Current Treatment Options for COVID-19 Associated Mucormycosis: Present Status and Future Perspectives
Abstract
1. Introduction
2. Mechanisms of Pathogenesis
3. Challenges in Control of Mucormycosis
4. Association of COVID-19 with Risk Factors of Mucormycosis and Their Role in Infection
4.1. Diabetes Mellitus and Diabetic Ketoacidosis
4.2. Immunosuppression
4.3. Nosocomial Sources
4.4. Other Factors
5. Diagnosis
5.1. Clinical Examination
5.2. Imaging
5.3. Histopathology
5.4. Culture
6. Current Recommended Strategies for Treatment of CAM
6.1. Reversal of Risk Factors
6.2. Surgical Debridement
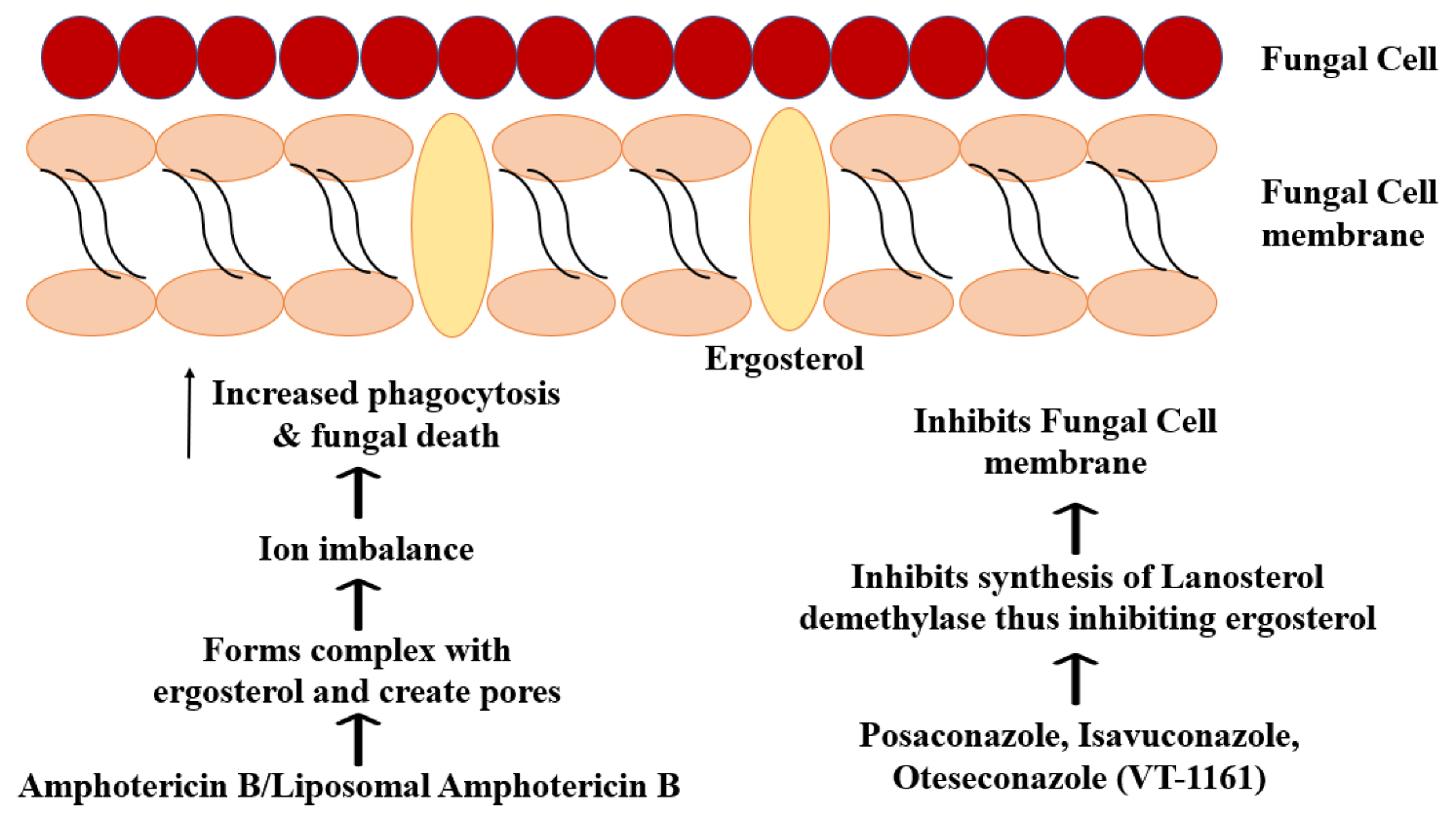
6.3. Systemic Antifungal Therapy
| Serial Number | Combination/Regimen | Type of Study | Type of Mucormycosis | Organism | Diagnostic Tests | Risk Factors (If Applicable) | Details of Combination/Regimen for Treatment of Mucormycosis (and Other Antifungals) | Other Concomitant Treatment (If Any) | Effect and/or Outcome | Addl Details | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LAmB + CAS + SD | Case report | RCM | Mucor | CT, Clinical Diagnosis, Histopathology | 1. Acute Myeloid Leukemia 2. Chemotherapy 3. Neutropenia | 1. Liposomal Amphotericin B 2. Liposomal Ampotericin B + Caspofungin (24 days) 3. Surgical Debridement 4. Caspofungin (45 days) | 1. Cytarabine 2. Idarubicin 3. Mitoxantrone 4. Broad-spectrum antibiotics 5. G-CSF 6. Potassium supplements | No infection after 3 months | Addition of Caspofungin was associated with improvement in patient’s conditions (LAmB monotherapy had no response) | [82] |
| 2 | (LAmB → ABLC) + CAS + SD | Case report | Oromandibular | Rhizopus oryzae | Clinical Suspicion, CT, Histopathology | 1. Diabetes mellitus 2. Acute Myeloid Leukemia 3. Chemotherapy | 1. AmB-deoxycholate 2. Fluconazole (stopped upon suspicion of mucormycosis) 3. Liposomal Amphotericin B + Caspofungin (56 days, maintained even after surgery) 4. Surgical Debridement 5. ABLC (5 Weeks) | 1. Idarubicin 2. Cytarabine 3. Tobramycin 4. Colimycin 5. Morphine 6. Imipenem 7. Amikacin 8. Vancomycin | Alive, no recurrence at 6-year follow-up | [83] | |
| 3 | LAmB + MCF + SD | Case report | ROM | Rhizopus oryzae | CT, Histopathology | 1. Diabetes mellitus 2. Hemodialysis for chronic renal failure | 1. Insulin therapy 2. Liposomal Amphotericin B 3. Surgical Debridement 4. Liposomal Amphotericin B + Micafungin (Oral) (2 + 4 weeks) 5. Amphotericin B (Sinus irrigation) | 1. Meropenem | No recurrence seen in 1 year follow-up | [84] | |
| 4 | HBO + LAmB + DEF + S | Case report | Hepatosplenic | Candida zeylanoides from blood cultures | Histopathology | 1. Febrile neutropenia 2. Minimally differentiated AML 3. Chemotherapy | 1. Voriconazole (9 days) 2. Voriconazole + Caspofungin 3. Liposomal Amphotericin B + Deferasirox 4. Hyperbaric Oxygen Therapy (60 sessions) + LAmB (21 days) + Deferasirox (Throughout) | 1. Cefepime → Meropenem 2. Vancomycin 3. Consolidation therapy—high-dose cytarabine | CT unremarkable after first consolidation therapy | [85] | |
| 5 | HBO + LAmB + PSZ + CAS + SD | Case report | ROM | Rhizopus | CT scan, Culture | 1. Acute Lymphoid Leukemia (ALL) 2. Chemotherapy | 1. Liposomal Amphotericin B 2. Sinus debridement 3. Liposomal Amphotericin B + Caspofungin + Posaconazole 4. Hyperbaric Oxygen Therapy (19 sessions) (Caspofungin stopped after 1 week of HBO, Amphotericin B continued for 2 months) 5. Discharged with oral posaconazole (4 months) | 1. Ceftazidime 2. Vancomycin 3. Consolidation chemotherapy | Favourable | [86] | |
| 6 | IFN-γ + NVB | Case report | Gastric | Histopathology | 1. Immunosuppression | 1. Liposomal Amphotericin B + Posaconazole 2. Gastrectomy 3. Splenectomy 4. Immunoadjuvant therapy 5. Nivolumab (1 dose) | Immunosuppression reversed. Patient discharged at 80 days | [87] | |||
| 7 | DAmB + LAmB + SD + VAC | Case report | Skin and Soft tissue | Rhizopus | Histopathology, Culture | 1. Bilineal leukemia (ALL and AML) 2. Chemotherapy | 1. Fluconazole (discontinued on diagnosis of mucormycosis) 2. Liposomal Amphotericin B (8 weeks) 3. Surgical Debridment 4. Vacuum-assisted closure (VAC) therapy 5. Deoxycholate amphotericin B (Topical) (3 weeks) | 1. Chemotherapy for AML (cytosine arabinoside, daunorubicin, and etoposide) 2. Chemotherapy for ALL (cytosine arabinoside and L -asparaginase) 3. Trimethoprim-sulfamethoxazole 4. Gentamicin 5. Vancomycin 6. Salvage chemotherapy (vinorelbine, thiotepa, gemcitabine, topotecan and dexamethasone) 7. Alternative salvage chemotherapy (6-mercaptopurine, imatinib and methotrexate) 8. Palliative chemotherapy—vincristine | Mucormycosis controlled; no recurrence. Patient died of unrelated causes | [88] | |
| 8 | LAmB (i.v.) + SD + AMB (N) | Case report | Sinonasal | Absidia corymbisera (Now Lichtheimia corymbifera) | Histopathology | 1. Acute promyelocytic leukemia 2. Chemotherapy | 1. Liposomal Amphotericin B (intravenous) 2. Amphotericin B (nebulisation) | Alive, no recurrence at 6-year follow-up | [75] | ||
| 9 | ABLC + (PSZ → ISZ) + CAS + SD | Case report | Disseminated | Cunninghamella | Clinical suspicion, Microscopic examination, Immunohistochemistry, PCR, Sanger sequencing, CT | 1. Acute Lymphoid Leukemia 2. Chemotherapy 3. Neutropenia | 1. Voriconazole (discontinued later) + Granulocyte colony-stimulating factor (G-CSF) 2. Amphotericin B Lipid Complex + Caspofungin 3. Posaconazole (3 days) 4. Isavuconazole (101 days, initially combination therapy, later monotherapy) | 1. Cefepime 2. Vancomycin 3. Clarithromycin | Patient observed to be well at 10-month check | [89] | |
| 10 | DAmB + MCF + PSZ | Case report | Disseminated | Rhizopus | Histopathology | 1. Preterm birth 2. Mother underwent chemotherapy before delivery | 1. Amphotericin B Deoxycholate + Caspofungin 2. Amphotericin B Deoxycholate + Caspofungin + Posaconazole 3. Micafungin discontinued subsequently (AMB—7 weeks; CAS—4 weeks, PSZ—3 weeks) | 1. Ampicillin + Gentamicin 2. Vancomycin + Gentamicin 3. Ampicillin + Gentamicin + Metronidazole | [90] | ||
| 11 | AMB + CAS + SD | Case report | RCM | Rhizopus arrhizus | Histopathology, Molecular identification | 1. Diabetes mellitus | 1. Amphotericin B (60 days) 2. Amphotericin B + Caspofungin (4 weeks) | 1. Targocid 2. Cefaxone 3. Flagyl | No recurrence in over 4 years | Caspofungin inclusion was associated with rapid improvement in symptoms | [91] |
| 12 | LAmB + PSZ + CAS + SD | Case report | Disseminated | Absidia corymbisera (Now Lichtheimia corymbifera) | Microscopic examination | 1. Chemotherapy 2. Osteosarcoma 3. Brief neutropenia 4. Malnutrition | 1. Surgical debridement—Multiple 2. Liposomal amphotericin B + Posaconazole 3. Liposomal amphotericin B + Posaconazole + Caspofungin (1 month) 4. Liposomal amphotericin B + Posaconazole (3 months) | 1. High-dose methotrexate and etoposide-ifosfamide | Culture negative after triple combination therapy | [92] | |
| 13 | (LAmB → PSZ) + S | Case report | Disseminated mixed invasive | Rhizopus | Histopathology | 1. Pancytopenia | 1. Fluconazole (discontinued eventually) 2. Liposomal Amphotericin B (discontinued on Day 100) 3. Surgical removal of fungal abcesses 4. Splenectomy 5. Nephrectomy (partial) 6. Lower lobe wedge resection (left) 7. Posaconazole (6 months, initiated on Day 100) | 1. Immunosuppressant therapy (rabbit anti-thymocyte globulin, methylprednisolone, G-CSF) 2. Imipenem–cilastatin 3. Vancomycin 4. Hematopoietic Stem Cell Transplantation 5. Cyclophosphamide 6. Rabbit anti-thymocyte globulin 7. Cyclosporin 8. Methotrexate | No residual abscess seen at 30-month follow-up MRI | [93] | |
| 14 | LAmB + PSZ + SD + S | Case report | Disseminated Cutaneous | Rhizomucor pussilus | Histopathology | 1. Acute Lymphoblastic Leukemia 2. Neutropenia 3. Chemotherapy 4. Steroid Therapy | 1. Surgical Debridement 2. Lung resection 3. Liposomal Amphotericin B + Posaconazole (12 weeks) | 1. Cefoperazone-sulbactam 2. Amikacin 3. Induction chemotherapy | Complete remission | [94] | |
| 15 | (LAmB + CAS + VOR) → (LAmB + PSZ + TER + SD + LAmB (N) + ABLC (i.pl)) | Case report | Disseminated | Cunninghamella bertholletiae | Histopathology, PCR | 1. Acute Lymphoblastic Leukemia 2. Pancytopenia | 1. Liposomal Amphotericin B 2. Voriconazole (Discontinued subsequently) 3. Caspofungin (Discontinued subsequently) 4. Posaconazole + Terbinafine 5. Surgical Debridement 6. Liposomal Amphotericin B (Nebulisation) 7. Amphotericin B Lipid Complex (Intrapleural) | 1. Broad spectrum antibiotics 2. Chemotherapy | No recurrence at 30 month follow up | [95] | |
| LAmB + TER + PSZ | Case report | Disseminated | Cunninghamella bertholletiae | PCR, Culture | 1. Acute Lymphoblastic Leukemia 2. Allogenic Stem Cell Transplant 3. Steroid Therapy 4. Diabetes mellitus 5. Iron overload | 1. Voriconazole (Discontinued later) 2. Liposomal Amphotericin B 3. Liposomal Amphotericin B + Terbinafine + Posaconazole | 1. Methylprednisolone 2. Etanercept 3. Mycophenolate mofetil 4. granulocyte-monocyte colony-stimulating factor (GM-CSF) 5. Simvastatin 6. Deferasirox | Patient died 3 years later (cause not mentioned) | [95] | ||
| 16 | LAmB + PSZ | Case report | Disseminated | Rhizopus microsporus | Culture, Clinical suspicion, | 1. AML | 1. Voriconazole (Discontinued later) 2. Caspofungin (Discontinued later) 3. Liposomal Amphotericin B + Posaconazole (5 months) 4. Allogenic HSCT 5. Posaconazole 6. Surgery | 1. Broad spectrum antibiotics 2. Antithymocyte globulin + Tacrolimus + Etanercept | No residual fungal lesions at 18 months | [96] | |
| 17 | LAmB + PSZ + DEF | Case report | Hepatic | Rhizomucor pusillus | Microscopic examination, Histopathology | 1. AML 2. Chemotherapy 3. Neutropenia 4. HSCT | 1. Liposomal Amphotericin B 2. Liposomal Amphotericin B + Posaconazole 3. Surgical Debridement 4. Discharged with posaconazole 5. Deferasirox | Favourable | [97] | ||
| 18 | LAmB + CAS + SD | Case report | RCM | Rhizopus oryzae | Histopathology | 1. Diabetes mellitus | 1. Liposomal Amphotericin B 2. Liposomal Amphotericin B + Caspofungin 3. Liposomal Amphotericin B (Discharge, 2nd hospitalization) | 1. Maxillectomy 2. Endoscopic decompression of orbita 3. Functional endoscopic sinus surgery 4. Meropenem 5. Ciprofloxacin | Recurrence due to patient non-compliance. Patient expired due to sepsis | [98] | |
| 19 | DAmB + RIF | Case report | Rhizopus oryzae | Bronchoscopy, Culture | 1. Diabetic Ketoacidosis | 1. Rifampicin + Amphotericin B | Culture and histopathology negative after 8 weeks. Died of unrelated causes 3 years later. | [99] | |||
| 20 | AMB + (PSZ → AFG) | Case report | Hepatic | Mucor spp. | Histopathology, Immunochemical testing | 1. AML 2. Neutropenia 3. Chemotherapy | 1. Amphotericin B (10 days) 2. Amphotericin B + Posaconazole (2 months) 3. Amphotericin B + Anidulafungin | 1. Chemotherapy-azacitadine 2. Moxifloxacin 3. Valacyclovir 4. Voriconazole 5. Levofloxacin 6. Metronidazole | Liver lesions improved. Patient expired due to complications | [100] | |
| 21 | AMB/LAmB + CAS | Retrospective study | ROCM, ROM (Coexisting pulmonary, cutaneous) | Rhizopus spp., Rhizomucor spp. | CT, MRI | 1. Diabetes mellitus 2. Neutropenia 3. Steroid therapy 4. Cancer 5. Transplant | 1. Caspofungin + Polyene (ABLC/LAmB) 2. Surgical Debridement | 1 Patient who received combination therapy expired within 30 days | [101] |
| Serial Number | Combination/Regimen | Type of Study | Type of CAM | Organism | Diagnostic Tests | Risk Factors Other Than COVID-19 (If Applicable) | Details of Combination/Regimen for Treatment of Mucormycosis | Other concomitant Treatment (If Any) | Effect and/or Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | AMB + (ISZ → PSZ) + TCR + HBO + SD + Maxillectomy | Case report | Rhinosinusal | Rhizopus oryzae | Endoscopy, Culture, Palate Biopsy | 1. Kidney Transplant 2. Immunosuppression 3. Prolonged history of isavuconazole use and IFIs 4. Diabetes mellitus (No DKA) 5. Steroid therapy | 1. Treatment with Amphotericin B and azole (initially Isavuconazole, later posaconazole to avoid resistance) for 5 months 2. Surgical Debridement—7 times 3. Total Maxillectomy 4. Reduction of steroid (prednisone) dosage 5. Tacrolimus (Before diagnosis of CAM and during CAM treatment) 6. Hyperbaric Chamber Therapy | Azithromycin Ceftriaxone Dexamethasone Piperacillin/Tazobactam | No recurrence of infection after 5 months | [44] |
| 2 | Fasciotomy + SD + LAmB + ISZ | Case report | Musculoskeletal | Lichtheimia ramosa | Culture | 1. Immunosuppression (Steroid Therapy—prednisone, mycophenolate and tacrolimus) 2. Kidney transplant (graft dysfunction) | 1. Liposomal Amphotericin B + Isavuconazole (24 days) 2. Isavuconazole for 3 months 3. Surgical Debridement—3 times 4. Fasciotomy | 1. Immunosuppressants (IS): prednisone, mycophenolate and TCR 2. Hydroxychloroquine 3. Azithromycin 4. Lopinavir/Ritonavir 5. Heparin 6. Tocilizumab (400 mg) | Favourable | [44] |
| 3 | FLU + AMB + SD | Case report | Sino-orbital | Rhizopus oryzae | Culture Histopathology MRI | None | 1. Surgical Debridement—2 times 2. Fluconazole 3. Amphotericin B (injection and lavage) 4. Discharged with prescription for continuation of Amphotericin B and Fluconazole | 1. Remdesivir 2. Methylprednisolone 3. Dexamethasone 4. Piptaz 5. Metronidazole 6. Tobramycin 7. Nepalact TDS 8. Monocef | Favourable at 2 month review | [59] |
| 4 | LAmB + PSZ + Sinus debridement without craniotomy | Case report | ROCM | Not Mentioned | MRI CT Culture of biopsy sample | 1. B-cell lymphoma 2. Chemotherapy 3. Neutropenia | 1. Liposomal Amphotericin B 2. Liposomal Amphotericin B + Posaconazole for 4 weeks 3. Surgical Debridement—Multiple | 1. R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) 2. CODOX-M/IVAC (cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate/ifosfamide, etoposide, and high-dose cytarabine) 3. Meropenem 4. Vancomycin | Patient discharged after 12 weeks. No recurrence for upto patient’s death, 3 months after discharge (unrelated to ROCM) | [74] |
| 5 | Amphotericin B + Azoles | Multicenter Epidemiologic Study | ROM, ROCM, Pulmonary, Renal, Disseminated, Others | Aspergillus and Mucorales | Microscopy Culture Histopathology | 1. Steroid Therapy 2. Diabetes mellitus | Amphotericin B + Posaconazole (Concurrent or sequential) | 1. Glucocorticoid drugs 2. Tocilizumab | The survival rates of sequential combination therapy were found to be better at 6 and 12 weeks compared to concurrent and single antifungal therapy | [39] |
| 6 | AMB + PSZ | Descriptive multicentre study (Cross-sectional) | Orbital | Not Mentioned | Not Mentioned | 1. Diabetes mellitus 2. Steroid Therapy 3. Neutropenia | 1. Amphotericin B 2. Posaconazole (2 weeks) 3. Orbital exenteration | Alive | [102] | |
| AMB + PSZ + SD | ROM | 1. Diabetes mellitus | 1. Amphotericin B 2. Posaconazole (2 weeks) 3. Surgical Debridement | Dexamethasone | Alive | |||||
| AMB + PSZ + CAS + SD | ROM | 1. Diabetes mellitus 2. Steroid Therapy | 1. Amphotericin B 2. Posaconazole (2 weeks) 3. Caspofungin (2 weeks) 4. Surgical Debridement | Dexamethasone | Alive | |||||
| AMB + CAS + SD | ROM | 1. Diabetes mellitus | 1. Amphotericin B 2. Caspofungin (2 weeks) 3. Surgical Debridement | Alive | ||||||
| AMB + PSZ + SD | Sino-orbital | 1. Diabetes mellitus 2. Steroid Therapy | 1. Amphotericin B 2. Posaconazole (2 weeks) 3. Surgical Debridement | Dexamethasone | Alive | |||||
| AMB + CAS + SD | Sinonasal | 1. Acute Myeloid Leukemia 2. Chemotherapy 3. Neutropenia | 1. Amphotericin B 2. Caspofungin (2 weeks) 3. Surgical Debridement | Dexamethasone | Alive | |||||
| 7 | AMB + Azoles | Review (Statistical Analysis) | ROM, ROCM, Pulmonary, Cutaneous, Gastrointestinal, Disseminated, Others | Rhizopus arrhizus, Rhizopus microsporus, Rhizopus spp., Lichtheimia spp. And Mucor spp. | - | 1. Glucocorticoid usage 2. Diabetes mellitus 3. Solid Organ Transplant 4. Immunosuppressive therapies | Amphotericin B + Azole (Isavuconazole or Posaconazole) (Sequential) Details of surgical debridement in combination with antifungal treatment not provided. | Details for individual cases unknown | Details for individual cases unknown | [103] |
| 8 | LAmB + VRZ + PSZ + SD | Retrospective Interventional study | ROCM | Not Mentioned | Histopathology, Imaging | 1. Diabetes mellitus 2. Steroid Therapy | 1. liposomal Amphotericin-B+ Voriconazole 2. Posaconazole 3. Orbital exenteration 4. Surgical Debridement | cefoperazone + sulbactam | [25] | |
| LAmB + PSZ + SD | ROCM | Histopathology, Culture | 1.Diabetes mellitus 2. Steroid Therapy | 1. Liposomal Amphotericin-B 2. Posaconazole 3. Surgical Debridement | 1. Methylprednisolone 2. Prednisolone | |||||
| LAmB + PSZ + SD | ROCM | Diagnosed as possible Mucor based on clinical evidence and imaging | 1.Diabetes mellitus 2. Steroid Therapy | 1. Liposomal Amphotericin-B 2. Posaconazole 3.Surgical Debridement | 1.Dexamethasone 2.Prednisolone 3. Gabapentin | |||||
| LAmB + PSZ + SD | ROCM | Histopathology, Culture | 1.Diabetes mellitus 2.Steroid Therapy 3.Existing Antifungal Therapy | 1. Liposomal Amphotericin B 2.Posaconazole 3.Surgical Debridement | 1. Prednisolone | |||||
| LAmB + PSZ + SD | ROCM | Histopathology, Culture | 1.Diabetes mellitus 2.Steroid Therapy 3.Existing Antifungal Therapy | 1. Liposomal Amphotericin B 2. Posaconazole 3. Orbital enteration 4. Surgical Debridement | 1. Dexamethasone | |||||
| 9 | AMB + PSZ | Case report | ROM | Not Mentioned | Histopathology | 1. Diabetes mellitus | 1. Insulin injections to control hyperglycemia 2.Surgical Debridement 3.Amphotericin B 4. Posaconazole | 1. Remdesivir 2. Levofloxacin 3. Dexamethasone 4. Vancomycin 5. Piperacillin-Tazobactam | Patient alive and stable at 2-month and 7-month follow up check | [58] |
| 10 | LAmB + CAS + PSZ | Case report | ROM | Rhizopus spp. | Histopathology CT Culture | 1. Hyperglycemia | 1. Liposomal Amphotericin B (4 days) 2. (Liposomal Amphotericin B → Posaconazole) + Caspofungin 3. Glucose Management 4. Surgical Debridement | 1. Remdesivir 2. Vancomycin 3. Cefepime 4. Dexamethasone | Patient died due to COVID-19 associated ARDS | [28] |
| 11 | AMB + ISZ + MCF | Case report | ROCM | Mucormycosis suspicion based on MRI | 1. Diabetes mellitus 2. Diabetic Ketoacidosis 3. Steroid Therapy | 1. Amphotericin B 2. Isavuconazole 3. Micafungin | 1. Remdesivir | Patient expired on Day 4 due to poor prognosis and rapid decline | [104] | |
| AMB + ISZ | Case report | ROCM | Rhizopus | CT Culture | 1. Diabetic Ketoacidosis | 1. Amphotericin B (3 weeks) 2. Amphotericin B + Isavuconazole (10 days) | 1. Remdesivir | Patient expired |
7. Successful Drugs and Combinational Therapies against CAM
7.1. Hyperbaric Therapy
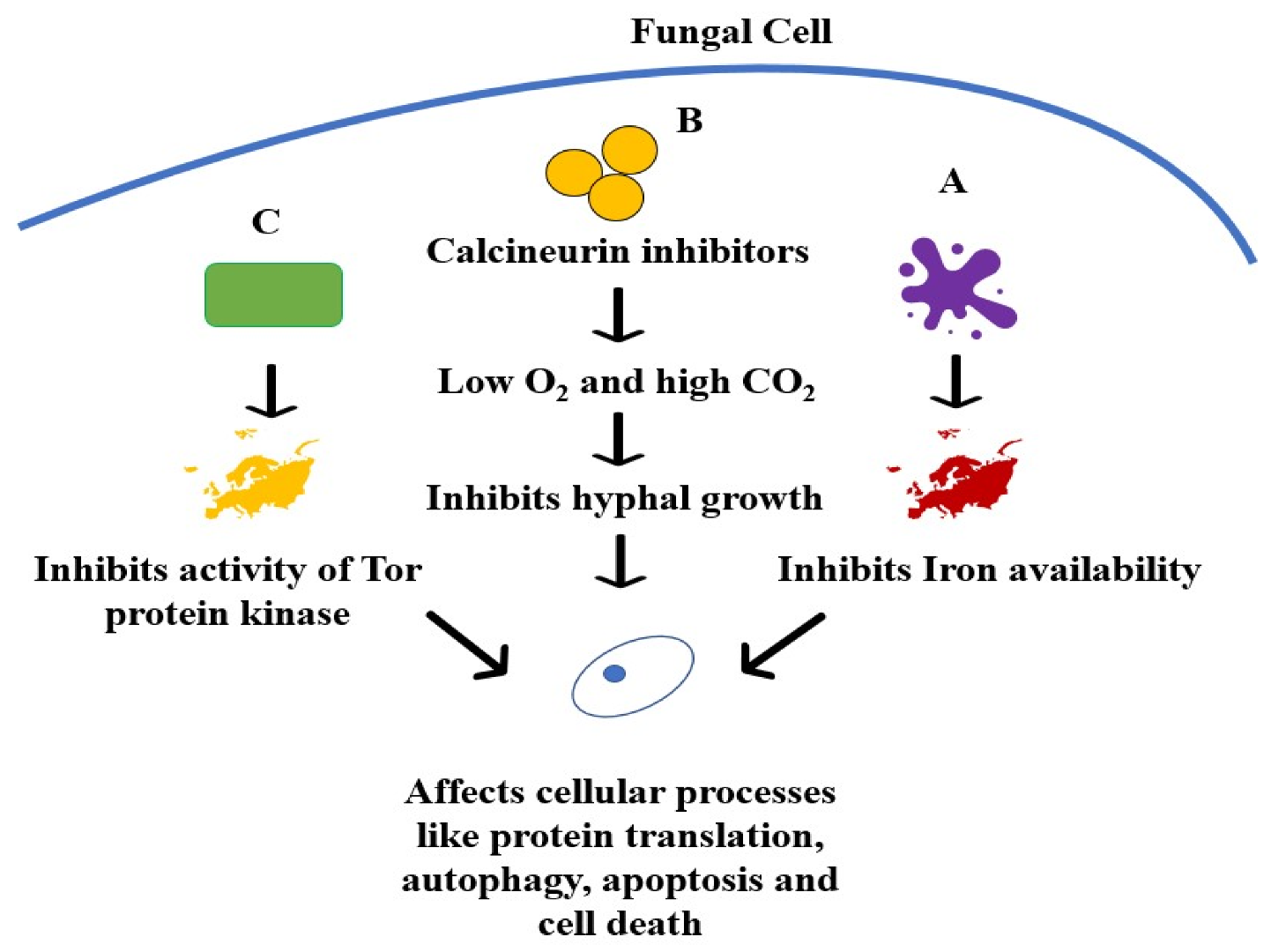
7.2. Immunosuppressants Used for Transplant Patients
7.3. Iron and Zinc Chelators
7.4. Echinocandins
8. New or Repurposed Drugs
8.1. Drugs Used in Monotherapies
8.1.1. VT-1161
8.1.2. Manogepix
8.1.3. Fosmanogepix (APX001)
8.1.4. Haemofungin
8.1.5. PC1244
8.1.6. EGFR Inhibitors
8.2. Potential adjunct Drugs for Treatment of CAM
8.2.1. Colistin
8.2.2. HDAC Inhibitors
8.2.3. Miltefosine
8.2.4. Statins
8.2.5. Rifampin
8.2.6. Terbinafine
8.2.7. Quinolones
8.3. Immunomodulating Strategies
8.3.1. Anti-CotH3 Antibodies
8.3.2. Anti-GRP78 Antibodies
8.3.3. Cytokine Administration
8.4. Other Therapies
8.4.1. Photodynamic Therapy
8.4.2. Hyperthermia
9. Insights from In Silico Studies
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pal, R.; Singh, B.; Bhadada, S.K.; Banerjee, M.; Bhogal, R.S.; Hage, N.; Kumar, A. COVID-19-Associated Mucormycosis: An Updated Systematic Review of Literature. Mycoses 2021, 64, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and Outcome of Zygomycosis: A Review of 929 Reported Cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef]
- Jeong, W.; Keighley, C.; Wolfe, R.; Lee, W.L.; Slavin, M.A.; Kong, D.C.M.; Chen, S.C.-A. The Epidemiology and Clinical Manifestations of Mucormycosis: A Systematic Review and Meta-Analysis of Case Reports. Clin. Microbiol. Infect. 2019, 25, 26–34. [Google Scholar] [CrossRef]
- Bhatt, K.; Agolli, A.; Patel, M.H.; Garimella, R.; Devi, M.; Garcia, E.; Amin, H.; Domingue, C.; Guerra Del Castillo, R.; Sanchez-Gonzalez, M. High Mortality Co-Infections of COVID-19 Patients: Mucormycosis and Other Fungal Infections. Discoveries 2021, 9, e126. [Google Scholar] [CrossRef] [PubMed]
- Gade, D.; Rahul, D.; Chandwani, N. Mucormycosis: Tsunami of Fungal Infection after Second Wave of COVID 19. Ann. Rom. Soc. Cell Biol. 2021, 25, 7231–7238. [Google Scholar]
- Bakshi, S.S.; Kalidoss, V.K. COVID 19 Infection and Mucormycosis—A Dangerously Increasing Combination. Egypt. J. Otolaryngol. 2021, 37, 53. [Google Scholar] [CrossRef]
- Waizel-Haiat, S.; Guerrero-Paz, J.A.; Sanchez-Hurtado, L.; Calleja-Alarcon, S.; Romero-Gutierrez, L. A Case of Fatal Rhino-Orbital Mucormycosis Associated With New Onset Diabetic Ketoacidosis and COVID-19. Cureus 2021, 13, e13163. [Google Scholar] [CrossRef] [PubMed]
- Baldin, C.; Ibrahim, A.S. Molecular Mechanisms of Mucormycosis-The Bitter and the Sweet. PLoS Pathog. 2017, 13, e1006408. [Google Scholar] [CrossRef]
- Khatri, A.; Chang, K.-M.; Berlinrut, I.; Wallach, F. Mucormycosis after Coronavirus Disease 2019 Infection in a Heart Transplant Recipient—Case Report and Review of Literature. J. Mycol. Med. 2021, 31, 101125. [Google Scholar] [CrossRef]
- Singh, R.P.; Gupta, N.; Kaur, T.; Gupta, A. Rare Case of Gastrointestinal Mucormycosis with Colonic Perforation in an Immunocompetent Patient with COVID-19. BMJ Case Rep. 2021, 14, e244096. [Google Scholar] [CrossRef]
- Do Monte Junior, E.S.; Santos, M.E.L.D.; Ribeiro, I.B.; de Oliveira Luz, G.; Baba, E.R.; Hirsch, B.S.; Funari, M.P.; de Moura, E.G.H. Rare and Fatal Gastrointestinal Mucormycosis (Zygomycosis) in a COVID-19 Patient: A Case Report. Clin. Endosc. 2020, 53, 746–749. [Google Scholar] [CrossRef]
- Soliman, M.; Harding, C.; El Haddad, H.; Mansour, A.; Anstead, M. Disseminated Mucormycosis with Extensive Cardiac Involvement. Cureus 2022, 11, e4760. [Google Scholar] [CrossRef]
- Kumar, P. How to Understand and Manage Mucormycosis Infections during COVID-19/SARS-CoV-2/Novel Coronavirus Pandemic Era in India & Developing Countries; Social Science Research Network: Rochester, NY, USA, 2021. [Google Scholar]
- Baruah, C. Mucormycosis and Aspergillosis Have Been Linked to COVID-19-Related Fungal Infections in India. Adv. Case Stud. 2021, 3. [Google Scholar] [CrossRef]
- Narayanan, S.; Chua, J.V.; Baddley, J.W. COVID-19 Associated Mucormycosis (CAM): Risk Factors and Mechanisms of Disease. Clin. Infect. Dis. 2022, 74, 1279–1283. [Google Scholar] [CrossRef]
- Morales-Franco, B.; Nava-Villalba, M.; Medina-Guerrero, E.O.; Sánchez-Nuño, Y.A.; Davila-Villa, P.; Anaya-Ambriz, E.J.; Charles-Niño, C.L. Host-Pathogen Molecular Factors Contribute to the Pathogenesis of Rhizopus Spp. in Diabetes Mellitus. Curr. Trop. Med. Rep. 2021, 8, 6–17. [Google Scholar] [CrossRef]
- Khan, N.; Gutierrez, C.G.; Martinez, D.V.; Proud, K.C. A Case Report of COVID-19 Associated Pulmonary Mucormycosis. Arch. Clin. Cases 2021, 7, 46–51. [Google Scholar] [CrossRef]
- Ibrahim, A.S. Host-Iron Assimilation: Pathogenesis and Novel Therapies of Mucormycosis. Mycoses 2014, 57 (Suppl. S3), 13–17. [Google Scholar] [CrossRef]
- Liu, M.; Spellberg, B.; Phan, Q.T.; Fu, Y.; Fu, Y.; Lee, A.S.; Edwards, J.E.; Filler, S.G.; Ibrahim, A.S. The Endothelial Cell Receptor GRP78 Is Required for Mucormycosis Pathogenesis in Diabetic Mice. J. Clin. Investig. 2010, 120, 1914–1924. [Google Scholar] [CrossRef]
- Alqarihi, A.; Gebremariam, T.; Gu, Y.; Swidergall, M.; Alkhazraji, S.; Soliman, S.S.M.; Bruno, V.M.; Edwards, J.E.; Filler, S.G.; Uppuluri, P.; et al. GRP78 and Integrins Play Different Roles in Host Cell Invasion during Mucormycosis. mBio 2020, 11, e01087-20. [Google Scholar] [CrossRef]
- Baldin, C.; Soliman, S.; Jeon, H.; Skory, C.; Edwards, J.; Ibrahim, A. Optimization of the CRISPR/Cas9 System to Manipulate Gene Function in Rhizopus Delemar. Open Forum Infect. Dis. 2017, 4, S116. [Google Scholar] [CrossRef]
- Soliman, S.S.M.; Baldin, C.; Gu, Y.; Singh, S.; Gebremariam, T.; Swidergall, M.; Alqarihi, A.; Youssef, E.G.; Alkhazraji, S.; Pikoulas, A.; et al. Mucoricin Is a Ricin-Like Toxin That Is Critical for the Pathogenesis of Mucormycosis. Nat. Microbiol. 2021, 6, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of Platelet-Derived Growth Factors in Physiology and Medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Galougahi, M.; Arastou, S.; Haseli, S. Fulminant Mucormycosis Complicating Coronavirus Disease 2019 (COVID-19). Int. Forum Allergy Rhinol. 2021, 11, 1029–1030. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.; Lahane, S.; Lahane, T.P.; Parekh, R.; Honavar, S.G. Mucor in a Viral Land: A Tale of Two Pathogens. Indian J. Ophthalmol. 2021, 69, 244–252. [Google Scholar] [CrossRef]
- Sen, M.; Honavar, S.G.; Sharma, N.; Sachdev, M.S. COVID-19 and Eye: A Review of Ophthalmic Manifestations of COVID-19. Indian J. Ophthalmol. 2021, 69, 488–509. [Google Scholar] [CrossRef]
- Hoenigl, M.; Seidel, D.; Carvalho, A.; Rudramurthy, S.M.; Arastehfar, A.; Gangneux, J.P.; Nasir, N.; Bonifaz, A.; Araiza, J.; Klimko, N.; et al. The Emergence of COVID-19 Associated Mucormycosis: Analysis of Cases From 18 Countries; Social Science Research Network: Rochester, NY, USA, 2021. [Google Scholar]
- Mekonnen, Z.K.; Ashraf, D.C.; Jankowski, T.; Grob, S.R.; Vagefi, M.R.; Kersten, R.C.; Simko, J.P.; Winn, B.J. Acute Invasive Rhino-Orbital Mucormycosis in a Patient With COVID-19-Associated Acute Respiratory Distress Syndrome. Ophthalmic Plast. Reconstr. Surg. 2021, 37, e40–e80. [Google Scholar] [CrossRef]
- Ahmadikia, K.; Hashemi, S.J.; Khodavaisy, S.; Getso, M.I.; Alijani, N.; Badali, H.; Mirhendi, H.; Salehi, M.; Tabari, A.; Mohammadi Ardehali, M.; et al. The Double-Edged Sword of Systemic Corticosteroid Therapy in Viral Pneumonia: A Case Report and Comparative Review of Influenza-Associated Mucormycosis versus COVID-19 Associated Mucormycosis. Mycoses 2021, 64, 798–808. [Google Scholar] [CrossRef]
- Pandiar, D.; Kumar, N.S.; Anand, R.; Kamboj, M.; Narwal, A.; Shameena, P.M. Does COVID 19 Generate a Milieu for Propagation of Mucormycosis? Med. Hypotheses 2021, 152, 110613. [Google Scholar] [CrossRef]
- Samanta, J.; Gupta, R.; Singh, M.P.; Patnaik, I.; Kumar, A.; Kochhar, R. Coronavirus Disease 2019 and the Pancreas. Pancreatology 2020, 20, 1567–1575. [Google Scholar] [CrossRef]
- Salameh, A.; Zöbisch, H.; Schröder, B.; Vigelahn, J.; Jahn, M.; Abraham, G.; Seeger, J.; Dähnert, I.; Dhein, S. Effects of Hypoxia and Acidosis on Cardiac Electrophysiology and Hemodynamics. Is NHE-Inhibition by Cariporide Still Advantageous? Front. Physiol. 2020, 11, 225. [Google Scholar] [CrossRef]
- Stone, N.; Gupta, N.; Schwartz, I. Mucormycosis: Time to Address This Deadly Fungal Infection. Lancet Microbe 2021, 2, e343–e344. [Google Scholar] [CrossRef]
- Artis, W.M.; Fountain, J.A.; Delcher, H.K.; Jones, H.E. A Mechanism of Susceptibility to Mucormycosis in Diabetic Ketoacidosis: Transferrin and Iron Availability. Diabetes 1982, 31, 1109–1114. [Google Scholar] [CrossRef]
- Ghosh, D.; Dey, S.; Chakraborty, H.; Mukherjee, S.; Halder, A.; Sarkar, A.; Chakraborty, P.; Ghosh, R.; Sarkar, J. Mucormycosis: A New Threat to Coronavirus Disease 2019 with Special Emphasis on India. Clin. Epidemiol. Glob. Health 2022, 15, 101013. [Google Scholar] [CrossRef]
- Allam, L.; Ghrifi, F.; Mohammed, H.; El Hafidi, N.; El Jaoudi, R.; El Harti, J.; Lmimouni, B.; Belyamani, L.; Ibrahimi, A. Targeting the GRP78-Dependant SARS-CoV-2 Cell Entry by Peptides and Small Molecules. Bioinform. Biol. Insights 2020, 14, 1177932220965505. [Google Scholar] [CrossRef]
- Carlos, A.J.; Ha, D.P.; Yeh, D.-W.; Krieken, R.V.; Tseng, C.-C.; Zhang, P.; Gill, P.; Machida, K.; Lee, A.S. The Chaperone GRP78 Is a Host Auxiliary Factor for SARS-CoV-2 and GRP78 Depleting Antibody Blocks Viral Entry and Infection. J. Biol. Chem. 2021, 296, 100759. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, R.; Joshi, S.R.; Misra, A. Mucormycosis in COVID-19: A Systematic Review of Cases Reported Worldwide and in India. Diabetes Metab. Syndr. 2021, 15, 102146. [Google Scholar] [CrossRef]
- Patel, A.; Agarwal, R.; Rudramurthy, S.M.; Shevkani, M.; Xess, I.; Sharma, R.; Savio, J.; Sethuraman, N.; Madan, S.; Shastri, P.; et al. Multicenter Epidemiologic Study of Coronavirus Disease-Associated Mucormycosis, India. Emerg. Infect. Dis. 2021, 27, 2349–2359. [Google Scholar] [CrossRef]
- Revannavar, S.M.; Supriya, P.S.; Samaga, L.; Vineeth, V. COVID-19 Triggering Mucormycosis in a Susceptible Patient: A New Phenomenon in the Developing World? BMJ Case Rep. 2021, 14, e241663. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High Expression of ACE2 Receptor of 2019-NCoV on the Epithelial Cells of Oral Mucosa. Int. J. Oral Sci. 2020, 12, 1–5. [Google Scholar] [CrossRef]
- Xiang, Q.; Feng, Z.; Diao, B.; Tu, C.; Qiao, Q.; Yang, H.; Zhang, Y.; Wang, G.; Wang, H.; Wang, C.; et al. SARS-CoV-2 Induces Lymphocytopenia by Promoting Inflammation and Decimates Secondary Lymphoid Organs. Front. Immunol. 2021, 12, 661052. [Google Scholar] [CrossRef]
- Fischer, K.; Hoffmann, P.; Voelkl, S.; Meidenbauer, N.; Ammer, J.; Edinger, M.; Gottfried, E.; Schwarz, S.; Rothe, G.; Hoves, S.; et al. Inhibitory Effect of Tumor Cell–Derived Lactic Acid on Human T Cells. Blood 2007, 109, 3812–3819. [Google Scholar] [CrossRef] [PubMed]
- Arana, C.; Cuevas Ramírez, R.E.; Xipell, M.; Casals, J.; Moreno, A.; Herrera, S.; Bodro, M.; Cofan, F.; Diekmann, F.; Esforzado, N. Mucormycosis Associated with COVID-19 in Two Kidney Transplant Patients. Transpl. Infect. Dis. 2021, e13652. [Google Scholar] [CrossRef] [PubMed]
- John, T.M.; Jacob, C.N.; Kontoyiannis, D.P. When Uncontrolled Diabetes Mellitus and Severe COVID-19 Converge: The Perfect Storm for Mucormycosis. J. Fungi 2021, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, S.; Muthuraju, S.; Vasugi, A.; Chandrasekar, M.; Murugan, R.; Inbasekaran, P.; Prabu, P. Clinicopathological Study of Mucormycosis in COVID-19 Patients: Experience From a Tertiary Care Center in South India. Cureus 2022, 14, e23016. [Google Scholar] [CrossRef]
- Muqeetadnan, M.; Rahman, A.; Amer, S.; Nusrat, S.; Hassan, S.; Hashmi, S. Pulmonary Mucormycosis: An Emerging Infection. Case Rep. Pulmonol. 2012, 2012, 120809. [Google Scholar] [CrossRef]
- Rammaert, B.; Lanternier, F.; Zahar, J.-R.; Dannaoui, E.; Bougnoux, M.-E.; Lecuit, M.; Lortholary, O. Healthcare-Associated Mucormycosis. Clini. Infect. Dis. 2012, 54, S44–S54. [Google Scholar] [CrossRef]
- Hartnett, K.P.; Jackson, B.R.; Perkins, K.M.; Glowicz, J.; Kerins, J.L.; Black, S.R.; Lockhart, S.R.; Christensen, B.E.; Beer, K.D. A Guide to Investigating Suspected Outbreaks of Mucormycosis in Healthcare. J. Fungi 2019, 5, 69. [Google Scholar] [CrossRef]
- Vasudevan, B.; Hazra, N.; Shijith, K.; Neema, S.; Vendhan, S. Mucormycosis: The Scathing Invader. Indian J. Dermatol. 2021, 66, 393–400. [Google Scholar] [CrossRef]
- Hasrat, N.; Farid, H.; Hashim, A. Rhinocerebral Mucormycosis as a COVID-19-Related Complication: A Case Report from Basra City, Southern Iraq. J. Sci. Res. 2021, 6, 1369. [Google Scholar]
- Ismaiel, W.F.; Abdelazim, M.H.; Eldsoky, I.; Ibrahim, A.A.; Alsobky, M.E.; Zafan, E.; Hasan, A. The Impact of COVID-19 Outbreak on the Incidence of Acute Invasive Fungal Rhinosinusitis. Am. J. Otolaryngol. 2021, 42, 103080. [Google Scholar] [CrossRef]
- Arora, S.; Hemmige, V.S.; Mandke, C.; Chansoria, M.; Rawat, S.K.; Dravid, A.; Sethi, Y.; Medikeri, G.; Jariwala, S.P.; Puius, Y.A.; et al. Online Registry of COVID-19-Associated Mucormycosis Cases, India, 2021. Emerg. Infect. Dis. 2021, 27, 2963–2965. [Google Scholar] [CrossRef]
- Nath, S.; Baidya, D.K. Mucormycosis in COVID-19: Is Zinc a Silent Killer in India? Indian J. Crit. Care Med. 2021, 25, 1079–1080. [Google Scholar] [CrossRef]
- Muthu, V.; Kumar, M.; Paul, R.A.; Zohmangaihi, D.; Choudhary, H.; Rudramurthy, S.M.; Panda, N.K.; Pannu, A.K.; Sharma, N.; Sharma, S.; et al. Is There an Association between Zinc and COVID-19-Associated Mucormycosis? Results of an Experimental and Clinical Study. Mycoses 2021, 64, 1291–1297. [Google Scholar] [CrossRef]
- Garg, D.; Muthu, V.; Sehgal, I.S.; Ramachandran, R.; Kaur, H.; Bhalla, A.; Puri, G.D.; Chakrabarti, A.; Agarwal, R. Coronavirus Disease (COVID-19) Associated Mucormycosis (CAM): Case Report and Systematic Review of Literature. Mycopathologia 2021, 186, 289–298. [Google Scholar] [CrossRef]
- Ravani, S.A.; Agrawal, G.A.; Leuva, P.A.; Modi, P.H.; Amin, K.D. Rise of the Phoenix: Mucormycosis in COVID-19 Times. Indian J. Ophthalmol. 2021, 69, 1563–1568. [Google Scholar] [CrossRef]
- Veisi, A.; Bagheri, A.; Eshaghi, M.; Rikhtehgar, M.H.; Rezaei Kanavi, M.; Farjad, R. Rhino-Orbital Mucormycosis during Steroid Therapy in COVID-19 Patients: A Case Report. Eur. J. Ophthalmol. 2021, 11206721211009450. [Google Scholar] [CrossRef]
- Maini, A.; Tomar, G.; Khanna, D.; Kini, Y.; Mehta, H.; Bhagyasree, V. Sino-Orbital Mucormycosis in a COVID-19 Patient: A Case Report. Int. J. Surg. Case Rep. 2021, 82, 105957. [Google Scholar] [CrossRef]
- Tabarsi, P.; Khalili, N.; Pourabdollah, M.; Sharifynia, S.; Naeini, A.S.; Ghorbani, J.; Mohamadnia, A.; Abtahian, Z.; Askari, E. Case Report: COVID-19-Associated Rhinosinusitis Mucormycosis Caused by Rhizopus Arrhizus: A Rare but Potentially Fatal Infection Occurring After Treatment with Corticosteroids. Am. J. Trop. Med. Hyg. 2021, 105, 449–453. [Google Scholar] [CrossRef]
- Preshaw, P.M. Detection and Diagnosis of Periodontal Conditions Amenable to Prevention. BMC Oral Health 2015, 15, S5. [Google Scholar] [CrossRef]
- Mehta, S.; Pandey, A. Rhino-Orbital Mucormycosis Associated With COVID-19. Cureus 2020, 12, e10726. [Google Scholar] [CrossRef]
- Alekseyev, K.; Didenko, L.; Chaudhry, B. Rhinocerebral Mucormycosis and COVID-19 Pneumonia. J. Med. Cases 2021, 12, 85–89. [Google Scholar] [CrossRef]
- Li, Y.; Xia, L. Coronavirus Disease 2019 (COVID-19): Role of Chest CT in Diagnosis and Management. AJR Am. J. Roentgenol. 2020, 214, 1280–1286. [Google Scholar] [CrossRef]
- Song, G.; Liang, G.; Liu, W. Fungal Co-Infections Associated with Global COVID-19 Pandemic: A Clinical and Diagnostic Perspective from China. Mycopathologia 2020, 185, 599–606. [Google Scholar] [CrossRef]
- Millon, L.; Reboux, G.; Bellanger, P.; Roussel, S.; Sornin, S.; Martin, C.; Deconinck, E.; Dalphin, J.-C.; Piarroux, R. Quantification de Stachybotrys chartarum par PCR en temps réel dans l’environnement domestique, hospitalier, et agricole. J. Mycol. Médicale 2006, 16, 183–188. [Google Scholar] [CrossRef]
- Legrand, M.; Gits-Muselli, M.; Boutin, L.; Garcia-Hermoso, D.; Maurel, V.; Soussi, S.; Benyamina, M.; Ferry, A.; Chaussard, M.; Hamane, S.; et al. Detection of Circulating Mucorales DNA in Critically Ill Burn Patients: Preliminary Report of a Screening Strategy for Early Diagnosis and Treatment. Clin. Infect. Dis. 2016, 63, 1312–1317. [Google Scholar] [CrossRef]
- Skiada, A.; Lass-Floerl, C.; Klimko, N.; Ibrahim, A.; Roilides, E.; Petrikkos, G. Challenges in the Diagnosis and Treatment of Mucormycosis. Med. Mycol. 2018, 56, 93–101. [Google Scholar] [CrossRef]
- Dannaoui, E. Antifungal Resistance in Mucorales. Int. J. Antimicrob. Agents 2017, 50, 617–621. [Google Scholar] [CrossRef]
- Rudramurthy, S.M.; Hoenigl, M.; Meis, J.F.; Cornely, O.A.; Muthu, V.; Gangneux, J.P.; Perfect, J.; Chakrabarti, A. ECMM and ISHAM ECMM/ISHAM Recommendations for Clinical Management of COVID-19 Associated Mucormycosis in Low- and Middle-Income Countries. Mycoses 2021, 64, 1028–1037. [Google Scholar] [CrossRef]
- Laturiya, R.; Badal, S.; Doiphode, A.; Nagargoje, G.; Bhale, S.; Sonare, M.; Student, P. Rising Incidence of Mucormycosis during Covid 19: A Review. J. Dent. Res. 2020, 2, 5. [Google Scholar]
- Saldanha, M.; Reddy, R.; Vincent, M.J. Title of the Article: Paranasal Mucormycosis in COVID-19 Patient. Indian J. Otolaryngol. Head Neck Surg. 2021, 1–4. [Google Scholar] [CrossRef]
- Noor, A.; Preuss, C.V. Amphotericin B. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Salehi, M.; Shahi, F.; Rizvi, F.S.; Ghaderkhani, S.; Zainaldain, H.; Khodavaisy, S.; Jamali-Moghaddam, S.R.; Dehghan Manshadi, S.A.; Rezahosseini, O. Combination Antifungal Therapy without Craniotomy in an Immunocompromised Patient with Rhino-Orbito-Cerebral Mucormycosis: A Case Report. Caspian J. Intern. Med. 2020, 11, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Raj, P.; Vella, E.J.; Bickerton, R.C. Successful Treatment of Rhinocerebral Mucormycosis by a Combination of Aggressive Surgical Debridement and the Use of Systemic Liposomal Amphotericin B and Local Therapy with Nebulized Amphotericin—A Case Report. J. Laryngol. Otol. 1998, 112, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Ibrahim, A.S. Recent Advances in the Treatment of Mucormycosis. Curr. Infect. Dis. Rep. 2010, 12, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Page, A.V.; Liles, W.C. Posaconazole: A New Agent for the Prevention and Management of Severe, Refractory or Invasive Fungal Infections. Can. J. Infect. Dis. Med. Microbiol. 2008, 19, 297–305. [Google Scholar] [CrossRef]
- Spellberg, B.; Ibrahim, A.S.; Chin-Hong, P.V.; Kontoyiannis, D.P.; Morris, M.I.; Perfect, J.R.; Fredricks, D.; Brass, E.P. The Deferasirox–AmBisome Therapy for Mucormycosis (DEFEAT Mucor) Study: A Randomized, Double-Blinded, Placebo-Controlled Trial. J. Antimicrob. Chemother. 2012, 67, 715–722. [Google Scholar] [CrossRef]
- Sun, Q.N.; Fothergill, A.W.; McCarthy, D.I.; Rinaldi, M.G.; Graybill, J.R. In Vitro Activities of Posaconazole, Itraconazole, Voriconazole, Amphotericin B, and Fluconazole against 37 Clinical Isolates of Zygomycetes. Antimicrob. Agents Chemother. 2002, 46, 1581–1582. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Chakrabarti, A.; Chowdhary, A.; Cordoba, S.; Dannaoui, E.; Dufresne, P.; Fothergill, A.; Ghannoum, M.; Gonzalez, G.M.; Guarro, J.; et al. Multicenter Evaluation of MIC Distributions for Epidemiologic Cutoff Value Definition to Detect Amphotericin B, Posaconazole, and Itraconazole Resistance among the Most Clinically Relevant Species of Mucorales. Antimicrob. Agents Chemother. 2015, 59, 1745–1750. [Google Scholar] [CrossRef]
- Zurl, C.; Hoenigl, M.; Schulz, E.; Hatzl, S.; Gorkiewicz, G.; Krause, R.; Eller, P.; Prattes, J. Autopsy Proven Pulmonary Mucormycosis Due to Rhizopus Microsporus in a Critically Ill COVID-19 Patient with Underlying Hematological Malignancy. J. Fungi 2021, 7, 88. [Google Scholar] [CrossRef]
- Vazquez, L.; Mateos, J.J.; Sanz-Rodriguez, C.; Perez, E.; Caballero, D.; San Miguel, J.F. Successful Treatment of Rhinocerebral Zygomycosis with a Combination of Caspofungin and Liposomal Amphotericin B. Haematologica 2005, 90, ECR39. [Google Scholar]
- Ojeda-Uribe, M.; Herbrecht, R.; Kiefer, M.H.; Schultz, P.; Chain, J.; Chenard, M.-P.; Servant, J.M.; Debry, C. Lessons from a Case of Oromandibular Mucormycosis Treated with Surgery and a Combination of Amphotericin B Lipid Formulation plus Caspofungin. Acta Haematol. 2010, 124, 98–102. [Google Scholar] [CrossRef]
- Ogawa, T.; Takezawa, K.; Tojima, I.; Shibayama, M.; Kouzaki, H.; Ishida, M.; Okabe, H.; Shimizu, T. Successful Treatment of Rhino-Orbital Mucormycosis by a New Combination Therapy with Liposomal Amphotericin B and Micafungin. Auris Nasus Larynx 2012, 39, 224–228. [Google Scholar] [CrossRef]
- Ribeiro, E.F.O.; dos Santos, V.M.; Paixão, G.T.G.; Cruz, L.R.; Danilow, M.Z.; Campos, V.F. Mucormycosis in a Patient with Acute Myeloid Leukemia Successfully Treated with Liposomal Amphotericin B Associated with Deferasirox and Hyperbaric Oxygen. Mycopathologia 2013, 175, 295–300. [Google Scholar] [CrossRef]
- Jensen, T.S.R.; Arendrup, M.C.; von Buchvald, C.; Frandsen, T.L.; Juhler, M.; Nygaard, U. Successful Treatment of Rhino-Orbital-Cerebral Mucormycosis in a Child With Leukemia. J. Pediatr. Hematol. Oncol. 2017, 39, e211–e215. [Google Scholar] [CrossRef]
- Grimaldi, D.; Pradier, O.; Hotchkiss, R.S.; Vincent, J.-L. Nivolumab plus Interferon-γ in the Treatment of Intractable Mucormycosis. Lancet Infect. Dis. 2017, 17, 18. [Google Scholar] [CrossRef]
- Di Pentima, M.C.; Chan, S.; Powell, J.; Napoli, J.A.; Walter, A.W.; Walsh, T.J. Topical Amphotericin B in Combination with Standard Therapy for Severe Necrotizing Skin and Soft-Tissue Mucormycosis in an Infant with Bilineal Leukemia: Case Report and Review. J. Pediatr. Hematol. Oncol. 2014, 36, e468–e470. [Google Scholar] [CrossRef]
- Pomorska, A.; Malecka, A.; Jaworski, R.; Radon-Proskura, J.; Hare, R.K.; Nielsen, H.V.; Andersen, L.O.; Jensen, H.E.; Arendrup, M.C.; Irga-Jaworska, N. Isavuconazole in a Successful Combination Treatment of Disseminated Mucormycosis in a Child with Acute Lymphoblastic Leukaemia and Generalized Haemochromatosis: A Case Report and Review of the Literature. Mycopathologia 2019, 184, 81–88. [Google Scholar] [CrossRef]
- Fatemizadeh, R.; Rodman, E.; Demmler-Harrison, G.J.; Dinu, D. Rhizopus Infection in a Preterm Infant: A Novel Use of Posaconazole. Pediatr. Infect. Dis. J. 2020, 39, 310–312. [Google Scholar] [CrossRef]
- Gargouri, M.; Marrakchi, C.; Feki, W.; Charfi, S.; Maaloul, I.; Lahiani, D.; Elleuch, E.; Koubaa, M.; Mnif, Z.; Ayadi, A.; et al. Combination of Amphotericin B and Caspofungin in the Treatment of Mucormycosis. Med. Mycol Case Rep. 2019, 26, 32–37. [Google Scholar] [CrossRef]
- Roux, B.G.-L.; Méchinaud, F.; Gay-Andrieu, F.; Lortholary, O.; Dannaoui, E.; Hoinard, D.; Corradini, N. Successful Triple Combination Therapy of Disseminated Absidia Corymbifera Infection in an Adolescent with Osteosarcoma. J. Pediatr. Hematol. Oncol. 2010, 32, 131–133. [Google Scholar] [CrossRef]
- Weng, T.-F.; Ho, M.-W.; Lin, H.-C.; Lu, M.-Y.; Peng, C.-T.; Wu, K.-H. Successful Treatment of Disseminated Mixed Invasive Fungal Infection after Hematopoietic Stem Cell Transplantation for Severe Aplastic Anemia. Pediatr. Transplant. 2012, 16, E35–E38. [Google Scholar] [CrossRef]
- Gupta, A.; Jain, S.; Agrawal, C.; Kapoor, G. Successful Outcome of Mucormycosis in Two Children on Induction Therapy for Acute Lymphoblastic Leukemia. Indian J. Med. Paediatr. Oncol. 2013, 34, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Carceller, F.; Oñoro, G.; Buitrago, M.J.; Herrero, B.; Lassaletta, Á.; Pérez-Martínez, A.; González-Vicent, M.; Madero, L. Cunninghamella Bertholletiae Infection in Children: Review and Report of 2 Cases with Disseminated Infection. J. Pediatr. Hematol. Oncol. 2014, 36, e109–e114. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, O.; Van Delden, C.; Garbino, J.; Robert, J.; Lamoth, F.; Passweg, J.; Chalandon, Y. Disseminated Rhizopus Microsporus Infection Cured by Salvage Allogeneic Hematopoietic Stem Cell Transplantation, Antifungal Combination Therapy, and Surgical Resection. Transpl. Infect. Dis. 2010, 12, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Busca, A.; Marmont, F.; Locatelli, F.; Limerutti, G.; Sorrentino, M.T.; Barbui, A.; Patrono, D.; Salizzoni, M.; David, E.; De Rosa, F. Combined Antifungal Therapy, Iron Chelation and Surgical Resection as Treatment of Hepatic Zygomycosis in a Patient with Haematological Malignancy. Mycoses 2010, 53, 275–278. [Google Scholar] [CrossRef]
- Kazak, E.; Aslan, E.; Akalın, H.; Saraydaroğlu, O.; Hakyemez, B.; Erişen, L.; Yazıcı, B.; Gürcüoğlu, E.; Yılmaz, E.; Ener, B.; et al. A Mucormycosis Case Treated with a Combination of Caspofungin and Amphotericin B. J. Mycol. Med. 2013, 23, 179–184. [Google Scholar] [CrossRef]
- Christenson, J.C.; Shalit, I.; Welch, D.F.; Guruswamy, A.; Marks, M.I. Synergistic Action of Amphotericin B and Rifampin against Rhizopus Species. Antimicrob. Agents Chemother. 1987, 31, 1775–1778. [Google Scholar] [CrossRef][Green Version]
- Bernardo, R.M.; Gurung, A.; Jain, D.; Malinis, M.F. Therapeutic Challenges of Hepatic Mucormycosis in Hematologic Malignancy: A Case Report and Review of the Literature. Am. J. Case Rep. 2016, 17, 484–489. [Google Scholar] [CrossRef]
- Reed, C.; Bryant, R.; Ibrahim, A.S.; Edwards, J.; Filler, S.G.; Goldberg, R.; Spellberg, B. Combination Polyene-Caspofungin Treatment of Rhino-Orbital-Cerebral Mucormycosis. Clin. Infect. Dis. 2008, 47, 364–371. [Google Scholar] [CrossRef]
- Pakdel, F.; Ahmadikia, K.; Salehi, M.; Tabari, A.; Jafari, R.; Mehrparvar, G.; Rezaie, Y.; Rajaeih, S.; Alijani, N.; Barac, A.; et al. Mucormycosis in Patients with COVID-19: A Cross-Sectional Descriptive Multicentre Study from Iran. Mycoses 2021, 64, 1238–1252. [Google Scholar] [CrossRef]
- Muthu, V.; Rudramurthy, S.M.; Chakrabarti, A.; Agarwal, R. Epidemiology and Pathophysiology of COVID-19-Associated Mucormycosis: India Versus the Rest of the World. Mycopathologia 2021, 186, 739–754. [Google Scholar] [CrossRef]
- Dallalzadeh, L.O.; Ozzello, D.J.; Liu, C.Y.; Kikkawa, D.O.; Korn, B.S. Secondary Infection with Rhino-Orbital Cerebral Mucormycosis Associated with COVID-19. Orbit 2021, 1–4. [Google Scholar] [CrossRef]
- Laihad, F.M.; Sudiana, I.K. Literature Review: Hyperbaric oxygen therapy on mucormycosis infection in oral cavity. Folia Med. Indones. 2017, 53, 163. [Google Scholar] [CrossRef]
- Senniappan, K.; Jeyabalan, S.; Rangappa, P.; Kanchi, M. Hyperbaric Oxygen Therapy: Can It Be a Novel Supportive Therapy in COVID-19? Indian J. Anaesth. 2020, 64, 835–841. [Google Scholar] [CrossRef]
- Malhotra, H.S.; Gupta, P.; Mehrotra, D.; Dandu, H.; Kohli, N.; Verma, V.; Kaur, A.; Kumar, N.; Prabhu, V.; Singh, M.K.; et al. COVID-19 Associated Mucormycosis: Staging and Management Recommendations (Report of a Multi-Disciplinary Expert Committee). J. Oral Biol. Craniofac. Res. 2021. [Google Scholar] [CrossRef]
- Brunet, K.; Rammaert, B. Mucormycosis Treatment: Recommendations, Latest Advances, and Perspectives. J. Mycol. Med. 2020, 30, 101007. [Google Scholar] [CrossRef]
- Cravedi, P.; Ruggenenti, P.; Remuzzi, G. Sirolimus for Calcineurin Inhibitors in Organ Transplantation: Contra. Kidney Int. 2010, 78, 1068–1074. [Google Scholar] [CrossRef]
- Vellanki, S.; Billmyre, R.B.; Lorenzen, A.; Campbell, M.; Turner, B.; Huh, E.Y.; Heitman, J.; Lee, S.C. A Novel Resistance Pathway for Calcineurin Inhibitors in the Human-Pathogenic Mucorales Mucor Circinelloides. mBio 2020, 11, e02949-19. [Google Scholar] [CrossRef]
- Calo, S.; Shertz-Wall, C.; Lee, S.C.; Bastidas, R.J.; Nicolás, F.E.; Granek, J.A.; Mieczkowski, P.; Torres-Martínez, S.; Ruiz-Vázquez, R.M.; Cardenas, M.E.; et al. Antifungal Drug Resistance Evoked via RNAi-Dependent Epimutations. Nature 2014, 513, 555–558. [Google Scholar] [CrossRef]
- Thakur, M.; Revankar, S.G. In Vitro Interaction of Caspofungin and Immunosuppressives against Agents of Mucormycosis. J Antimicrob. Chemother. 2011, 66, 2312–2314. [Google Scholar] [CrossRef]
- Lewis, R.E.; Ben-Ami, R.; Best, L.; Albert, N.; Walsh, T.J.; Kontoyiannis, D.P. Tacrolimus Enhances the Potency of Posaconazole against Rhizopus Oryzae in Vitro and in an Experimental Model of Mucormycosis. J. Infect. Dis. 2013, 207, 834–841. [Google Scholar] [CrossRef]
- Singh, N.; Aguado, J.M.; Bonatti, H.; Forrest, G.; Gupta, K.L.; Safdar, N.; John, G.T.; Pursell, K.J.; Muñoz, P.; Patel, R.; et al. Zygomycosis in Solid Organ Transplant Recipients: A Prospective, Matched Case-Control Study to Assess Risks for Disease and Outcome. J. Infect. Dis. 2009, 200, 1002–1011. [Google Scholar] [CrossRef]
- Bastidas, R.J.; Shertz, C.A.; Lee, S.C.; Heitman, J.; Cardenas, M.E. Rapamycin Exerts Antifungal Activity in Vitro and in Vivo against Mucor Circinelloides via FKBP12-Dependent Inhibition of Tor. Eukaryot. Cell 2012, 11, 270–281. [Google Scholar] [CrossRef]
- Cruz, M.C.; Goldstein, A.L.; Blankenship, J.; Del Poeta, M.; Perfect, J.R.; McCusker, J.H.; Bennani, Y.L.; Cardenas, M.E.; Heitman, J. Rapamycin and Less Immunosuppressive Analogs Are Toxic to Candida Albicans and Cryptococcus Neoformans via FKBP12-Dependent Inhibition of TOR. Antimicrob. Agents Chemother. 2001, 45, 3162–3170. [Google Scholar] [CrossRef]
- Vakil, R.; Knilans, K.; Andes, D.; Kwon, G.S. Combination Antifungal Therapy Involving Amphotericin B, Rapamycin and 5-Fluorocytosine Using PEG-Phospholipid Micelles. Pharm. Res. 2008, 25, 2056–2064. [Google Scholar] [CrossRef]
- Leonardelli, F.; Macedo, D.; Dudiuk, C.; Theill, L.; Cabeza, M.S.; Gamarra, S.; Garcia-Effron, G. In Vitro Activity of Combinations of Zinc Chelators with Amphotericin B and Posaconazole against Six Mucorales Species. Antimicrob. Agents Chemother. 2019, 63, e00266-19. [Google Scholar] [CrossRef]
- Drogari-Apiranthitou, M.; Mantopoulou, F.-D.; Skiada, A.; Kanioura, L.; Grammatikou, M.; Vrioni, G.; Mitroussia-Ziouva, A.; Tsakris, A.; Petrikkos, G. In Vitro Antifungal Susceptibility of Filamentous Fungi Causing Rare Infections: Synergy Testing of Amphotericin B, Posaconazole and Anidulafungin in Pairs. J. Antimicrob. Chemother. 2012, 67, 1937–1940. [Google Scholar] [CrossRef]
- Gebremariam, T.; Wiederhold, N.P.; Fothergill, A.W.; Garvey, E.P.; Hoekstra, W.J.; Schotzinger, R.J.; Patterson, T.F.; Filler, S.G.; Ibrahim, A.S. VT-1161 Protects Immunosuppressed Mice from Rhizopus Arrhizus Var. Arrhizus Infection. Antimicrob. Agents Chemother. 2015, 59, 7815–7817. [Google Scholar] [CrossRef]
- Gebremariam, T.; Alkhazraji, S.; Lin, L.; Wiederhold, N.P.; Garvey, E.P.; Hoekstra, W.J.; Schotzinger, R.J.; Patterson, T.F.; Filler, S.G.; Ibrahim, A.S. Prophylactic Treatment with VT-1161 Protects Immunosuppressed Mice from Rhizopus Arrhizus Var. Arrhizus Infection. Antimicrob. Agents Chemother. 2017, 61, e00390-17. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.J.; Ibrahim, A.S. Fosmanogepix: A Review of the First-in-Class Broad Spectrum Agent for the Treatment of Invasive Fungal Infections. J. Fungi 2020, 6, 239. [Google Scholar] [CrossRef] [PubMed]
- Covel, J.; Soltow, Q.; Kapoor, M.; Moloney, M.; Webb, P.; Trzoss, M.; Sharp, M.; Shaw, K. The Discovery of Manogepix/Fosmanogepix and Other Gwt1 Inhibitors for the Treatment of Invasive Fungal Infections. In 2019 Medicinal Chemistry Reviews; MEDI: Randolph, MA, USA, 2019; pp. 221–237. ISBN 978-0-9962932-8-0. [Google Scholar]
- Gebremariam, T.; Alkhazraji, S.; Alqarihi, A.; Wiederhold, N.P.; Shaw, K.J.; Patterson, T.F.; Filler, S.G.; Ibrahim, A.S. Fosmanogepix (APX001) Is Effective in the Treatment of Pulmonary Murine Mucormycosis Due to Rhizopus Arrhizus. Antimicrob. Agents Chemother. 2020, 64, e00178-20. [Google Scholar] [CrossRef] [PubMed]
- Amplyx Pharmaceuticals. A Phase 2, Open-Label Study to Evaluate the Safety and Efficacy of APX001 in the Treatment of Patients with Invasive Mold Infections Caused by Aspergillus Species or Rare Molds; 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04240886 (accessed on 12 February 2022).
- Ben Yaakov, D.; Rivkin, A.; Mircus, G.; Albert, N.; Dietl, A.-M.; Kovalerchick, D.; Carmeli, S.; Haas, H.; Kontoyiannis, D.P.; Osherov, N. Identification and Characterization of Haemofungin, a Novel Antifungal Compound That Inhibits the Final Step of Haem Biosynthesis. J. Antimicrob. Chemother. 2016, 71, 946–952. [Google Scholar] [CrossRef]
- Colley, T.; Sehra, G.; Chowdhary, A.; Alanio, A.; Kelly, S.L.; Kizawa, Y.; Armstrong-James, D.; Fisher, M.C.; Warrilow, A.G.S.; Parker, J.E.; et al. In Vitro and In Vivo Efficacy of a Novel and Long-Acting Fungicidal Azole, PC1244, on Aspergillus Fumigatus Infection. Antimicrob. Agents Chemother. 2018, 62, e01941-17. [Google Scholar] [CrossRef]
- Watkins, T.N.; Gebremariam, T.; Swidergall, M.; Shetty, A.C.; Graf, K.T.; Alqarihi, A.; Alkhazraji, S.; Alsaadi, A.I.; Edwards, V.L.; Filler, S.G.; et al. Inhibition of EGFR Signaling Protects from Mucormycosis. mBio 2018, 9, e01384-18. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Lewis, R.E.; Tarrand, J.; Leventakos, K.; Kontoyiannis, D.P. Antifungal Activity of Colistin against Mucorales Species in Vitro and in a Murine Model of Rhizopus Oryzae Pulmonary Infection. Antimicrob. Agents Chemother. 2010, 54, 484–490. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Messer, S.A.; Georgopapadakou, N.; Martell, L.A.; Besterman, J.M.; Diekema, D.J. Activity of MGCD290, a Hos2 Histone Deacetylase Inhibitor, in Combination with Azole Antifungals against Opportunistic Fungal Pathogens. J. Clin. Microbiol. 2009, 47, 3797–3804. [Google Scholar] [CrossRef]
- Biswas, C.; Sorrell, T.C.; Djordjevic, J.T.; Zuo, X.; Jolliffe, K.A.; Chen, S.C.-A. In Vitro Activity of Miltefosine as a Single Agent and in Combination with Voriconazole or Posaconazole against Uncommon Filamentous Fungal Pathogens. J. Antimicrob. Chemother. 2013, 68, 2842–2846. [Google Scholar] [CrossRef]
- Roze, L.V.; Linz, J.E. Lovastatin Triggers an Apoptosis-like Cell Death Process in the Fungus Mucor Racemosus. Fungal. Genet. Biol. 1998, 25, 119–133. [Google Scholar] [CrossRef]
- Chamilos, G.; Lewis, R.E.; Kontoyiannis, D.P. Lovastatin Has Significant Activity against Zygomycetes and Interacts Synergistically with Voriconazole. Antimicrob. Agents Chemother. 2006, 50, 96–103. [Google Scholar] [CrossRef]
- Naeimi Eshkaleti, M.; Kordbacheh, P.; Hashemi, S.J.; Falahati, M.; Zaini, F.; Mirhendi, H.; Safara, M.; Hosseinpoor, L. In Vitro Activity of Amphotericin B in Combination with Statins against Clinical and Environmental Rhizopus Oryzae Strains. Iran. J. Public Health 2019, 48, 943–948. [Google Scholar] [CrossRef]
- Dannaoui, E.; Afeltra, J.; Meis, J.F.G.M.; Verweij, P.E. Eurofung Network In Vitro Susceptibilities of Zygomycetes to Combinations of Antimicrobial Agents. Antimicrob. Agents Chemother. 2002, 46, 2708–2711. [Google Scholar] [CrossRef]
- Sugar, A.M.; Liu, X.P. Combination Antifungal Therapy in Treatment of Murine Pulmonary Mucormycosis: Roles of Quinolones and Azoles. Antimicrob. Agents Chemother. 2000, 44, 2004–2006. [Google Scholar] [CrossRef] [PubMed]
- Gebremariam, T.; Alkhazraji, S.; Soliman, S.S.M.; Gu, Y.; Jeon, H.H.; Zhang, L.; French, S.W.; Stevens, D.A.; Edwards, J.E.; Filler, S.G.; et al. Anti-CotH3 Antibodies Protect Mice from Mucormycosis by Prevention of Invasion and Augmenting Opsonophagocytosis. Sci. Adv. 2019, 5, eaaw1327. [Google Scholar] [CrossRef]
- Abzug, M.J.; Walsh, T.J. Interferon-Gamma and Colony-Stimulating Factors as Adjuvant Therapy for Refractory Fungal Infections in Children. Pediatr. Infect. Dis. J. 2004, 23, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Sam, Q.H.; Yew, W.S.; Seneviratne, C.J.; Chang, M.W.; Chai, L.Y.A. Immunomodulation as Therapy for Fungal Infection: Are We Closer? Front. Microbiol. 2018, 9, 1612. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tang, J.; Sun, Y.; Gao, L. Effects of Photodynamic Inactivation on the Growth and Antifungal Susceptibility of Rhizopus Oryzae. Mycopathologia 2019, 184, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, F.; Pontikos, M.A.; Walsh, T.J.; Albert, N.; Lewis, R.E.; Kontoyiannis, D.P. Hyperthermia Sensitizes Rhizopus Oryzae to Posaconazole and Itraconazole Action through Apoptosis. Antimicrob. Agents Chemother. 2013, 57, 4360–4368. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.K.; Dasgupta, A.; Taneja, N.; Chaubey, S.; Gabrani, R.; Sharma, S.K.; Gupta, S. Putative Drug Targets in Rhizopus Oryzae: In-Silico Insight. Int. J. Bioinform. Res. Appl. 2013, 9, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.D.; Kaur, I. Targeting β-Glucan Synthase for Mucormycosis “The 'Black Fungus” Maiming COVID Patients in India: Computational Insights. J. Drug Deliv. Ther. 2021, 11, 9–14. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madhavan, Y.; Sai, K.V.; Shanmugam, D.K.; Manimaran, A.; Guruviah, K.; Mohanta, Y.K.; Venugopal, D.C.; Mohanta, T.K.; Sharma, N.; Muthupandian, S. Current Treatment Options for COVID-19 Associated Mucormycosis: Present Status and Future Perspectives. J. Clin. Med. 2022, 11, 3620. https://doi.org/10.3390/jcm11133620
Madhavan Y, Sai KV, Shanmugam DK, Manimaran A, Guruviah K, Mohanta YK, Venugopal DC, Mohanta TK, Sharma N, Muthupandian S. Current Treatment Options for COVID-19 Associated Mucormycosis: Present Status and Future Perspectives. Journal of Clinical Medicine. 2022; 11(13):3620. https://doi.org/10.3390/jcm11133620
Chicago/Turabian StyleMadhavan, Yasasve, Kadambari Vijay Sai, Dilip Kumar Shanmugam, Aashabharathi Manimaran, Karthigadevi Guruviah, Yugal Kishore Mohanta, Divyambika Catakapatri Venugopal, Tapan Kumar Mohanta, Nanaocha Sharma, and Saravanan Muthupandian. 2022. "Current Treatment Options for COVID-19 Associated Mucormycosis: Present Status and Future Perspectives" Journal of Clinical Medicine 11, no. 13: 3620. https://doi.org/10.3390/jcm11133620
APA StyleMadhavan, Y., Sai, K. V., Shanmugam, D. K., Manimaran, A., Guruviah, K., Mohanta, Y. K., Venugopal, D. C., Mohanta, T. K., Sharma, N., & Muthupandian, S. (2022). Current Treatment Options for COVID-19 Associated Mucormycosis: Present Status and Future Perspectives. Journal of Clinical Medicine, 11(13), 3620. https://doi.org/10.3390/jcm11133620











