Using TBAg/PHA Ratio for Monitoring TB Treatment: A Prospective Multicenter Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Clinical and Follow-Up Procedures
2.3. Assessment of TB Treatment Outcomes
2.4. Serial T-SPOT Assay and Calculation of TBAg/PHA Ratio
2.5. Statistical Analysis
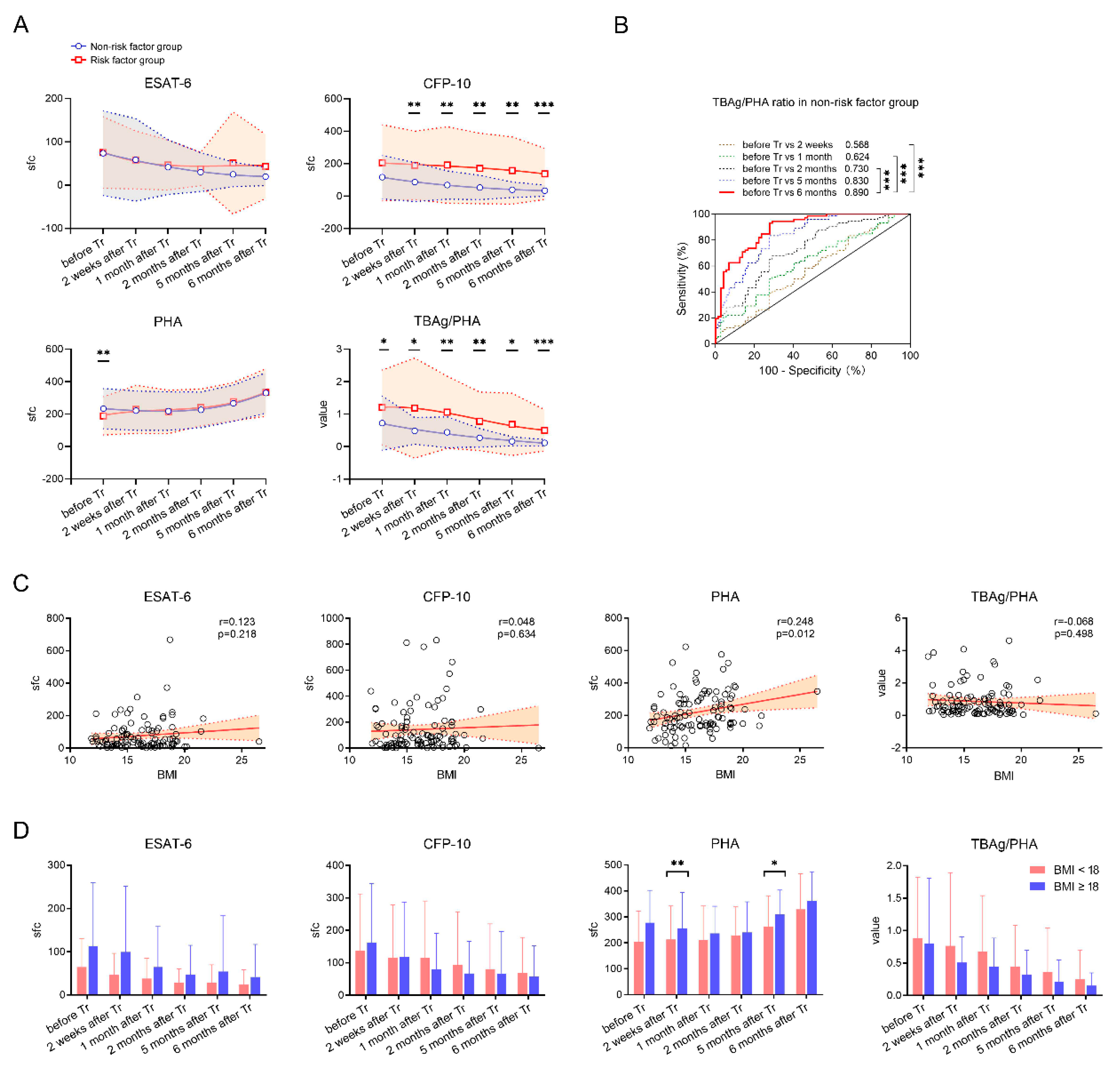
3. Results
3.1. Patients’ Characteristics
3.2. T-SPOT Results in Patients with Successful Treatment Outcomes
3.3. Classification Groups in Patients with Successful Treatment Outcomes
3.4. T-SPOT Results in Patients with Unsuccessful Treatment Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation (WHO). Global Tuberculosis Report 2020; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Bocchino, M.; Chairadonna, P.; Matarese, A.; Bruzzese, D.; Salvatores, M.; Tronci, M.; Moscariello, E.; Galati, D.; Alma, M.G.; Sanduzzi, A.; et al. Limited usefulness of QuantiFERON-TB Gold In-Tube for monitoring anti-tuberculosis therapy. Respir. Med. 2010, 104, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Ndzi, E.N.; Nkenfou, C.N.; Mekue, L.M.; Zentilin, L.; Tamgue, O.; Pefura, E.W.Y.; Kuiate, J.R.; Giacca, M.; Ndjolo, A. MicroRNA hsa-miR-29a-3p is a plasma biomarker for the differential diagnosis and monitoring of tuberculosis. Tuberculosis 2019, 114, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Satproedprai, N.; Wichukchinda, N.; Suphankong, S.; Inunchot, W.; Kuntima, T.; Kumpeerasart, S.; Wattanapokayakit, S.; Nedsuwan, S.; Yanai, H.; Higuchi, K.; et al. Diagnostic value of blood gene expression signatures in active tuberculosis in Thais: A pilot study. Genes Immun. 2015, 16, 253–260. [Google Scholar] [CrossRef]
- Wang, F.; Hou, H.Y.; Wu, S.J.; Zhu, Q.; Huang, M.; Yin, B.; Huang, J.; Pan, Y.Y.; Mao, L.; Sun, Z.Y. Using the TBAg/PHA ratio in the T-SPOT((R)).TB assay to distinguish TB disease from LTBI in an endemic area. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Tuberc. Lung Dis. 2016, 20, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, J.; Zhou, Y.; Luo, Y.; Wu, S.; Huang, M.; Yin, B.; Huang, J.; Mao, L.; Sun, Z. The Use of TB-Specific Antigen/Phytohemagglutinin Ratio for Diagnosis and Treatment Monitoring of Extrapulmonary Tuberculosis. Front. Immunol. 2018, 9, 1047. [Google Scholar] [CrossRef]
- Zhou, Y.; Du, J.; Hou, H.Y.; Lu, Y.F.; Yu, J.; Mao, L.Y.; Wang, F.; Sun, Z.Y. Application of ImmunoScore Model for the Differentiation between Active Tuberculosis and Latent Tuberculosis Infection as Well as Monitoring Anti-tuberculosis Therapy. Front. Cell. Infect. Microbiol. 2017, 7, 457. [Google Scholar] [CrossRef]
- Katakura, S.; Kobayashi, N.; Hashimoto, H.; Kamimaki, C.; Tanaka, K.; Kubo, S.; Nakashima, K.; Teranishi, S.; Watanabe, K.; Hara, Y.; et al. Identification of a novel biomarker based on lymphocyte count, albumin level, and TBAg/PHA ratio for differentiation between active and latent tuberculosis infection in Japan. Tuberculosis 2020, 125, 101992. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, L.; Wang, F.; Sun, Z.; Tan, Y.; Sha, W. The TBAg/PHA ratio in T-SPOT.TB assay has high prospective value in the diagnosis of active tuberculosis: A multicenter study in China. Respir. Res. 2021, 22, 165. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Treatment of Tuberculosis: Guidelines, 4th ed.; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Van’t Boveneind-Vrubleuskaya, N.; Daskapan, A.; Kosterink, J.G.; van der Werf, T.S.; van den Hof, S.; Alffenaar, J.C. Predictors of Prolonged TB Treatment in a Dutch Outpatient Setting. PLoS ONE 2016, 11, e0166030. [Google Scholar] [CrossRef]
- Shin, H.J.; Kwon, Y.S. Treatment of Drug Susceptible Pulmonary Tuberculosis. Tuberc. Respir. Dis. 2015, 78, 161–167. [Google Scholar] [CrossRef]
- Lanoix, J.P.; Guimard, T.; Ettahar, N.; Grannec, A.; Flateau, C.; Chapuzet, C.; Bentayeb, H.; Tattevin, P.; Schmit, J.L. Risk factors for prolonged treatment of lymph node tuberculosis. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Tuberc. Lung Dis. 2012, 16, 373–375. [Google Scholar] [CrossRef]
- Adekambi, T.; Ibegbu, C.C.; Cagle, S.; Kalokhe, A.S.; Wang, Y.F.; Hu, Y.; Day, C.L.; Ray, S.M.; Rengarajan, J. Biomarkers on patient T cells diagnose active tuberculosis and monitor treatment response. J. Clin. Investig. 2015, 125, 3723. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.I.M.; Ntinginya, N.E.; Kibiki, G.; Mtafya, B.A.; Semvua, H.; Mpagama, S.; Mtabho, C.; Saathoff, E.; Held, K.; Loose, R.; et al. Phenotypic Changes on Mycobacterium Tuberculosis-Specific CD4 T Cells as Surrogate Markers for Tuberculosis Treatment Efficacy. Front. Immunol. 2018, 9, 2247. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.I.M.; Ziegler, C.; Held, K.; Dubinski, I.; Ley-Zaporozhan, J.; Geldmacher, C.; von Both, U. The TAM-TB Assay-A Promising TB Immune-Diagnostic Test with a Potential for Treatment Monitoring. Front. Pediatrics 2019, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.J.; Li, H.M.; Yang, Y.R.; Zhang, J.X.; Liang, Y.; Wu, X.Q. Cytokine and soluble adhesion molecule profiles and biomarkers for treatment monitoring in Re-treated smear-positive patients with pulmonary tuberculosis. Cytokine 2018, 108, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Hur, Y.G.; Kang, Y.A.; Jang, S.H.; Hong, J.Y.; Kim, A.; Lee, S.A.; Kim, Y.; Cho, S.N. Adjunctive biomarkers for improving diagnosis of tuberculosis and monitoring therapeutic effects. J. Infect. 2015, 70, 346–355. [Google Scholar] [CrossRef]
- Kassa, D.; de Jager, W.; Gebremichael, G.; Alemayehu, Y.; Ran, L.; Fransen, J.; Wolday, D.; Messele, T.; Tegbaru, B.; Ottenhoff, T.H.; et al. The effect of HIV coinfection, HAART and TB treatment on cytokine/chemokine responses to Mycobacterium tuberculosis (Mtb) antigens in active TB patients and latently Mtb infected individuals. Tuberculosis 2016, 96, 131–140. [Google Scholar] [CrossRef]
- Mihret, A.; Bekele, Y.; Bobosha, K.; Kidd, M.; Aseffa, A.; Howe, R.; Walzl, G. Plasma cytokines and chemokines differentiate between active disease and non-active tuberculosis infection. J. Infect. 2013, 66, 357–365. [Google Scholar] [CrossRef]
- Feruglio, S.L.; Troseid, M.; Damas, J.K.; Kvale, D.; Dyrhol-Riise, A.M. Soluble markers of the Toll-like receptor 4 pathway differentiate between active and latent tuberculosis and are associated with treatment responses. PLoS ONE 2013, 8, e69896. [Google Scholar] [CrossRef]
- Wergeland, I.; Pullar, N.; Assmus, J.; Ueland, T.; Tonby, K.; Feruglio, S.; Kvale, D.; Damas, J.K.; Aukrust, P.; Mollnes, T.E.; et al. IP-10 differentiates between active and latent tuberculosis irrespective of HIV status and declines during therapy. J. Infect. 2015, 70, 381–391. [Google Scholar] [CrossRef]
- Jiang, H.; Gong, H.; Zhang, Q.; Gu, J.; Liang, L.; Zhang, J. Decreased expression of perforin in CD8(+) T lymphocytes in patients with Mycobacterium tuberculosis infection and its potential value as a marker for efficacy of treatment. J. Thorac. Dis. 2017, 9, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Brodie, D.; Lederer, D.J.; Gallardo, J.S.; Trivedi, S.H.; Burzynski, J.N.; Schluger, N.W. Use of an interferon-gamma release assay to diagnose latent tuberculosis infection in foreign-born patients. Chest 2008, 133, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Campainha, S.; Gomes, T.; Carvalho, A.; Duarte, R. Negative predictive value of TST and IGRA in anti-TNF treated patients. Eur. Respir. J. 2012, 40, 790–791. [Google Scholar] [CrossRef] [PubMed]
- Sollai, S.; Galli, L.; de Martino, M.; Chiappini, E. Systematic review and meta-analysis on the utility of Interferon-gamma release assays for the diagnosis of Mycobacterium tuberculosis infection in children: A 2013 update. BMC Infect. Dis. 2014, 14 Suppl 1, S6. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, E.; Bonsignori, F.; Mangone, G.; Galli, L.; Mazzantini, R.; Sollai, S.; Azzari, C.; de Martino, M. Serial T-SPOT.TB and quantiFERON-TB-Gold In-Tube assays to monitor response to antitubercular treatment in Italian children with active or latent tuberculosis infection. Pediatric Infect. Dis. J. 2012, 31, 974–977. [Google Scholar] [CrossRef]
- Chiappini, E.; Fossi, F.; Bonsignori, F.; Sollai, S.; Galli, L.; de Martino, M. Utility of interferon-gamma release assay results to monitor anti-tubercular treatment in adults and children. Clin. Ther. 2012, 34, 1041–1048. [Google Scholar] [CrossRef]
- Bosshard, V.; Roux-Lombard, P.; Perneger, T.; Metzger, M.; Vivien, R.; Rochat, T.; Janssens, J.P. Do results of the T-SPOT.TB interferon-gamma release assay change after treatment of tuberculosis? Respir. Med. 2009, 103, 30–34. [Google Scholar] [CrossRef]
- Chee, C.B.; KhinMar, K.W.; Gan, S.H.; Barkham, T.M.; Koh, C.K.; Shen, L.; Wang, Y.T. Tuberculosis treatment effect on T-cell interferon-gamma responses to Mycobacterium tuberculosis-specific antigens. Eur. Respir. J. 2010, 36, 355–361. [Google Scholar] [CrossRef]
- Clifford, V.; He, Y.; Zufferey, C.; Connell, T.; Curtis, N. Interferon gamma release assays for monitoring the response to treatment for tuberculosis: A systematic review. Tuberculosis 2015, 95, 639–650. [Google Scholar] [CrossRef]
- Park, I.N.; Shim, T.S. Qualitative and quantitative results of interferon-gamma release assays for monitoring the response to anti-tuberculosis treatment. Korean J. Intern. Med. 2017, 32, 302–308. [Google Scholar] [CrossRef][Green Version]
- Bosco, M.J.; Hou, H.; Mao, L.; Wu, X.; Ramroop, K.D.; Lu, Y.; Mao, L.; Zhou, Y.; Sun, Z.; Wang, F. The performance of the TBAg/PHA ratio in the diagnosis of active TB disease in immunocompromised patients. Intern. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2017, 59, 55–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hadaya, K.; Bridevaux, P.O.; Roux-Lombard, P.; Delort, A.; Saudan, P.; Martin, P.Y.; Janssens, J.P. Contribution of interferon-gamma release assays (IGRAs) to the diagnosis of latent tuberculosis infection after renal transplantation. Transplantation 2013, 95, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Jia, H.; Liu, F.; Sun, H.; Gao, M.; Du, F.; Xing, A.; Du, B.; Sun, Q.; Wei, R.; et al. Risk factors for false-negative T-SPOT.TB assay results in patients with pulmonary and extra-pulmonary TB. J. Infect. 2015, 70, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Al-Attiyah, R.; Mustafa, A.S.; Abal, A.T.; Madi, N.M.; Andersen, P. Restoration of mycobacterial antigen-induced proliferation and interferon-gamma responses in peripheral blood mononuclear cells of tuberculosis patients upon effective chemotherapy. FEMS Immunol. Med. Microbiol. 2003, 38, 249–256. [Google Scholar] [CrossRef]
- Luo, X.; Wu, F.; Ma, J.; Xiao, H.; Cui, H. Immunological recovery in patients with pulmonary tuberculosis after intensive phase treatment. J. Int. Med. Res. 2018, 46, 3539–3551. [Google Scholar] [CrossRef]
- Wang, F.; Hou, H.; Wu, S.; Tang, Q.; Huang, M.; Yin, B.; Huang, J.; Liu, W.; Mao, L.; Lu, Y.; et al. Tim-3 pathway affects NK cell impairment in patients with active tuberculosis. Cytokine 2015, 76, 270–279. [Google Scholar] [CrossRef]
- Potter, M.R.; Moore, M. PHA stimulation of separated human lymphocyte populations. Clin. Exp. Immunol. 1975, 21, 456–467. [Google Scholar]



| Characteristic | Successful Outcome (n = 102) | Unsuccessful Outcome (n = 9) | p Value | |
|---|---|---|---|---|
| Mean age (mean ± SD), years | 39.22 ± 15.28 | 44.89 ± 20.20 | 0.401 | |
| Male sex | 70 (68.62) | 6 (66.67) | 0.903 | |
| Immunosuppressive conditions | ||||
| HIV infection | 2 (1.96) | 0 | ||
| malignancy | 4 (3.92) | 0 | ||
| autoimmune disease receiving treatment | 3 (2.94) | 0 | ||
| transplantation receiving treatment | 1 (0.98) | 0 | ||
| diabetes | 5 (4.90) | 1 (11.11) | 0.405 | |
| chronic renal failure | 4 (3.92) | 0 | ||
| BMI | 16.14 ± 2.507 | 16.42 ± 1.578 | 0.642 | |
| Blood pressure | ||||
| systolic pressure | 113.4 ± 15.65 | 108.1 ± 8.100 | 0.311 | |
| diastolic pressure | 71.79 ± 9.669 | 69.22 ± 7.823 | 0.531 | |
| WBC count (mean ± SD), ×109/L | 7.2 ± 2.008 | 7.034 ± 2.202 | 0.682 | |
| Lymphocyte count, ×109/L | 1.672 ± 0.569 | 1.611 ± 0.405 | 0.823 | |
| Optimal AUC (95% CI) | Cutoff Value | Sensitivity % (95% CI) | Specificity % (95% CI) | |
|---|---|---|---|---|
| ESAT-6 | 0.753 (0.687–0.819) | 21.5 | 65.69 (55.63–74.81) | 72.55 (62.82–80.92) |
| CFP-10 | 0.702 (0.630–0.774) | 25.5 | 51.96 (41.84–61.96) | 83.33 (74.66–89.98) |
| PHA | 0.753 (0.687–0.820) | 195.5 | 89.22 (81.52–94.49) | 51.96 (41.84–61.96) |
| TBAg/PHA ratio | 0.839 (0.785–0.894) | 0.193 | 76.47 (67.04–84.31) | 81.37 (72.45–88.40) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, M.; Liu, G.; Wu, X.; Wan, R.; Hou, H.; Wu, S.; Sun, Z.; Kuang, H.; Wang, F. Using TBAg/PHA Ratio for Monitoring TB Treatment: A Prospective Multicenter Study. J. Clin. Med. 2022, 11, 3780. https://doi.org/10.3390/jcm11133780
Wang X, Li M, Liu G, Wu X, Wan R, Hou H, Wu S, Sun Z, Kuang H, Wang F. Using TBAg/PHA Ratio for Monitoring TB Treatment: A Prospective Multicenter Study. Journal of Clinical Medicine. 2022; 11(13):3780. https://doi.org/10.3390/jcm11133780
Chicago/Turabian StyleWang, Xiaochen, Mingwu Li, Guobiao Liu, Xiaoying Wu, Rong Wan, Hongyan Hou, Shiji Wu, Ziyong Sun, Haobin Kuang, and Feng Wang. 2022. "Using TBAg/PHA Ratio for Monitoring TB Treatment: A Prospective Multicenter Study" Journal of Clinical Medicine 11, no. 13: 3780. https://doi.org/10.3390/jcm11133780
APA StyleWang, X., Li, M., Liu, G., Wu, X., Wan, R., Hou, H., Wu, S., Sun, Z., Kuang, H., & Wang, F. (2022). Using TBAg/PHA Ratio for Monitoring TB Treatment: A Prospective Multicenter Study. Journal of Clinical Medicine, 11(13), 3780. https://doi.org/10.3390/jcm11133780






