18 (12.4) |
| Peripheral arterial occlusive disease, n (%) | 17 (7.5) | 11 (7.6) |
| Atrial fibrillation, n (%) | 12 (6.6) | 9 (6.2) |
| Carcinoma, n (%) | 11 (4.8) | 6 (4.1) |
| BAS Baseline | FAS Baseline | FAS 12-Month FU | p-Value | |
|---|---|---|---|---|
| (n = 233) | (n = 145) | (n = 145) | FAS 12 vs. FAS | |
| Fibrates, n (%) | 5 (2.2) | 2 (1.4) | 1 (0.7) | 0.3173 |
| Ezetimibe, n (%) | 74 (31.8) | 55 (37.9) | 51 (35.2) | 0.0153 |
| PCSK9 antibody, n (%) | 43 (18.5) | 34 (23.4) | 36 (24.8) | 0.5637 |
| Statin, n (%) | 191 (82.0) | 125 (86.2) | 117 (80.7) | <0.0001 |
| Other lipid-lowering therapy, n (%) | 22 (9.4) | 14 (9.7) | 14 (9.7) | 0.0679 |
| None, n (%) | 26 (11.2) | 11 (7.6) | 6 (4.1) | 1.000 |
| Physical exercise * | (n = 233) | (n = 145) | (n = 145) | <0.0001 |
| None, n (%) | 99 (42.5) | 57 (39.3) | 53 (36.6) | |
| 1× per week, n (%) | 70 (30.0) | 55 (37.9) | 47 (32.4) | |
| 2–3× per week, n (%) | 35 (15.0) | 23 (15.9) | 34 (23.5) | |
| >3× per week, n (%) | 23 (9.9) | 10 (6.9) | 10 (6.9) | |
| Fruit and vegetable consumption * | (n = 233) | (n = 145) | (n = 145) | 0.3991 |
| ≥3× per week, n (%) | 146 (62.7) | 91 (62.8) | 86 (59.3) | |
| <3× per week, n (%) | 75 (32.2) | 50 (34.5) | 52 (35.9) | |
| None, n (%) | 6 (2.6) | 4 (2.8) | 6 (4.2) | |
| Fish consumption * | (n = 233) | (n = 145) | (n = 145) | 0.3092 |
| ≥2× per week, n (%) | 49 (21.0) | 29 (20.0) | 30 (20.7) | |
| <2× per week, n (%) | 149 (64.0) | 99 (68.3) | 90 (62.1) | |
| None, n (%) | 29 (12.5) | 17 (11.7) | 25 (17.2) | |
| Alcohol consumption (number of alcoholic beverages) * | (n = 233) | (n = 145) | (n = 145) | 0.0779 |
| ≥2× per day, n (%) | 24 (10.3) | 15 (10.3) | 15 (10.3) | |
| <2× per day, n (%) | 103 (44.2) | 65 (44.8) | 70 (48.3) | |
| None, n (%) | 101 (43.4) | 65 (44.8) | 59 (40.7) | |
| Smoking status * | (n = 233) | (n = 145) | (n = 145) | 0.0609 |
| Non-smoker, n (%) | 153 (65.7) | 98 (67.6) | 107 (73.8) | |
| Current smoker, n (%) | 23 (9.9) | 13 (9.0) | 11 (7.6) | |
| Ex-smoker, n (%) | 51 (21.9) | 34 (23.5) | 27 (18.6) ** |
| BAS Baseline (n = 233) | FAS Baseline (n = 145) | FAS 12-Month FU (n = 145) | |
|---|---|---|---|
| P2Y12 antagonist, n (%) | 18 (7.7) | 13 (9.0) | 7 (4.8) |
| Other platelet aggregation inhibitors, n (%) | 94 (40.3) | 62 (42.8) | 72 (49.7) |
| Vitamin-K antagonist, n (%) | 6 (2.6) | 6 (4.2) | 3 (2.1) |
| Direct oral anticoagulant, n (%) | 7 (3.0) | 5 (3.5) | 5 (3.5) |
| Beta blocker, n (%) | 65 (27.9) | 37 (25.5) | 53 (36.6) |
| Angiotensin II receptor blocker, n (%) | 58 (24.9) | 46 (31.7) | 47 (32.4) |
| ACE inhibitor, n (%) | 84 (36.1) | 47 (32.4) | 46 (31.7) |
| Diuretic, n (%) | 47 (20.2) | 29 (20.0) | 24 (16.6) |
| If channel inhibitor, n (%) | 12 (5.2) | 9 (6.2) | 3 (2.1) |
| Calcium channel blocker, n (%) | 52 (22.3) | 38 (26.2) | 44 (30.3) |
| Oral antidiabetic, n (%) | 55 (23.6) | 37 (25.5) | 30 (20.7) |
| GLP-1 receptor agonist, n (%) | 12 (5.2) | 11 (7.6) | 10 (6.9) |
| Insulin, n (%) | 32 (13.7) | 17 (11.7) | 15 (10.3) |
| Anti-angina drug, n (%) | 6 (2.6) | 5 (3.5) | 4 (2.8) |
| BAS (n = 233) | FAS (n = 145) | |||||
|---|---|---|---|---|---|---|
| Baseline Median (IQR) | Baseline Median (IQR) | 12 Months Median (IQR) | p-Value 12 Months vs. BL | Abs. Δ BL—12 Months | Rel. Δ BL—12 Months | |
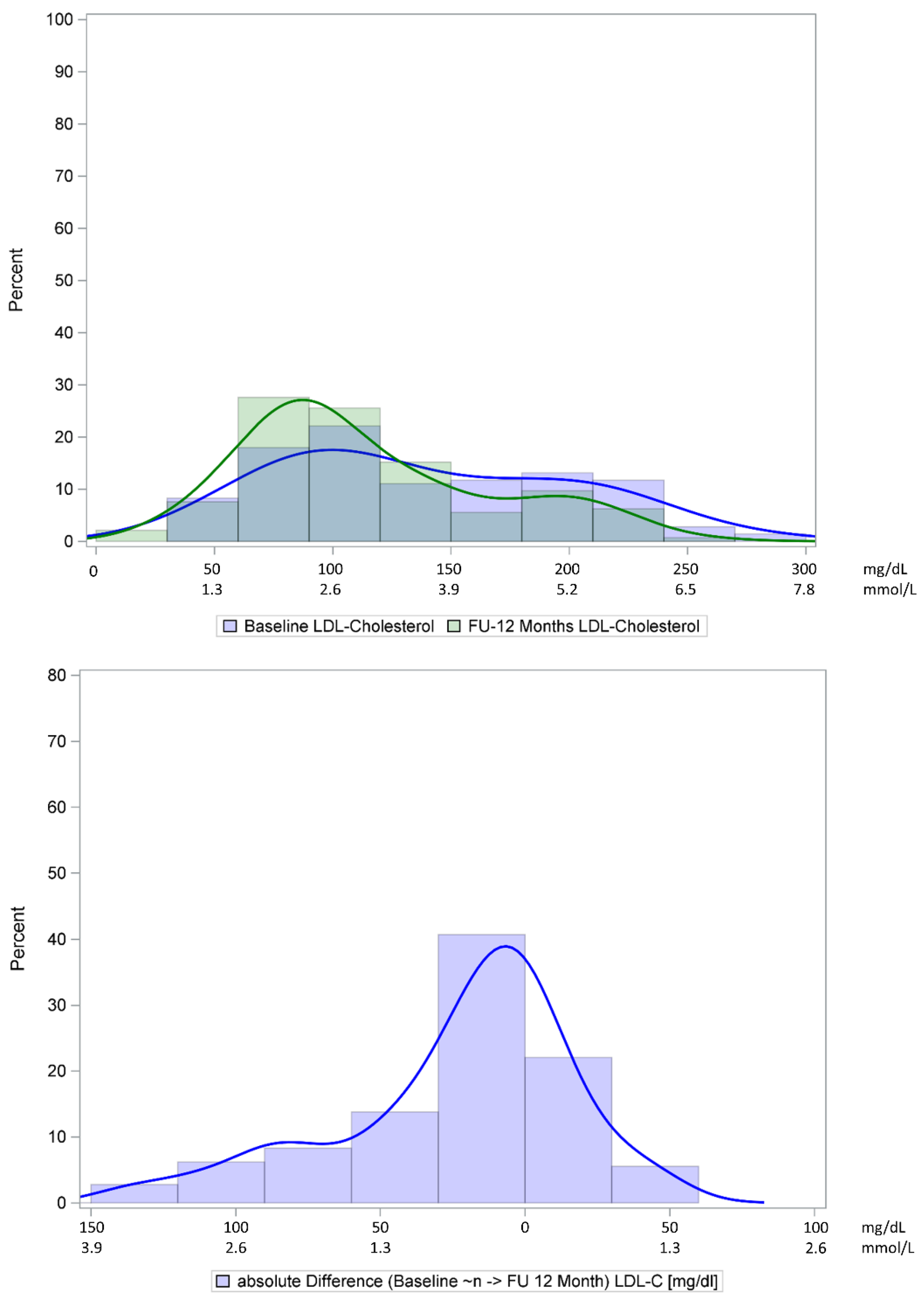
| LDL-C, mg/dL | 134 (92, 194) | 128 (89, 194) | 99 (78, 144) | <0.0001 | −14 (−43, 1) | −10 (−33, 2) |
| mmol/L | 3.5 (2.4, 5.0) | 3.3 (2.3, 5.0) | 2.6 (2.0, 3.7) | |||
| >150 mg/dL, n (%) | 94/219 (72.9) | 59 (40.7) | 32/145 (22.1) | <0.0001 | −18.6 | - |
| <70 mg/dL, n (%) | 24/219 (11.0) | 15/145 (10.3) | 25/145 (17.2) | <0.0001 | +6.9 | - |
| <70 mg/dL OR 50% red. | - | - | 30/145 (20.7) | |||
| Total cholesterol, mg/dL | 211 (169, 267) | 204 (167, 267) | 188 (144, 216) | <0.0001 | −15 (−48, −1) | −6 (−23, −0) |
| mmol/L | 5.5 (4.4, 6.9) | 5.3 (4.3, 6.9) | 4.9 (3.7, 5.6) | |||
| >260 mg/dL, n (%) | 65/219 (29.7) | 42/143 (29.4) | 26/141 (18.4) | <0.0001 | ||
| HDL-C, mg/dL | 48 (40, 59) | 47 (40, 59) | 46 (41, 55) | 0.3266 | 0 (−4, 2) | 0 (−8, 5) |
| mmol/L | 1.2 (1.0, 1.5) | 1.2 (1.0, 1.5) | 1.2 (1.1, 1.4) | |||
| m < 45/f < 55 mg/dL, n (%) | 112/212 (52.8) | 77/140 (55.0) | 79/141 (56.0) | 0.3778 | ||
| Triglycerides, mg/dL | 160 (114, 214) | 165 (115, 211) | 155 (101, 206) | 0.0027 | −8 (−31, 20) | −4 (−21, 19) |
| mmol/L | 1.8 (1.3, 2.4) | 1.9 (1.3, 2.4) | 1.8 (1.1, 2.3) | |||
| >172 mg/dL, n (%) | 98/218 (45.0) | 68/144 (47.2) | 58/133 (43.6) | 0.0124 | ||
| FAS at 12 Months (n = 145) | |
|---|---|
| Myocardial infarction, n (%) [95% CI] | 2 (1.4) [0.4–4.9] |
| Cardiac catheter without PCI, n (%) | 0 (0.0) |
| Percutaneous coronary intervention, n (%) [95% CI] | 3 (2.1) [0.7–5.9] |
| Bypass surgery, n (%) | 0 (0.0) |
| Stroke, n (%) | 0 (0.0) |
| Transitory ischemic attack, n (%) | 0 (0.0) |
| Hospitalization due to event, n (%) [95% CI] | 4 (2.8) [1.1–6.9] |
| Duration of hospitalization due to event, days | |
| mean ± SD | 4.5 ± 3.7 |
| median (IQR) | 3 (3, 7) |
| Rehabilitation, n (%) | 0 (0.0) |
| Other inpatient stay, n (%) [95% CI] | 3 (2.1) [0.7–5.9] |
| Duration of other inpatient stay, days | |
| mean ± SD | 9.3 ± 8.1 |
| median (IQR) | 8 (2, 18) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gitt, A.K.; Laufs, U.; März, W.; Paar, W.D.; Bramlage, P.; Marx, N.; Parhofer, K.G. Hypercholesterolemia Diagnosis, Treatment Patterns, and 12-Month Target Achievement in Clinical Practice in Germany in Patients with Familial Hypercholesterolemia. J. Clin. Med. 2022, 11, 3810. https://doi.org/10.3390/jcm11133810
Gitt AK, Laufs U, März W, Paar WD, Bramlage P, Marx N, Parhofer KG. Hypercholesterolemia Diagnosis, Treatment Patterns, and 12-Month Target Achievement in Clinical Practice in Germany in Patients with Familial Hypercholesterolemia. Journal of Clinical Medicine. 2022; 11(13):3810. https://doi.org/10.3390/jcm11133810
Chicago/Turabian StyleGitt, Anselm K., Ulrich Laufs, Winfried März, W. Dieter Paar, Peter Bramlage, Nikolaus Marx, and Klaus G. Parhofer. 2022. "Hypercholesterolemia Diagnosis, Treatment Patterns, and 12-Month Target Achievement in Clinical Practice in Germany in Patients with Familial Hypercholesterolemia" Journal of Clinical Medicine 11, no. 13: 3810. https://doi.org/10.3390/jcm11133810
APA StyleGitt, A. K., Laufs, U., März, W., Paar, W. D., Bramlage, P., Marx, N., & Parhofer, K. G. (2022). Hypercholesterolemia Diagnosis, Treatment Patterns, and 12-Month Target Achievement in Clinical Practice in Germany in Patients with Familial Hypercholesterolemia. Journal of Clinical Medicine, 11(13), 3810. https://doi.org/10.3390/jcm11133810






