Association Analysis of the Cerebral Fractional Tissue Oxygen Extraction (cFTOE) and the Cerebral Oxygen Saturation (crSaO2) with Perinatal Factors in Preterm Neonates: A Single Centre Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Analysed Parameters
2.2.1. Brain Oxygenation
2.2.2. Oxygen Saturation and Blood Pressure
2.2.3. Head Ultrasound
2.2.4. pH Gas Value
2.2.5. Clinical Variables
2.3. Statistical Analysis
3. Results
3.1. Description of Preterm Neonates Sample
3.2. Relationship between cFTOE, crSaO2 and Maternal Pathology
3.3. Relationship between Cerebral Fractional Tissue Oxygen Extraction (cFTOE) and Cerebral Oxygen Saturation (crSaO2) with Demographic and Clinical Characteristics of Premature Neonates: Bivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lemmers, P.M.A.; Toet, M.; Van Schelven, L.J.; Van Bel, F. Cerebral oxygenation and cerebral oxygen extraction in the preterm infant: The impact of respiratory distress syndrome. Exp. Brain Res. 2006, 173, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Matyas, M.; Hasmasanu, M.; Silaghi, C.N.; Samasca, G.; Lupan, I.; Orsolya, K.; Zaharie, G. Early Preeclampsia Effect on Preterm Newborns Outcome. J. Clin. Med. 2022, 11, 452. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, E.A.; Keating, P.; Ter Horst, H.J.; Martijn, A.; Bos, A.F. Cerebral oxygen saturation and extraction in preterm infants with transient periventricular echodensities. Pediatrics 2009, 124, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Naulaers, G.; Meyns, B.; Miserez, M.; Leunens, V.; Van Huffel, S.; Casaer, P.; Weindling, M.; Devlieger, H. Use of tissue oxygenation index and fractional tissue oxygen extraction as non-invasive parameters for cerebral oxygenation. A validation study in piglets. Neonatology 2007, 92, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.W.; Hadway, J.; Lee, T.Y. Near-infrared spectroscopy measurement of oxygen extraction fraction and cerebral metabolic rate of oxygen in newborn piglets. Pediatric Res. 2003, 54, 861–867. [Google Scholar] [CrossRef] [Green Version]
- Dix, L.M.L.; van Bel, F.; Lemmers, P.M.A. Monitoring cerebral oxygenation in neonates: An update. Front. Pediatrics 2017, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Vesoulis, Z.A.; Mintzer, J.P.; Chock, V.Y. Neonatal NIRS monitoring: Recommendations for data capture and review of analytics. J. Perinatol. 2021, 41, 675–688. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy Hypertension in Pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Plavka, R.; Saugstad, O.D.; Simeoni, U.; Speer, C.P.; Vento, M.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2016 Update. Neonatology 2017, 111, 107–125. [Google Scholar] [CrossRef] [Green Version]
- Jeffrey, M.; Perlman, J.J.V. Chapter 24—Preterm Intraventricular hemorrhage. In Volpe’s Neurology of the Newborn; Elsevier: Amsterdam, The Netherlands, 2018; pp. 637–745.e4. [Google Scholar]
- Robertson, C.; Perlamn, M. Follow-up of the term infant after hypoxic–ischemic encephalopathy. Paediatrics Child Health 2006, 11, 278–282. [Google Scholar] [CrossRef]
- Thewissen, L.; Pistorius, L.; Baerts, W.; Naulaers, G.; Van Bel, F.; Lemmers, P. Neonatal haemodynamic effects following foetal exposure to labetalol in hypertensive disorders of pregnancy. J. Matern. Fetal Neonatal Med. 2017, 30, 1533–1538. [Google Scholar] [CrossRef] [Green Version]
- Walter, L.M.; Ahmed, B.; Odoi, A.; Cooney, H.; Horne, R.S.C.; Wong, F.Y. Bradycardias are associated with more severe effects on cerebral oxygenation in very preterm infants than in late preterm infants. Early Hum. Dev. 2018, 127, 33–41. [Google Scholar] [CrossRef]
- Pichler, G.; Urlesberger, B.; Müller, W. Impact of bradycardia on cerebral oxygenation and cerebral blood volume during apnoea in preterm infants. Physiol. Meas. 2003, 24, 671–680. [Google Scholar] [CrossRef]
- Chock, V.Y.; Kwon, S.H.; Ambalavanan, N.; Batton, B.; Nelin, L.D.; Chalak, L.F.; Tian, L.; Van Meurs, K.P. Cerebral Oxygenation and Autoregulation in Preterm Infants (Early NIRS Study). J. Pediatrics 2020, 227, 94–100.e1. [Google Scholar] [CrossRef]
- Lazea, C.; Sur, L.; Florea, M. ROHHAD (Rapid-onset Obesity with Hypoventilation, Hypothalamic Dysfunction, Autonomic Dysregulation) Syndrome-What Every Pediatrician Should Know About the Etiopathogenesis, Diagnosis and Treatment: A Review. Int. J. Gen. Med. 2021, 14, 319–326. [Google Scholar] [CrossRef]
- Liao, S.M.C.; Rao, R.; Mathur, A.M. Head position change is not associated with acute changes in bilateral cerebral oxygenation in stable preterm infants during the first three days of life. Am. J. Perinatol. 2015, 32, 645–652. [Google Scholar] [CrossRef] [Green Version]
- Alderliesten, T.; van Bel, F.; van der Aa, N.E.; Steendijk, P.; van Haastert, I.C.; de Vries, L.S.; Groenendaal, F.; Lemmers, P. Low Cerebral Oxygenation in Preterm Infants Is Associated with Adverse Neurodevelopmental Outcome. J. Pediatrics 2019, 207, 109–116.e2. [Google Scholar] [CrossRef]
- Martini, S.; Corvaglia, L. Splanchnic NIRS monitoring in neonatal care: Rationale, current applications and future perspectives. J. Perinatol. 2018, 38, 431–443. [Google Scholar] [CrossRef]
- Pichler, G.; Baumgartner, S.; Biermayr, M.; Dempsey, E.; Fuchs, H.; Goos, T.G.; Lista, G.; Lorenz, L.; Karpinski, L.; Mitra, S.; et al. Cerebral regional tissue Oxygen Saturation to Guide Oxygen Delivery in preterm neonates during immediate transition after birth (COSGOD III): An investigator-initiated, randomized, multi-center, multi-national, clinical trial on additional cerebral tissue oxygen saturation monitoring combined with defined treatment guidelines versus standard monitoring and treatment as usual in premature infants during immediate transition: Study protocol for a randomized controlled trial. Trials 2019, 20, 178. [Google Scholar] [CrossRef]
- Schmid, M.B.; Hopfner, R.J.; Lenhof, S.; Hummler, H.D.; Fuchs, H. Cerebral oxygenation during intermittent hypoxemia and bradycardia in preterm infants. Neonatology 2015, 107, 137–146. [Google Scholar] [CrossRef]
- Noori, S.; McCoy, M.; Anderson, M.P.; Ramji, F.; Seri, I. Changes in cardiac function and cerebral blood flow in relation to peri/intraventricular hemorrhage in extremely preterm infants. J. Pediatrics 2014, 164, 264–270.e3. [Google Scholar] [CrossRef]
- Zaharie, G.C.; Hăşmăşanu, M.G.; Blaga, L.; Matyas, M.; Mureșan, D.; Bolboacă, S.D. Cardiac left heart morphology and function in newborns with intrauterine growth restriction: Relevance for long-term assessment. Med. Ultrason. 2019, 21, 62–68. [Google Scholar] [CrossRef]
- Sortica da Costa, C.; Cardim, D.; Molnar, Z.; Kelsall, W.; Ng, I.; Czosnyka, M.; Smielewski, P.; Austin, T. Changes in hemodynamics, cerebral oxygenation and cerebrovascular reactivity during the early transitional circulation in preterm infants. Pediatric Res. 2019, 86, 247–253. [Google Scholar] [CrossRef]
- Kenosi, M.; O’Toole, J.M.; Livingston, V.; Hawkes, G.A.; Boylan, G.B.; O’Halloran, K.D.; Ryan, A.C.; Dempsey, E.M. Effects of Fractional Inspired Oxygen on Cerebral Oxygenation in Preterm Infants following Delivery. J. Pediatrics 2015, 167, 1007–1012.e1. [Google Scholar] [CrossRef]
- Verhagen, E.A.; Ter Horst, H.J.; Keating, P.; Martijn, A.; Van Braeckel, K.N.J.A.; Bos, A.F. Cerebral oxygenation in preterm infants with germinal matrix-intraventricular hemorrhages. Stroke 2010, 41, 2901–2907. [Google Scholar] [CrossRef] [Green Version]
- Ng, I.H.X.; Da Costa, C.S.; Zeiler, F.A.; Wong, F.Y.; Smielewski, P.; Czosnyka, M.; Austin, T. Burden of hypoxia and intraventricular haemorrhage in extremely preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 242–247. [Google Scholar] [CrossRef]
- Lin, P.Y.; Hagan, K.; Fenoglio, A.; Grant, P.E.; Franceschini, M.A. Reduced cerebral blood flow and oxygen metabolism in extremely preterm neonates with low-grade germinal matrix- intraventricular hemorrhage. Sci. Rep. 2016, 6, 25903. [Google Scholar] [CrossRef] [Green Version]
- Hyttel-Sorensen, S.; Pellicer, A.; Alderliesten, T.; Austin, T.; Van Bel, F.; Benders, M.; Claris, O.; Dempsey, E.; Franz, A.R.; Fumagalli, M.; et al. Cerebral near infrared spectroscopy oximetry in extremely preterm infants: Phase II randomised clinical trial. BMJ 2015, 350, g7635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, E.; Baerts, W.; Alderliesten, T.; Derks, J.; Lemmers, P.; van Bel, F. Growth restriction and gender influence cerebral oxygenation in preterm neonates. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F156–F161. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Frasketi, M.J.; Aly, S.; El-Dib, M.; Hoffman, H.J.; Aly, H. Changes in cerebral tissue oxygenation and fractional oxygen extraction with gestational age and postnatal maturation in preterm infants. J. Perinatol. 2021, 41, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Alderliesten, T.; Dix, L.; Baerts, W.; Caicedo, A.; Van Huffel, S.; Naulaers, G.; Groenendaal, F.; Van Bel, F.; Lemmers, P. Reference values of regional cerebral oxygen saturation during the first 3 days of life in preterm neonates. Pediatric Res. 2016, 79, 55–64. [Google Scholar] [CrossRef]
- Lorenz, L.; Marulli, A.; Dawson, J.A.; Owen, L.S.; Manley, B.J.; Donath, S.M.; Davis, P.G.; Kamlin, C.O.F. Cerebral oxygenation during skin-to-skin care in preterm infants not receiving respiratory support. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F137–F142. [Google Scholar] [CrossRef]
- Alosh, H.; Ramirez, A.; Mink, R. The correlation between brain near-infrared spectroscopy and cerebral blood flow in piglets with intracranial hypertension. J. Appl. Physiol. 2016, 121, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Eriksen, V.R.; Hahn, G.H.; Greisen, G. Dopamine therapy is associated with impaired cerebral autoregulation in preterm infants. Acta Paediatr. 2014, 103, 1221–1226. [Google Scholar] [CrossRef]
- Gilmore, M.M.; Stone, B.S.; Shepard, J.A.; Czosnyka, M.; Easley, R.B.; Brady, K.M. Relationship between cerebrovascular dysautoregulation and arterial blood pressure in the premature infant. J. Perinatol. 2011, 31, 722–729. [Google Scholar] [CrossRef] [Green Version]
| Variables | Sample Statistics |
|---|---|
| Gestational Age (weeks) | 27.98 ± 2.37 |
| Extremely premature (1) | 19 (39.58) |
| Birth weight (g) | 1035 [740, 1270] |
| Extremely_low_birth weight (2) | 23 (47.92) |
| Gender (male) | 26 (54.17) |
| RDS category | |
| No/mild | 5 (10.42) |
| Moderate | 13 (27.08) |
| Severe | 30 (62.50) |
| Asphyxia | 17 (35.42) |
| Anaemia | 21 (43.75) |
| Sepsis | 24 (50.00) |
| IVH severity | |
| Absent | 24 (50.00) |
| I–II | 17 (35.42) |
| III–IV | 7 (14.58) |
| Variables | Average crStO2 | p-Value | cFTOE | p-Value |
|---|---|---|---|---|
| Sex (b) | 0.212 | 0.282 | ||
| Male | 60.54 ± 9.31 | 0.36 ± 0.10 | ||
| Female | 64.36 ± 11.61 | 0.33 ± 0.12 | ||
| Extremely premature | 0.106 | 0.138 | ||
| Gestational age < 28 weeks | 59.26 ± 9.13 | 0.37 ± 0.09 | ||
| Gestational age ≥ 28 weeks | 64.28 ± 10.99 | 0.33 ± 0.14 | ||
| Low birth weight | 0.631 | 0.756 | ||
| Birth weight < 1000 g | 61.52 ± 9.76 | 0.35 ± 0.10 | ||
| Birth weight ≥ 1000 g | 63.00 ± 11.28 | 0.34 ± 0.12 | ||
| IVH | 0.215 | 0.244 | ||
| absent | 64.92 ± 10.16 | 0.32 ± 0.11 | ||
| I–II | 59.24 ± 11.09 | 0.38 ± 0.12 | ||
| III–IV | 60.71 ± 9.05 | 0.36 ± 0.09 | ||
| Surfactant | 0.076 | 0.129 | ||
| Yes | 59.93 ± 9.37 | 0.37 ± 0.10 | ||
| No | 65.33 ± 11.28 | 0.32 ± 0.12 | ||
| Apnoea | 0.0026 * | 0.0039 * | ||
| Yes | 56.84 ± 9.83 | 0.40 ± 0.10 | ||
| No | 65.86 ± 9.45 | 0.31 ± 0.10 | ||
| Inotropic iv | <0.0001 * | <0.0001 * | ||
| Yes | 54.29 ± 7.04 | 0.42 ± 0.08 | ||
| No | 66.68 ± 9.48 | 0.30 ± 0.10 | ||
| Neonatal sepsis | 0.106 | 0.138 | ||
| Yes | 59.26 ± 9.13 | 0.37 ± 0.09 | ||
| No | 64.28 ± 10.99 | 0.33 ± 0.14 | ||
| Metabolic acidosis | 0.147 | 0.155 | ||
| No/mild (pH > 7.2) | 66.19 ± 11.33 | 0.31 ± 0.12 | ||
| Moderate (7.0 < pH < 7.2) | 60.86 ± 9.37 | 0.36 ± 0.10 | ||
| Severe (pH < 7) | 56.75 ± 12.34 | 0.41 ± 0.12 | ||
| Hypocapnia | 0.047 * | 0.043 * | ||
| Yes | 65.04 ± 9.07 | 0.32 ± 0.09 | ||
| No | 59.05 ± 11.32 | 0.38 ± 0.12 |
| Average crSaO2 | cFTOE | |
|---|---|---|
| Variables | Correlation Coefficient ρ [95% CI] | Correlation Coefficient ρ [95% CI] |
| IR | −0.06 [−0.35, 0.25] | 0.04 [−0.26, 0.36] |
| MAP | ||
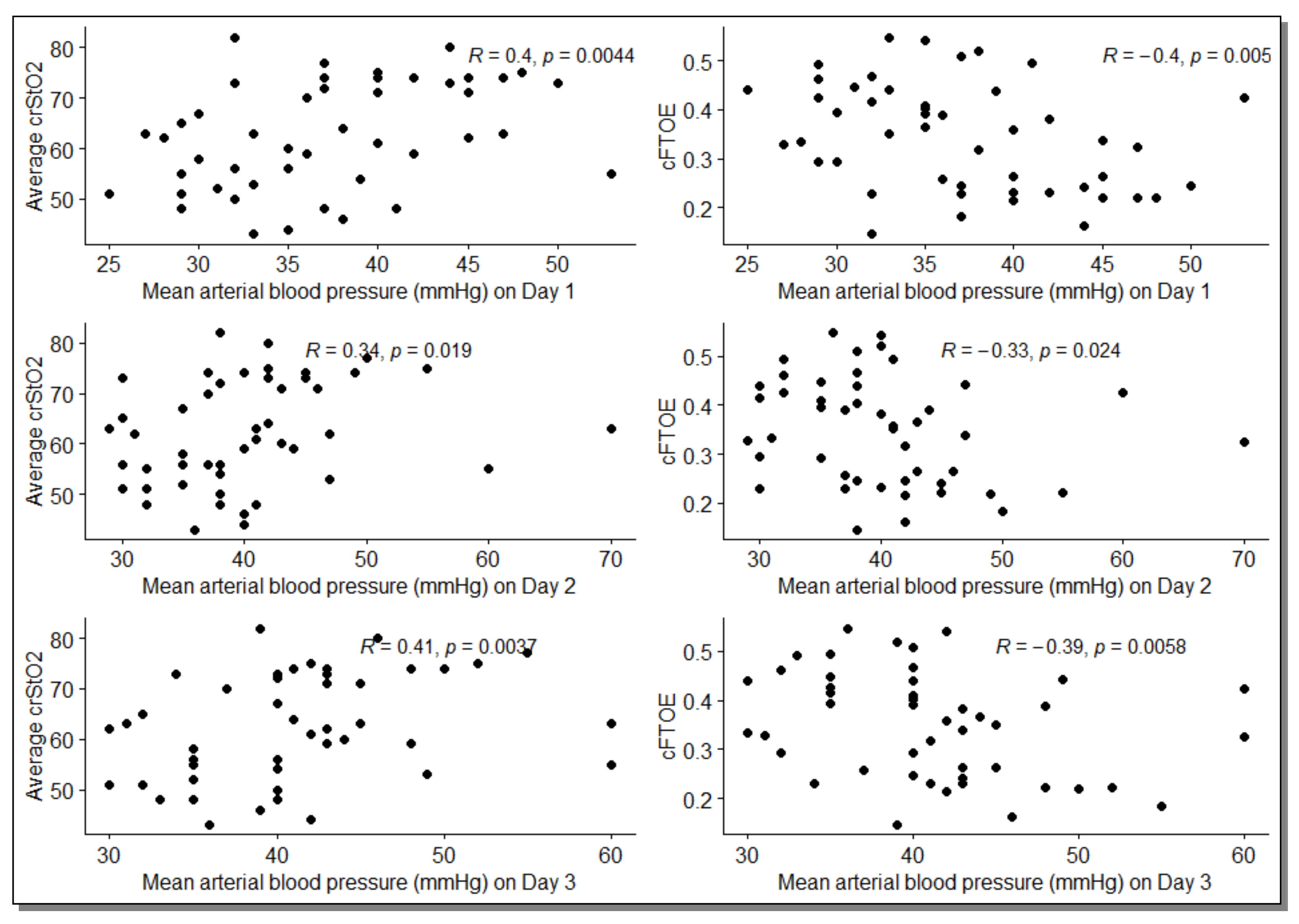
| Day 1 | 0.40 [0.15, 0.61] * | −0.40 [−0.61, −0.14] * |
| Day 2 | 0.34 [0.07, 0.56] * | −0.33 [−0.53, −0.08] * |
| Day 3 | 0.41 [0.16, 0.63] * | −0.39 [−0.62, −0.15] * |
| Day 4 (a) | 0.44 [0.15, 0.69] * | −0.41 [−0.65, −0.14] * |
| SatO2 | ||
| Day 1 | 0.10 [−0.15, 0.33] | −0.05 [−0.28, 0.21] |
| Day 2 | 0.05 [−0.25, 0.32] | 0.01 [−0.26, 0.28] |
| Day 3 | 0.32 [0.01, 0.59] * | −0.28 [−0.54, 0.04] |
| Day 4 | 0.03 [−0.31, 0.34] | 0.0001 [−0.31, 0.31] |
| SatO2_FiO2 | ||
| Day 1 (b) | 0.04 [−0.23, 0.31] | −0.02 [−0.29, 0.29] |
| Day 2 | 0.17 [−0.11, 0.43] | −0.15 [−0.41, 0.14] |
| Day 3 | 0.23 [−0.07, 0.50] | −0.23 [−0.47, 0.04] |
| Day 4 (c) | 0.21 [−0.12, 0.48] | −0.21 [−0.48, 0.09] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matyas, M.; Iancu, M.; Hasmasanu, M.; Man, A.; Zaharie, G. Association Analysis of the Cerebral Fractional Tissue Oxygen Extraction (cFTOE) and the Cerebral Oxygen Saturation (crSaO2) with Perinatal Factors in Preterm Neonates: A Single Centre Study. J. Clin. Med. 2022, 11, 3546. https://doi.org/10.3390/jcm11123546
Matyas M, Iancu M, Hasmasanu M, Man A, Zaharie G. Association Analysis of the Cerebral Fractional Tissue Oxygen Extraction (cFTOE) and the Cerebral Oxygen Saturation (crSaO2) with Perinatal Factors in Preterm Neonates: A Single Centre Study. Journal of Clinical Medicine. 2022; 11(12):3546. https://doi.org/10.3390/jcm11123546
Chicago/Turabian StyleMatyas, Melinda, Mihaela Iancu, Monica Hasmasanu, Anca Man, and Gabriela Zaharie. 2022. "Association Analysis of the Cerebral Fractional Tissue Oxygen Extraction (cFTOE) and the Cerebral Oxygen Saturation (crSaO2) with Perinatal Factors in Preterm Neonates: A Single Centre Study" Journal of Clinical Medicine 11, no. 12: 3546. https://doi.org/10.3390/jcm11123546
APA StyleMatyas, M., Iancu, M., Hasmasanu, M., Man, A., & Zaharie, G. (2022). Association Analysis of the Cerebral Fractional Tissue Oxygen Extraction (cFTOE) and the Cerebral Oxygen Saturation (crSaO2) with Perinatal Factors in Preterm Neonates: A Single Centre Study. Journal of Clinical Medicine, 11(12), 3546. https://doi.org/10.3390/jcm11123546







