SARS-CoV-2 Specific Antibody Response and T Cell-Immunity in Immunocompromised Patients up to Six Months Post COVID: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients and Blood Sampling
2.3. Real-Time PCR for Quantification of SARS-CoV-2 Viral Load
2.4. Analysis of Longitudinal SARS-CoV-2 IgG Antibodies
2.5. Lymphocyte Phenotyping by Flow Cytometry
2.6. T Cell Mediated Anti-SARS-CoV-2 Specific Immune Responses
2.7. Statistical Analyses
3. Results
3.1. Clinical Characteristics of the Study Participants
3.2. SARS-CoV-2 Viral Load
3.3. Longitudinal SARS-CoV-2 IgG Antibodies
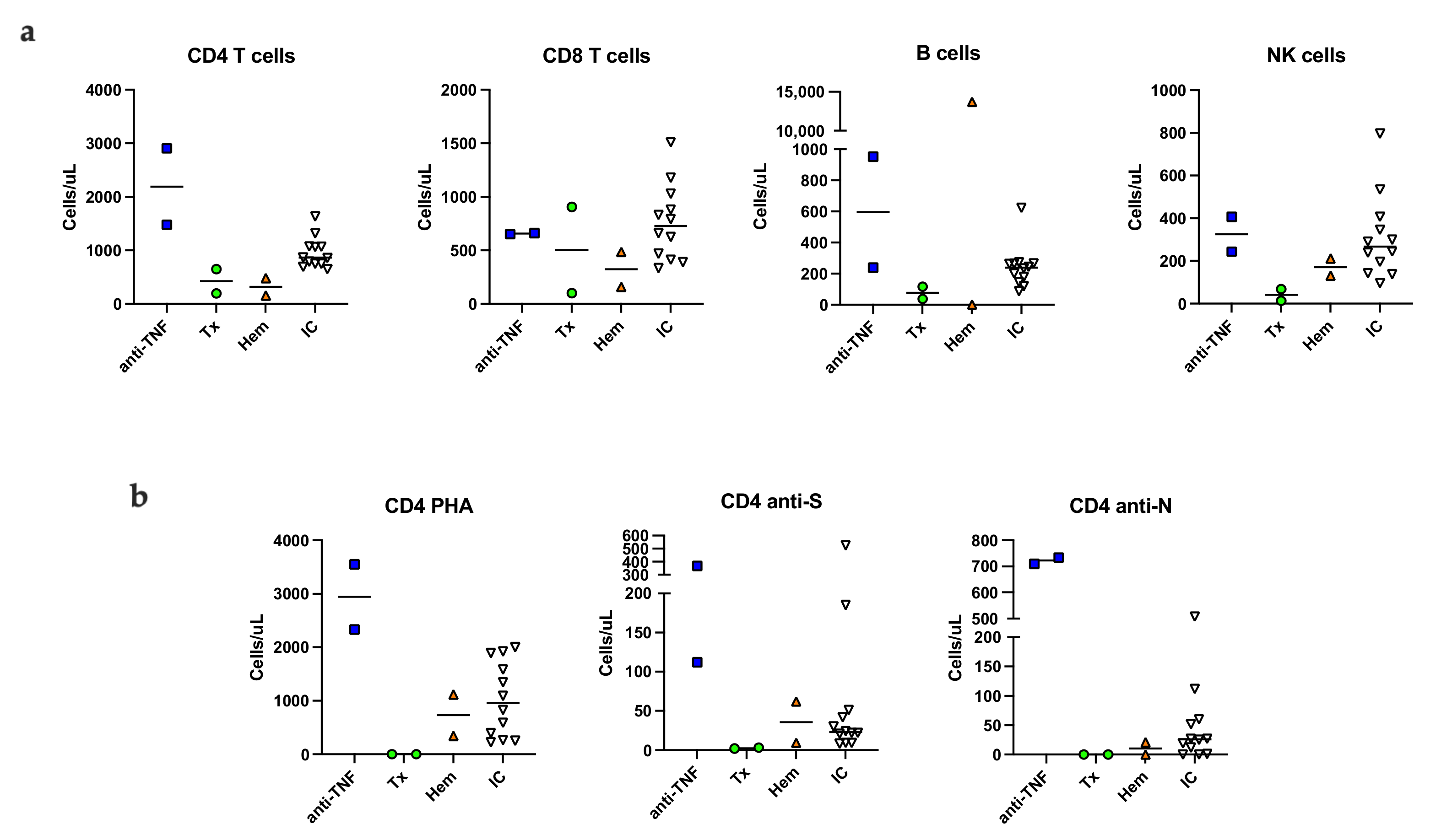
3.4. Immunological Phenotype Six Months Post Infection
3.5. SARS-CoV-2 Specific Memory T Cells Six Months Post Infection
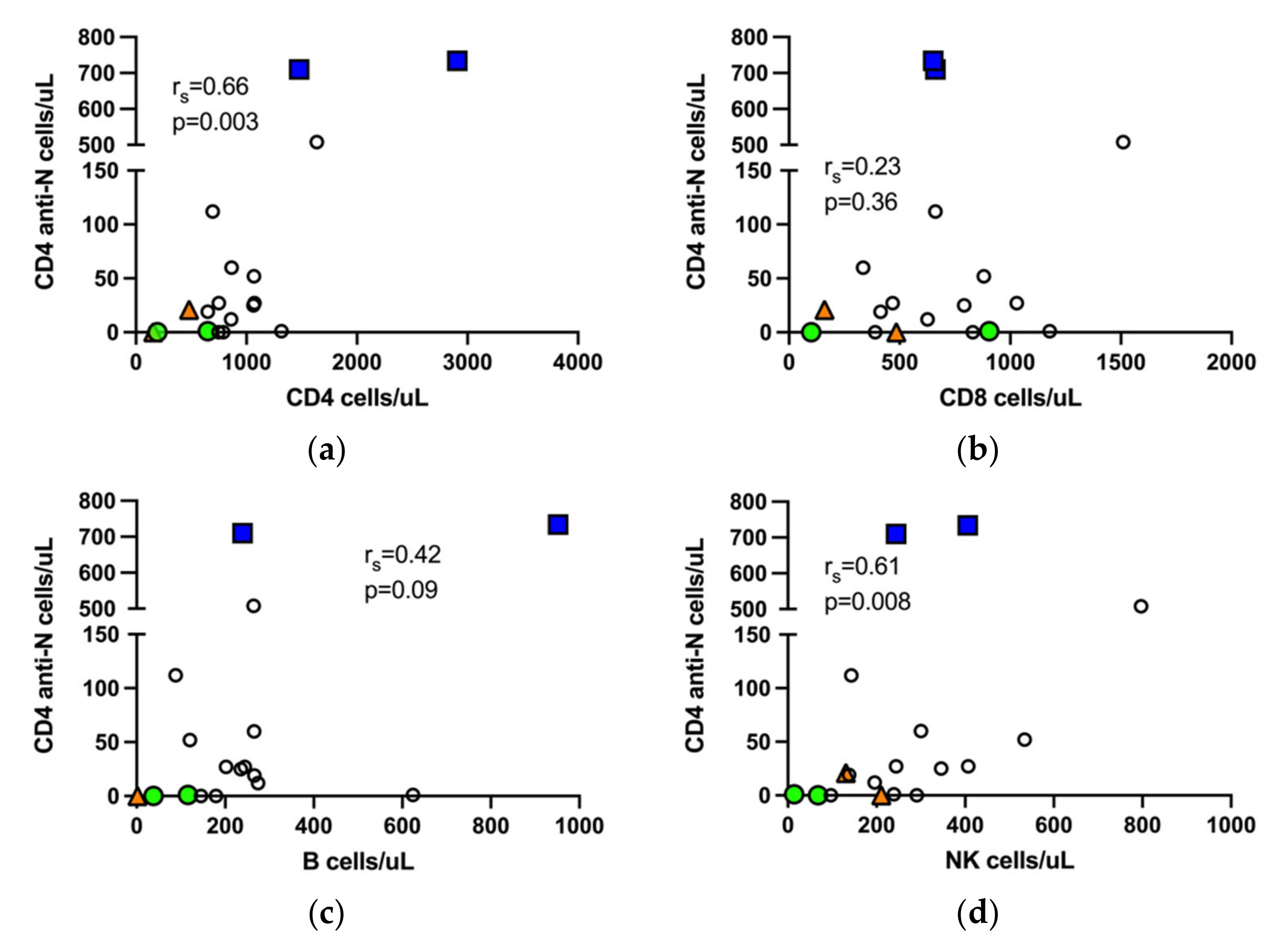
3.6. Correlation of SARS-CoV-2 Specific T Cells with NK Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baek, M.S.; Lee, M.-T.; Kim, W.-Y.; Choi, J.C.; Jung, S.-Y. COVID-19-related outcomes in immunocompromised patients: A nationwide study in Korea. PLoS ONE 2021, 16, e0257641. [Google Scholar] [CrossRef] [PubMed]
- Suárez-García, I.; Perales-Fraile, I.; González-García, A.; Muñoz-Blanco, A.; Manzano, L.; Fabregate, M.; Díez-Manglano, J.; Aizpuru, E.F.; Fernández, F.A.; García, A.G.; et al. In-hospital mortality among immunosuppressed patients with COVID-19: Analysis from a national cohort in Spain. PLoS ONE 2021, 16, e0255524. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.M.; Schlauch, D.; Mulloy, M.; Dao, A.; Reyad, A.I.; Correll, M.; Fromell, G.J.; Pittman, J.; Bingaman, A.W.; Sankarapandian, B.; et al. Outcomes of COVID-19 in hospitalized solid organ transplant recipients compared to a matched cohort of non-transplant patients at a national healthcare system in the United States. Clin. Transplant. 2021, 35, e14216. [Google Scholar] [CrossRef]
- Di Felice, G.; Visci, G.; Teglia, F.; Angelini, M.; Boffetta, P. Effect of cancer on outcome of COVID-19 patients: A systematic review and meta-analysis of studies of unvaccinated patients. eLife 2022, 11, e74634. [Google Scholar] [CrossRef] [PubMed]
- Grainger, R.; Kim, A.H.J.; Conway, R.; Yazdany, J.; Robinson, P.C. COVID-19 in people with rheumatic diseases: Risks, outcomes, treatment considerations. Nat. Rev. Rheumatol. 2022, 18, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhao, J.; Han, M.; Meng, F.; Zhou, J. SARS-CoV-2 infection in immunocompromised patients: Humoral versus cell-mediated immunity. J. Immunother. Cancer 2020, 8, e000862. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.; Suffiotti, M.; Pellaton, C.; Bouchaab, H.; Cairoli, A.; Salvadé, V.; Stevenel, C.; Hottinger, R.; Pythoud, C.; Coutechier, L.; et al. Humoral Responses Against Variants of Concern by COVID-19 mRNA Vaccines in Immunocompromised Patients. JAMA Oncol. 2022, 8, e220446. [Google Scholar] [CrossRef]
- Crombie, J.L.; Sherman, A.C.; Cheng, C.-A.; Ryan, C.E.; Zon, R.; Desjardins, M.; Baker, P.; McDonough, M.; Izaguirre, N.; Bausk, B.; et al. Activity of mRNA COVID-19 vaccines in patients with lymphoid malignancies. Blood Adv. 2021, 5, 3062–3065. [Google Scholar] [CrossRef]
- Moor, M.B.; Suter-Riniker, F.; Horn, M.P.; Aeberli, D.; Amsler, J.; Moller, B.; Njue, L.M.; Medri, C.; Angelillo-Scherrer, A.; Borradori, L.; et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): An investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021, 3, e789–e797. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA 2021, 325, 2204–2206. [Google Scholar] [CrossRef]
- Harrington, D.; Azim, T.; Rosmarin, C.; Cutino-Moguel, T.; Hopkins, M. Evaluation of 2 commercial anti-SARS-CoV-2 antibody assays in an immunocompetent and immunocompromised inpatient population with COVID-19. Diagn Microbiol. Infect. Dis. 2021, 101, 115449. [Google Scholar] [CrossRef]
- Levavi, H.; Lancman, G.; Gabrilove, J. Impact of rituximab on COVID-19 outcomes. Ann. Hematol. 2021, 100, 2805–2812. [Google Scholar] [CrossRef]
- Bin Lee, A.R.Y.; Wong, S.Y.; Chai, L.Y.A.; Lee, S.C.; Lee, M.X.; Muthiah, M.D.; Tay, S.H.; Teo, C.B.; Tan, B.K.J.; Chan, Y.H.; et al. Efficacy of covid-19 vaccines in immunocompromised patients: Systematic review and meta-analysis. BMJ 2022, 376, e068632. [Google Scholar] [CrossRef]
- Sjowall, J.; Azharuddin, M.; Frodlund, M.; Zhang, Y.; Sandner, L.; Dahle, C.; Hinkula, J.; Sjöwall, C. SARS-CoV-2 Antibody Isotypes in Systemic Lupus Erythematosus Patients Prior to Vaccination: Associations With Disease Activity, Antinuclear Antibodies, and Immunomodulatory Drugs During the First Year of the Pandemic. Front Immunol. 2021, 12, 724047. [Google Scholar] [CrossRef]
- Huang, A.; Cicin-Sain, C.; Pasin, C.; Epp, S.; Audige, A.; Muller, N.J.; Nilsson, J.; Bankova, A.; Wolfensberger, N.; Vilinovszki, O.; et al. Antibody Response to SARS-CoV-2 Vaccination in Patients following Allogeneic Hematopoietic Cell Transplantation. Transplant Cell Ther. 2022, 28, 214.e1–214.e11. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Mohanraj, D.; Baldwin, S.; Singh, S.; Gordon, A.; Whitelegg, A. Cellular and humoral responses to SARS-CoV-2 vaccination in immunosuppressed patients. Cell Immunol. 2022, 373, 104501. [Google Scholar] [CrossRef]
- Enocsson, H.; Idoff, C.; Gustafsson, A.; Govender, M.; Hopkins, F.; Larsson, M.; Nilsdotter-Augustinsson, Å.; Sjöwall, J. Soluble Urokinase Plasminogen Activator Receptor (suPAR) Independently Predicts Severity and Length of Hospitalisation in Patients With COVID-19. Front. Med. 2021, 8, 791716. [Google Scholar] [CrossRef]
- Hagbom, M.; Carmona-Vicente, N.; Sharma, S.; Olsson, H.; Jamtberg, M.; Nilsdotter-Augustinsson, A.; Sjöwall, J.; Nordgren, J. Evaluation of SARS-CoV-2 rapid antigen diagnostic tests for saliva samples. Heliyon 2022, 8, e08998. [Google Scholar] [CrossRef]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.W.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Ko, J.H.; Park, J.; Moon, H.W.; Baek, J.Y.; Jung, S.; Lim, H.Y.; Kim, K.C.; Huh, K.; Cho, S.Y.; et al. Estimating the Neutralizing Effect and Titer Correlation of Semi-Quantitative Anti-SARS-CoV-2 Antibody Immunoassays. Front. Cell Infect. Microbiol. 2022, 12, 822599. [Google Scholar] [CrossRef] [PubMed]
- Gaines, H.; Andersson, L.; Biberfeld, G. A new method for measuring lymphoproliferation at the single-cell level in whole blood cultures by flow cytometry. J. Immunol. Methods 1996, 195, 63–72. [Google Scholar] [CrossRef]
- Tan, A.T.; Linster, M.; Tan, C.W.; Le Bert, N.; Chia, W.N.; Kunasegaran, K.; Zhuang, Y.; Tham, C.Y.L.; Chia, A.; Smith, G.J.D.; et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021, 34, 108728. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, 6529. [Google Scholar] [CrossRef]
- Sherina, N.; Piralla, A.; Du, L.; Wan, H.; Kumagai-Braesch, M.; Andrell, J.; Braesch-Andersen, S.; Cassaniti, I.; Percivalle, E.; Sarasini, A.; et al. Persistence of SARS-CoV-2-specific B and T cell responses in convalescent COVID-19 patients 6–8 months after the infection. Med (N Y) 2021, 2, 281–295.e4. [Google Scholar] [CrossRef]
- Chavarot, N.; Leruez-Ville, M.; Scemla, A.; Burger, C.; Amrouche, L.; Rouzaud, C.; Lebreton, X.; Martinez, F.; Sberro-Soussan, R.; Legendre, C.; et al. Decline and loss of anti-SARS-CoV-2 antibodies in kidney transplant recipients in the 6 months following SARS-CoV-2 infection. Kidney Int. 2021, 99, 486–488. [Google Scholar] [CrossRef]
- Seow, J.; Graham, C.; Merrick, B.; Acors, S.; Pickering, S.; Steel, K.J.A.; Hemmings, O.; O’Byrne, A.; Kouphou, N.; Galao, R.P.; et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020, 5, 1598–1607. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Rha, M.S.; Sa, M.; Choi, H.K.; Jeon, J.H.; Seok, H.; Park, D.W.; Park, S.H.; Jeong, H.W.; Choi, W.S.; et al. SARS-CoV-2-specific T cell memory is sustained in COVID-19 convalescent patients for 10 months with successful development of stem cell-like memory T cells. Nat. Commun. 2021, 12, 4043. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Dowell, A.C.; Pearce, H.; Verma, K.; Long, H.M.; Begum, J.; Aiano, F.; Amin-Chowdhury, Z.; Hoschler, K.; Brooks, T.; et al. Robust SARS-CoV-2-specific T cell immunity is maintained at 6 months following primary infection. Nat. Immunol. 2021, 22, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Svahn, A.; Linde, A.; Thorstensson, R.; Karlén, K.; Andersson, L.; Gaines, H. Development and evaluation of a flow-cytometric assay of specific cell-mediated immune response in activated whole blood for the detection of cell-mediated immunity against varicella-zoster virus. J. Immunol. Methods 2003, 277, 17–25. [Google Scholar] [CrossRef]
- Mellergård, J.; Edström, M.; Jenmalm, M.C.; Dahle, C.; Vrethem, M.; Ernerudh, J. Increased B Cell and Cytotoxic NK Cell Proportions and Increased T Cell Responsiveness in Blood of Natalizumab-Treated Multiple Sclerosis Patients. PLoS ONE 2013, 8, e81685. [Google Scholar] [CrossRef]
- Li, L.; Muftuoglu, M.; Liang, S.; Basyal, M.; Lv, J.; Akdogan, M.E.; Chen, K.; Andreeff, M.; Flowers, C.R.; Parmar, S. In-depth analysis of SARS-CoV-2-specific T cells reveals diverse differentiation hierarchies in vaccinated individuals. JCI Insight 2022, 7, e156559. [Google Scholar] [CrossRef]
- Morris, A.B.; Adams, L.E.; Ford, M.L. Influence of T Cell Coinhibitory Molecules on CD8+ Recall Responses. Front. Immunol. 2018, 9, 1810. [Google Scholar] [CrossRef]
- Erickson, J.J.; Gilchuk, P.; Hastings, A.K.; Tollefson, S.J.; Johnson, M.; Downing, M.B.; Boyd, K.L.; Johnson, J.E.; Kim, A.S.; Joyce, S.; et al. Viral acute lower respiratory infections impair CD8+ T cells through PD-1. J. Clin. Investig. 2012, 122, 2967–2982. [Google Scholar] [CrossRef] [Green Version]
- Tavukcuoglu, E.; Horzum, U.; Inkaya, A.C.; Unal, S.; Esendagli, G. Functional responsiveness of memory T cells from COVID-19 patients. Cell. Immunol. 2021, 365, 104363. [Google Scholar] [CrossRef]
- Mehta, A.K.; Gracias, D.; Croft, M. TNF activity and T cells. Cytokine 2016, 101, 14–18. [Google Scholar] [CrossRef]
- Izadi, Z.; Brenner, E.J.; Mahil, S.K.; Dand, N.; Yiu, Z.Z.N.; Yates, M.; Ungaro, R.C.; Zhang, X.; Agrawal, M.; Colombel, J.-F.; et al. Association Between Tumor Necrosis Factor Inhibitors and the Risk of Hospitalization or Death Among Patients With Immune-Mediated Inflammatory Disease and COVID-19. JAMA Netw. Open 2021, 4, e2129639. [Google Scholar] [CrossRef]
- Li, D.; Xu, A.; Mengesha, E.; Elyanow, R.; Gittelman, R.M.; Chapman, H.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Pozdnyakova, V.; et al. The T-Cell Response to SARS-CoV-2 Vaccination in Inflammatory Bowel Disease is Augmented with Anti-TNF Therapy. Inflamm. Bowel Dis. 2022, izac071. [Google Scholar] [CrossRef]
- Björkström, N.K.; Ponzetta, A. Natural killer cells and unconventional T cells in COVID-19. Curr. Opin. Virol. 2021, 49, 176–182. [Google Scholar] [CrossRef]
- Maucourant, C.; Filipovic, I.; Ponzetta, A.; Aleman, S.; Cornillet, M.; Hertwig, L.; Strunz, B.; Lentini, A.; Reinius, B.; Brownlie, D.; et al. Natural killer cell immunotypes related to COVID-19 disease severity. Sci. Immunol. 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Wilk, A.J.; Rustagi, A.; Zhao, N.Q.; Roque, J.; Martínez-Colón, G.J.; McKechnie, J.L.; Ivison, G.T.; Ranganath, T.; Vergara, R.; Hollis, T.; et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat. Med. 2020, 26, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.; Katkar, G.D.; Khandelwal, S.; Behroozikhah, M.; Claire, A.; Castillo, V.; Tindle, C.; Fuller, M.; Taheri, S.; Rogers, T.F.; et al. AI-guided discovery of the invariant host response to viral pandemics. eBioMedicine 2021, 68, 103390. [Google Scholar] [CrossRef] [PubMed]
- Whitmire, J.K.; Eam, B.; Benning, N.; Whitton, J.L. Direct interferon-gamma signaling dramatically enhances CD4+ and CD8+ T cell memory. J. Immunol. 2007, 179, 1190–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerosa, F.; Baldani-Guerra, B.; Nisii, C.; Marchesini, V.; Carra, G.; Trinchieri, G. Reciprocal Activating Interaction between Natural Killer Cells and Dendritic Cells. J. Exp. Med. 2002, 195, 327–333. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.B.; Latz, E.; Mills, K.H.G.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.J.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Verges, S.; Milush, J.M.; Schwartz, B.S.; Pando, M.J.; Jarjoura, J.; York, V.A.; Houchins, J.P.; Miller, S.; Kang, S.M.; Norris, P.J.; et al. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 14725–14732. [Google Scholar] [CrossRef] [Green Version]
- Conway, S.; Keller, M.D.; Bollard, C.M. Cellular therapies for the treatment and prevention of SARS-CoV-2 infection. Blood 2022. [Google Scholar] [CrossRef]
| Immunocompromised n = 6 | Controls n = 12 | |
|---|---|---|
| Sex, male, n (%) | 3 (50) | 6 (50) |
| Age, years, median (range) | 62 (44–73) | 64 (46–75) |
| Ever smoker, n (%) | 3 (60) | 7 (58) |
| Ischemic heart disease, n (%) | 2 (33) | 6 (50) |
| Lung disease, n (%) | 2 (33) | 3 (25) |
| Diabetes, n (%) | 0 | 3 (25) |
| BMI, median (range) | 26 (22–37) | 30 (25–43) |
| Symptom duration at inclusion, days median (range) | 12 (5–16) | 10 (6–30) |
| Hospitalization, days median (range) | 7 (2–54) | 7 (3–15) |
| Intensive care #, n (%) | 1 (17) | 0 |
| Oxygen therapy, n (%) | 3 (50) | 11 (92) |
| HFNOT therapy, n (%) | 2 (33) | 5 (42) |
| Mechanical ventilation, n (%) | 1 (17) | 0 |
| Remdesivir, n (%) | 3 (50) | 5 (42) |
| Dexamethasone, n (%) | 4 (67) | 8 (67) |
| Biochemical variables at inclusion | ||
| Hemoglobin (g/L), median (range) | 115 (87–126) | 133 (98–154) |
| WBC (×109/L), median (range) | 6.1 (0.4–49) | 6.9 (3.5–12) |
| Platelets (×109/L), median (range) | 154 (20–668) | 282 (121–543) |
| Lymphocytes (×109/L), median (range) | 1.1 (0.3–33) | 1.1 (0.3–2.8) |
| Neutrophils:lymphocytes, median (range) | 2.1 (0.2–14.7) | 4.0 (1.8–20) |
| C-reactive protein, (mg/L) | 34 (11–376) | 43 (7–258) |
| Plasma creatinine (umol/L,) median (range) | 110 (54–341) | 65 (51–188) |
| LDH (ukat/L), median (range) | 6.3 (3.7–9.4) | 6.8 (4.1–7.7) |
| SARS-CoV-2 serology at inclusion | ||
| Anti-SARS-CoV-2 nucleocapsid IgG, n (%) | 4 (67) | 7 (58) |
| Anti-SARS-CoV-2 spike IgG, n (%) | 2 (33) | 7 (58) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sjöwall, J.; Hjorth, M.; Gustafsson, A.; Göransson, R.; Larsson, M.; Waller, H.; Nordgren, J.; Nilsdotter-Augustinsson, Å.; Nyström, S. SARS-CoV-2 Specific Antibody Response and T Cell-Immunity in Immunocompromised Patients up to Six Months Post COVID: A Pilot Study. J. Clin. Med. 2022, 11, 3535. https://doi.org/10.3390/jcm11123535
Sjöwall J, Hjorth M, Gustafsson A, Göransson R, Larsson M, Waller H, Nordgren J, Nilsdotter-Augustinsson Å, Nyström S. SARS-CoV-2 Specific Antibody Response and T Cell-Immunity in Immunocompromised Patients up to Six Months Post COVID: A Pilot Study. Journal of Clinical Medicine. 2022; 11(12):3535. https://doi.org/10.3390/jcm11123535
Chicago/Turabian StyleSjöwall, Johanna, Maria Hjorth, Annette Gustafsson, Robin Göransson, Marie Larsson, Hjalmar Waller, Johan Nordgren, Åsa Nilsdotter-Augustinsson, and Sofia Nyström. 2022. "SARS-CoV-2 Specific Antibody Response and T Cell-Immunity in Immunocompromised Patients up to Six Months Post COVID: A Pilot Study" Journal of Clinical Medicine 11, no. 12: 3535. https://doi.org/10.3390/jcm11123535
APA StyleSjöwall, J., Hjorth, M., Gustafsson, A., Göransson, R., Larsson, M., Waller, H., Nordgren, J., Nilsdotter-Augustinsson, Å., & Nyström, S. (2022). SARS-CoV-2 Specific Antibody Response and T Cell-Immunity in Immunocompromised Patients up to Six Months Post COVID: A Pilot Study. Journal of Clinical Medicine, 11(12), 3535. https://doi.org/10.3390/jcm11123535






