Collapsing Glomerulonephritis in a Kidney Transplant Recipient after mRNA SARS-CoV-2 Vaccination
Abstract
:1. Introduction
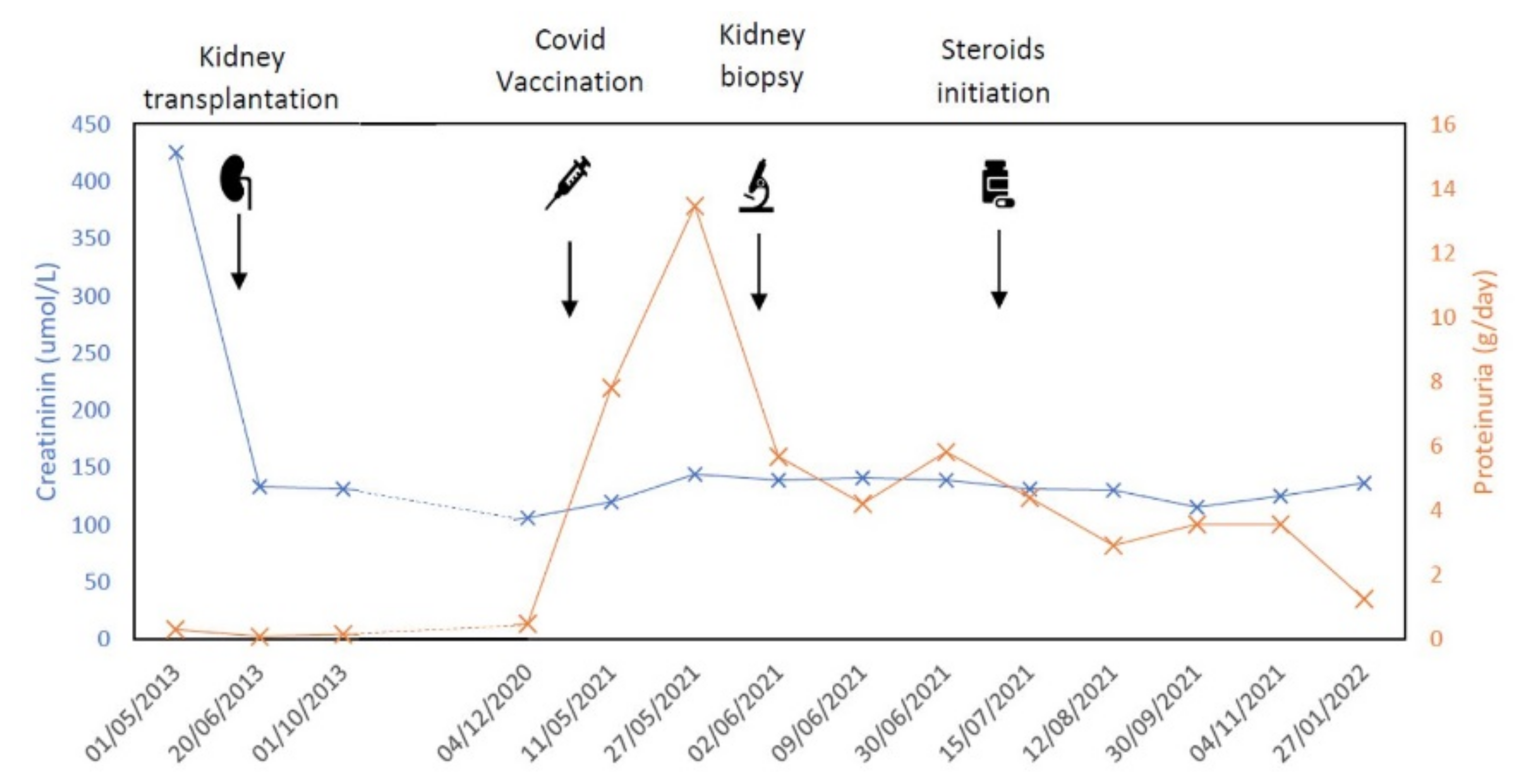
2. Case Report
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- May, R.M.; Cassol, C.; Hannoudi, A.; Larsen, C.P.; Lerma, E.V.; Haun, R.S.; Braga, J.R.; Hassen, S.I.; Wilson, J.; VanBeek, C.; et al. A multi-center retrospective cohort study defines the spectrum of kidney pathology in Coronavirus 2019 Disease (COVID-19). Kidney Int. 2021, 100, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.A.; Tawhari, I.; Safar-Boueri, L.; Seif, N.; Alahmadi, A.; Gargiulo, R.; Aggarwal, V.; Usman, I.; Kisselev, S.; Gharavi, A.G.; et al. COVID-19–Associated Glomerular Disease. J. Am. Soc. Nephrol. 2020, 32, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Jeyalan, V.; Storrar, J.; Wu, H.H.L.; Ponnusamy, A.; Sinha, S.; Kalra, P.A.; Chinnadurai, R. Native and transplant kidney histopathological manifestations in association with COVID-19 infection: A systematic review. World J. Transplant. 2021, 11, 480–502. [Google Scholar] [CrossRef] [PubMed]
- Caza, T.N.; Cassol, C.A.; Messias, N.; Hannoudi, A.; Haun, R.S.; Walker, P.D.; May, R.M.; Seipp, R.M.; Betchick, E.J.; Amin, H.; et al. Glomerular Disease in Temporal Association with SARS-CoV-2 Vaccination: A Series of 29 Cases. Kidney360 2021, 2, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Neves, P.D.; Caires, R.A.; Guimarães, M.P.; Costalonga, E.C.; Cavalcante, L.B.; e Silva, V.T.C.; Mattedi, F.Z.; Santana, L.F.; Teixeira-Júnior, A.A.; Gomes, O.V.; et al. Collapsing glomerulopathy following SARS-CoV-2 adenovirus-vector–based vaccine: Report of 2 cases. Kidney Int. 2022, 101, 637–639. [Google Scholar] [CrossRef]
- Jefferis, J.; Kassianos, A.J.; Grivei, A.; Doucet, B.; Healy, H.; Francis, L.; Mon, S.Y.; John, G.T. SARS-CoV-2 vaccination–associated collapsing glomerulopathy in a kidney transplant recipient. Kidney Int. 2022, 101, 635–636. [Google Scholar] [CrossRef] [PubMed]
- Favà, A.; Donadeu, L.; Sabé, N.; Pernin, V.; González-Costello, J.; Lladó, L.; Meneghini, M.; Charmetant, X.; García-Romero, E.; Cachero, A.; et al. SARS-CoV-2-Specific serological and functional T cell immune responses during acute and early COVID-19 convalescence in solid organ transplant patients. Am. J. Transplant. 2021, 21, 2749–2761. [Google Scholar] [CrossRef]
- De Vriese, A.S.; Sethi, S.; Nath, K.A.; Glassock, R.J.; Fervenza, F.C. Differentiating Primary, Genetic, and Secondary FSGS in Adults: A Clinicopathologic Approach. J. Am. Soc. Nephrol. 2018, 29, 759–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Agati, V.D.; Kaskel, F.J.; Falk, R.J. Focal Segmental Glomerulosclerosis. N. Engl. J. Med. 2011, 365, 2398–2411. [Google Scholar] [CrossRef] [Green Version]
- Korbet, S.M. Treatment of Primary FSGS in Adults. J. Am. Soc. Nephrol. 2012, 23, 1769–1776. [Google Scholar] [CrossRef] [Green Version]
- Bruggeman, L.A.; Dikman, S.; Meng, C.; Quaggin, S.E.; Coffman, T.M.; Klotman, P.E. Nephropathy in human immunodeficiency virus-1 transgenic mice is due to renal transgene expression. J. Clin. Investig. 1997, 100, 84–92. [Google Scholar] [CrossRef]
- Genovese, G.; Friedman, D.J.; Ross, M.D.; Lecordier, L.; Uzureau, P.; Freedman, B.I.; Bowden, D.W.; Langefeld, C.D.; Oleksyk, T.K.; Knob, A.L.U.; et al. Association of Trypanolytic ApoL1 Variants with Kidney Disease in African Americans. Science 2010, 329, 841–845. [Google Scholar] [CrossRef] [Green Version]
- Nichols, B.; Jog, P.; Lee, J.H.; Blackler, D.; Wilmot, M.; D’Agati, V.; Markowitz, G.; Kopp, J.B.; Alper, S.L.; Pollak, M.R.; et al. Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int. 2015, 87, 332–342. [Google Scholar] [CrossRef] [Green Version]
- Chandra, P.; Kopp, J.B. Viruses and collapsing glomerulopathy: A brief critical review: Table 1. Clin. Kidney J. 2012, 6, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Daniel, E.; Sekulic, M.; Kudose, S.; Kubin, C.; Ye, X.; Shayan, K.; Patel, A.; Cohen, D.J.; Ratner, L.E.; Santoriello, D.; et al. Kidney allograft biopsy findings after COVID-19. Am. J. Transplant. 2021, 21, 4032–4042. [Google Scholar] [CrossRef]
- Hassler, L.; Reyes, F.; Sparks, M.A.; Welling, P.; Batlle, D. Evidence for and against Direct Kidney Infection by SARS-CoV-2 in Patients with COVID-19. Clin. J. Am. Soc. Nephrol. 2021, 16, 1755–1765. [Google Scholar] [CrossRef] [PubMed]
- Kapp, M.E.; Fogo, A.B.; Roufouse, C.; Najafian, B.; Radhakrishnan, J.; Mohan, S.; Miller, S.E.; D’Agati, V.D.; Silberzweig, J.I.; Barbar, T.; et al. Renal Considerations in COVID-19. ASAIO J. 2021, 67, 1087. [Google Scholar] [CrossRef]
- Kudose, S.; Santoriello, D.; Bomback, A.S.; Sekulic, M.; Batal, I.; Stokes, M.B.; Ghavami, I.A.; Kim, J.S.; Marasa, M.; Xu, K.; et al. Longitudinal Outcomes of COVID-19–Associated Collapsing Glomerulopathy and Other Podocytopathies. J. Am. Soc. Nephrol. 2021, 32, 2958–2969. [Google Scholar] [CrossRef]
- Luque, S.; Lúcia, M.; Crespo, E.; Jarque, M.; Grinyó, J.M.; Bestard, O. A multicolour HLA-specific B-cell FluoroSpot assay to functionally track circulating HLA-specific memory B cells. J. Immunol. Methods 2018, 462, 23–33. [Google Scholar] [CrossRef]
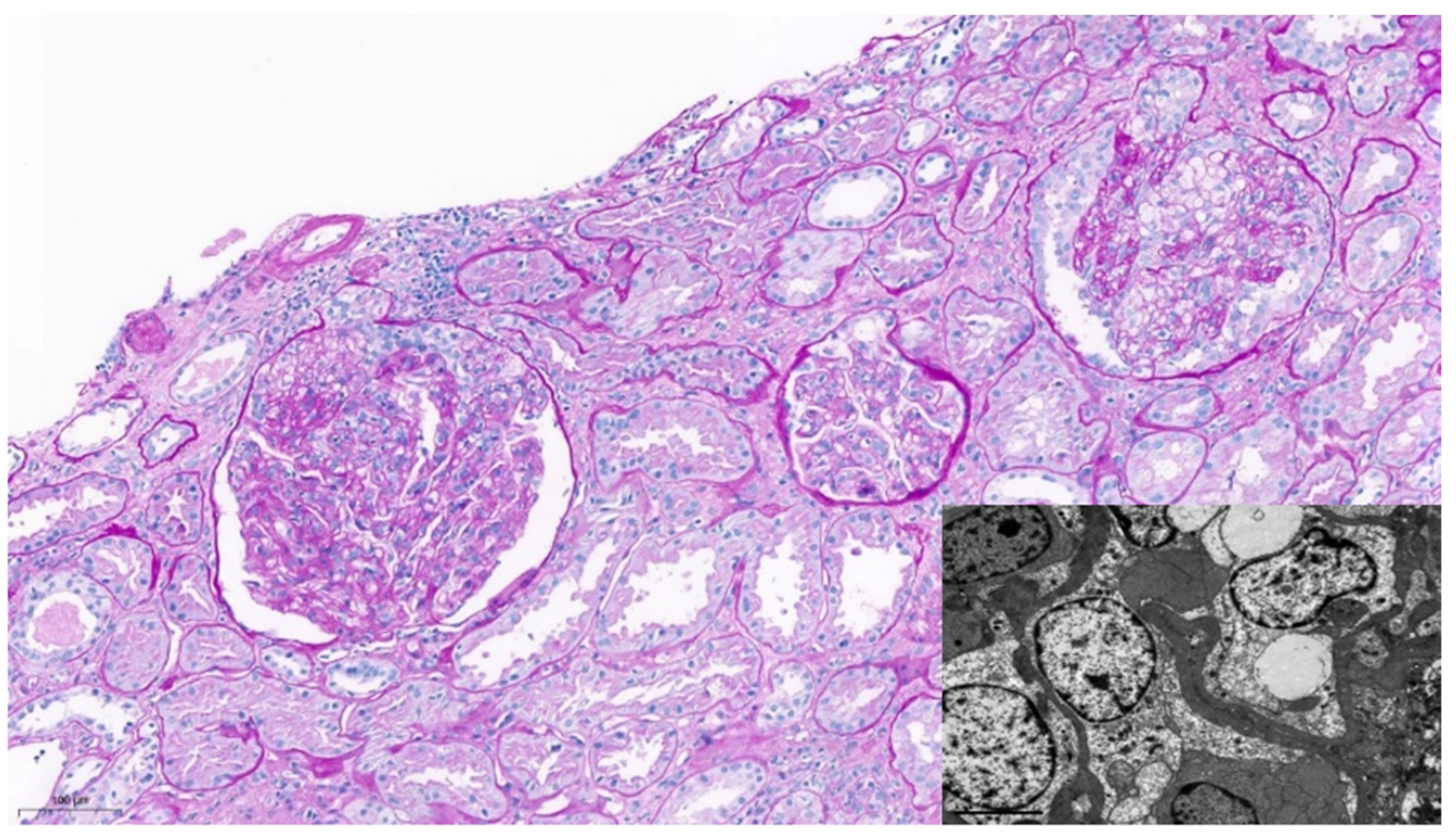
| Case | Ref. | Age | Sex | Ethnicity | APOL1 Risk Alleles | Kidney Transplant Recipient | Comorbidities |
|---|---|---|---|---|---|---|---|
| 1 | Caza et al. [4] | 67 | F | Black | G1/G1 | No | N/S |
| 2 | Caza et al. [4] | 26 | F | Black | G1/G2 | No | N/S |
| 3 | Neves et al. [5] | 63 | F | Black | G1/G0 | No | Hypertension, heart failure, dyslipidemia |
| 4 | Neves et al. [5] | 58 | F | N/S | G2/G2 | No | Multiple myeloma with cast nephropathy |
| 5 | Jefferis et al. [6] | N/S | F | N/S | N/S | Yes | N/S |
| Case | Vaccine Type | Dose Number | Renal Presentation | Baseline Creatinine (mg/dL) | Presentation Creatinine (mg/dL) | Renal Outcome |
|---|---|---|---|---|---|---|
| 1 | Moderna (RNA) | 2 | NS, AKI | Normal | 6.7 | Dialysis |
| 2 | Moderna (RNA) | 2 | NS, AKI | Normal | 7.7 | Partial remission |
| 3 | ChAdOx1 (AdV) | 1 | NS | Normal | 0.88 | Complete remission |
| 4 | ChAdOx1 (AdV) | 1 | NS, AKI | 2–3 | N/S | Dialysis |
| 5 | ChAdOx1 (AdV) | 1 | NS, AKI | 1.41–2.11 | 6.03 | Dialysis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez Preciado, F.; De Carvalho Ovalles, R.A.; Codina, S.; Donadeu, L.; Favà, A.; Martinez Valenzuela, L.; Sandoval, D.; Fernández-Cidón, B.; Bestard, O.; Alia-Ramos, P.; et al. Collapsing Glomerulonephritis in a Kidney Transplant Recipient after mRNA SARS-CoV-2 Vaccination. J. Clin. Med. 2022, 11, 3651. https://doi.org/10.3390/jcm11133651
Gómez Preciado F, De Carvalho Ovalles RA, Codina S, Donadeu L, Favà A, Martinez Valenzuela L, Sandoval D, Fernández-Cidón B, Bestard O, Alia-Ramos P, et al. Collapsing Glomerulonephritis in a Kidney Transplant Recipient after mRNA SARS-CoV-2 Vaccination. Journal of Clinical Medicine. 2022; 11(13):3651. https://doi.org/10.3390/jcm11133651
Chicago/Turabian StyleGómez Preciado, Francisco, Rosa Alejandra De Carvalho Ovalles, Sergi Codina, Laura Donadeu, Alexandre Favà, Laura Martinez Valenzuela, Diego Sandoval, Bárbara Fernández-Cidón, Oriol Bestard, Pedro Alia-Ramos, and et al. 2022. "Collapsing Glomerulonephritis in a Kidney Transplant Recipient after mRNA SARS-CoV-2 Vaccination" Journal of Clinical Medicine 11, no. 13: 3651. https://doi.org/10.3390/jcm11133651
APA StyleGómez Preciado, F., De Carvalho Ovalles, R. A., Codina, S., Donadeu, L., Favà, A., Martinez Valenzuela, L., Sandoval, D., Fernández-Cidón, B., Bestard, O., Alia-Ramos, P., Gomà, M., Melilli, E., & Cruzado, J. M. (2022). Collapsing Glomerulonephritis in a Kidney Transplant Recipient after mRNA SARS-CoV-2 Vaccination. Journal of Clinical Medicine, 11(13), 3651. https://doi.org/10.3390/jcm11133651






