Significance of Autoantibodies to Ki/SL as Biomarkers for Systemic Lupus Erythematosus and Sicca Syndrome
Abstract
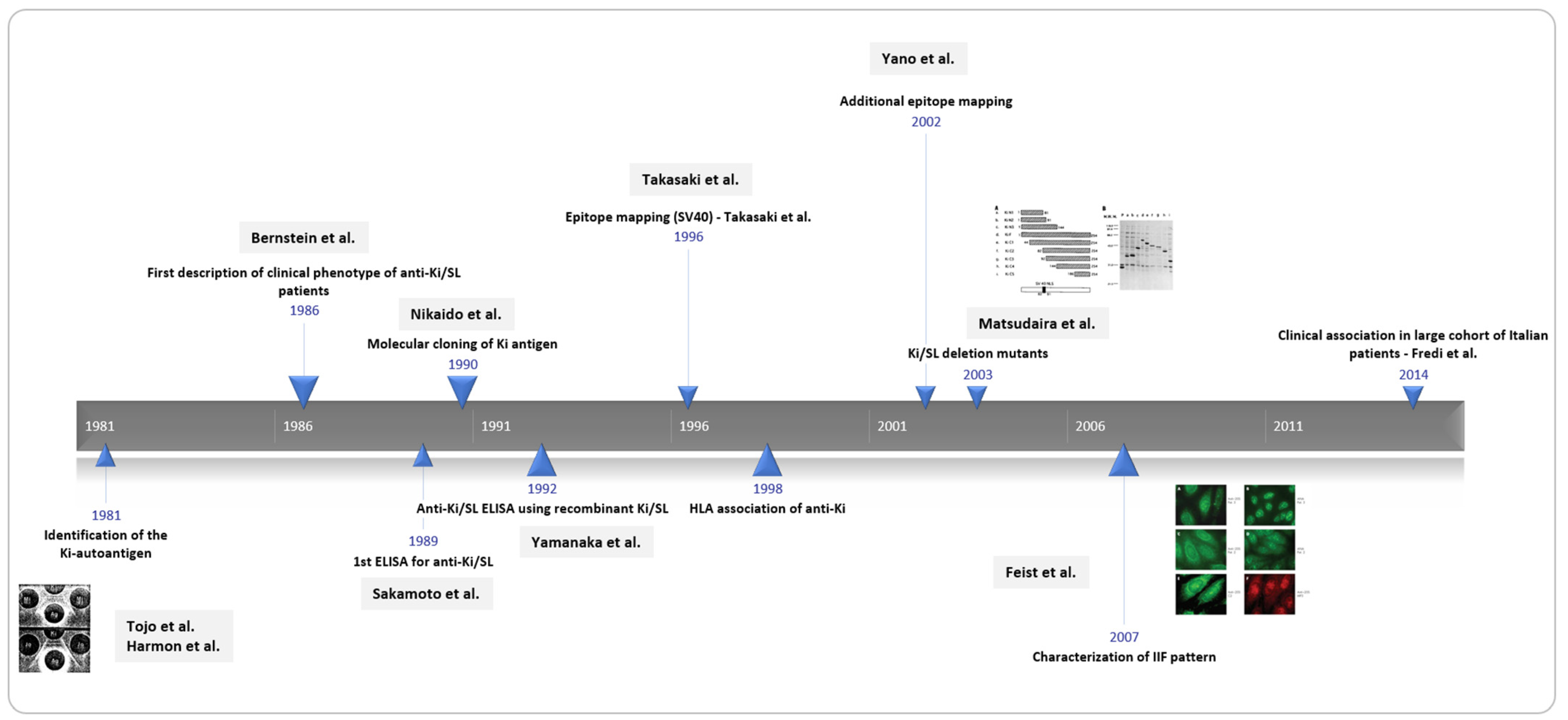
1. Introduction
2. Materials and Methods
3. Clinical and Demographic Association of Anti-Ki/SL Antibodies
4. Case Reports and Longitudinal Analysis of anti-Ki/SL Antibodies
5. Epitope Distribution on the Proteasome Complex and on Ki/SL
6. Detection Methods for Anti-Ki/SL Antibodies
6.1. Indirect Immunofluorescence Pattern of Anti-Ki/SL Antibodies
6.2. Other Detection Methods for Anti-Ki/SL Antibodies
7. Co-Expression of Anti-Ki/SL and Other Autoantibodies
8. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bernstein, R.M.; Bunn, C.C.; Hughes, G.R.; Francoeur, A.M.; Mathews, M.B. Cellular protein and RNA antigens in autoimmune disease. Mol. Biol. Med. 1984, 2, 105–120. [Google Scholar] [PubMed]
- Bernstein, R.M.; Morgan, S.H.; Bunn, C.C.; Gainey, R.C.; Hughes, G.R.; Mathews, M.B. The SL autoantibody-antigen system: Clinical and biochemical studies. Ann. Rheum. Dis. 1986, 45, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Tojo, T.; Kaburaki, J.; Hayakawa, M.; Okamoto, T.; Tomii, M.; Homma, M. Precipitating antibody to a soluble nuclear antigen "Ki" with specificity for systemic lupus erythematosus. Ryumachi 1981, 21, 129–140. [Google Scholar] [PubMed]
- Harmon, C.; Peebles, C.; Tan, E.M. SL-a new precipitating system. Arthritis Rheumatol. 1981, 24, S122. [Google Scholar]
- Francoeur, A.M.; Peebles, C.L.; Gompper, P.T.; Tan, E.M. Identification of Ki (Ku, p70/p80) autoantigens and analysis of anti-Ki autoantibody reactivity. J. Immunol. 1986, 136, 1648–1653. [Google Scholar]
- Muro, Y.; Kano, T.; Sugiura, K.; Hagiwara, M. Low frequency of autoantibodies against Ki-67 antigen in Japanese patients with systemic autoimmune diseases. J. Autoimmun. 1997, 10, 499–503. [Google Scholar] [CrossRef]
- Cavazzana, I.; Franceschini, F.; Vassalini, C.; Danieli, E.; Quinzanini, M.; Airo, P.; Cattaneo, R. Clinical and serological features of 35 patients with anti-Ki autoantibodies. Lupus 2005, 14, 837–841. [Google Scholar] [CrossRef]
- Yamanaka, K.; Takasaki, Y.; Nishida, Y.; Shimada, K.; Shibata, M.; Hashimoto, H. Detection and quantification of anti-Ki antibodies by enzyme-linked immunosorbent assay using recombinant Ki antigen. Arthritis Rheum. 1992, 35, 667–671. [Google Scholar] [CrossRef]
- Sherer, Y.; Gorstein, A.; Fritzler, M.J.; Shoenfeld, Y. Autoantibody explosion in systemic lupus erythematosus: More than 100 different antibodies found in SLE patients. Semin. Arthritis Rheum. 2004, 34, 501–537. [Google Scholar] [CrossRef]
- Sakamoto, M.; Takasaki, Y.; Yamanaka, K.; Kodama, A.; Hashimoto, H.; Hirose, S. Purification and characterization of Ki antigen and detection of anti-Ki antibody by enzyme-linked immunosorbent assay in patients with systemic lupus erythematosus. Arthritis Rheum. 1989, 32, 1554–1562. [Google Scholar] [CrossRef]
- Riboldi, P.; Asero, R.; Origgi, L.; Crespi, S. The SL/Ki system in connective tissue diseases: Incidence and clinical associations. Clin. Exp. Rheumatol. 1987, 5, 29–33. [Google Scholar] [PubMed]
- Matsunaga, K.; Yawata, M.; Tsuji, T.; Tani, K. HLA class II antigens associated with anti-Ki autoantibody positive connective tissue disease in Japanese. J. Rheumatol. 1998, 25, 1446–1447. [Google Scholar] [PubMed]
- Parodi, A.; Nigro, A.; Rebora, A. Anti-SL-Ki antibody in a patient with fatal connective tissue overlap disease. Br. J. Dermatol. 1989, 121, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Fredi, M.; Cavazzana, I.; Quinzanini, M.; Taraborelli, M.; Cartella, S.; Tincani, A.; Franceschini, F. Rare autoantibodies to cellular antigens in systemic lupus erythematosus. Lupus 2014, 23, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Boey, M.L.; Peebles, C.L.; Tsay, G.; Feng, P.H.; Tan, E.M. Clinical and autoantibody correlations in Orientals with systemic lupus erythematosus. Ann. Rheum. Dis. 1988, 47, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Colmegna, I.; Sainz, B., Jr.; Citera, G.; Maldonado-Cocco, J.A.; Garry, R.F.; Espinoza, L.R. Anti-20S proteasome antibodies in psoriatic arthritis. J. Rheumatol. 2008, 35, 674–676. [Google Scholar]
- Miyachi, K.; Hankins, R.W.; Mimori, T.; Okano, Y.; Akizuki, M. Prospective study of a systemic sclerosis/dermatomyositis overlap patient presenting with anti-Ku and anti-Ki antibodies. Mod. Rheumatol. 2002, 12, 253–255. [Google Scholar] [CrossRef]
- Oide, T.; Iwamura, A.; Yamamoto, H.; Aizawa, K.; Inoue, K.; Itoh, N.; Ikeda, S. Elderly-onset anticentromere antibody-positive pulmonary-renal syndrome: Report of an autopsy case. Nihon Kokyuki Gakkai Zasshi 2001, 39, 498–503. [Google Scholar]
- Wakasugi, M.; Sato, T.; Maruyama, Y.; Ueno, M.; Arakawa, M. A case of systemic lupus erythematosus diagnosed 7 years after epileptic seizure and developed chorea during prednisolone treatment. Ryumachi 1996, 36, 545–550. [Google Scholar]
- Ishiyama, K.; Suwa, A.; Ohta, S.; Moriguchi, M.; Suzuki, T.; Miyachi, K.; Hara, M.; Kashiwazaki, S. A case of systemic sclerosis associated with interstitial pneumonia with various autoantibodies: Improvement by intravenous cyclophosphamide therapy. Nihon Rinsho Meneki Gakkai Kaishi 1996, 19, 512–518. [Google Scholar] [CrossRef][Green Version]
- Feist, E.; Dorner, T.; Kuckelkorn, U.; Scheffler, S.; Burmester, G.; Kloetzel, P. Diagnostic importance of anti-proteasome antibodies. Int. Arch. Allergy Immunol. 2000, 123, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Kordonouri, O.; Meyer, K.; Egerer, K.; Hartmann, R.; Scheffler, S.; Burmester, G.R.; Kuckelkorn, U.; Danne, T.; Feist, E. Prevalence of 20S proteasome, anti-nuclear and thyroid antibodies in young patients at onset of type 1 diabetes mellitus and the risk of autoimmune thyroiditis. J. Pediatric Endocrinol. Metab. 2004, 17, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Brychcy, M.; Kuckelkorn, U.; Hausdorf, G.; Egerer, K.; Kloetzel, P.M.; Burmester, G.R.; Feist, E. Anti-20S proteasome autoantibodies inhibit proteasome stimulation by proteasome activator PA28. Arthritis Rheum. 2006, 54, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Feist, E.; Brychcy, M.; Hausdorf, G.; Hoyer, B.; Egerer, K.; Dorner, T.; Kuckelkorn, U.; Burmester, G.R. Anti-proteasome autoantibodies contribute to anti-nuclear antibody patterns on human larynx carcinoma cells. Ann. Rheum. Dis. 2007, 66, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Feist, E.; Burmester, G.R.; Kruger, E. The proteasome—victim or culprit in autoimmunity. Clin. Immunol. 2016, 172, 83–89. [Google Scholar] [CrossRef]
- Takasaki, Y.; Yano, T.; Hirokawa, K.; Takeuchi, K.; Ando, S.; Takahashi, T.; Shimada, K.; Hashimoto, H. An epitope on Ki antigen recognized by autoantibodies from lupus patients shows homology with the SV40 large T antigen nuclear localization signal. Arthritis Rheum. 1996, 39, 855–862. [Google Scholar] [CrossRef]
- Matsudaira, R.; Takeuchi, K.; Takasaki, Y.; Yano, T.; Matsushita, M.; Hashimoto, H. Relationships between autoantibody responses to deletion mutants of Ki antigen and clinical manifestations of lupus. J. Rheumatol. 2003, 30, 1208–1214. [Google Scholar]
- Yano, T.; Takasaki, Y.; Takeuchi, K.; Hirokawa, K.; Yamanaka, K.; Hashimoto, H. Anti-Ki antibodies recognize an epitope homologous with SV40 nuclear localization signal: Clinical significance and reactivities in various immunoassays. Mod. Rheumatol. 2002, 12, 50–55. [Google Scholar] [CrossRef]
- Matsushita, M.; Matsudaira, R.; Ikeda, K.; Nawata, M.; Tamura, N.; Takasaki, Y. Anti-proteasome activator 28alpha is a novel anti-cytoplasmic antibody in patients with systemic lupus erythematosus and Sjogren’s syndrome. Mod. Rheumatol. 2009, 19, 622–628. [Google Scholar] [CrossRef]
- Matsushita, M.; Takasaki, Y.; Takeuchi, K.; Yamada, H.; Matsudaira, R.; Hashimoto, H. Autoimmune response to proteasome activator 28alpha in patients with connective tissue diseases. J. Rheumatol. 2004, 31, 252–259. [Google Scholar]
- Damoiseaux, J.; von Muhlen, C.A.; Garcia-De La Torre, I.; Carballo, O.G.; de Melo, C.W.; Francescantonio, P.L.; Fritzler, M.J.; Herold, M.; Mimori, T.; Satoh, M.; et al. International consensus on ANA patterns (ICAP): The bumpy road towards a consensus on reporting ANA results. Autoimmun. Highlights 2016, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Röber, N.; Dellavance, A.; Ingénito, F.; Reimer, M.L.; Carballo, O.G.; Conrad, K.; Chan, E.K.L.; Andrade, L.E.C. Strong Association of the Myriad Discrete Speckled Nuclear Pattern With Anti-SS-A/Ro60 Antibodies: Consensus Experience of Four International Expert Centers. Front. Immunol. 2021, 12, 730102. [Google Scholar] [CrossRef] [PubMed]
- Walhelm, T.; Gunnarsson, I.; Heijke, R.; Leonard, D.; Trysberg, E.; Eriksson, P.; Sjöwall, C. Clinical Experience of Proteasome Inhibitor Bortezomib Regarding Efficacy and Safety in Severe Systemic Lupus Erythematosus: A Nationwide Study. Front. Immunol. 2021, 12, 756941. [Google Scholar] [CrossRef] [PubMed]
- Segarra, A.; Arredondo, K.V.; Jaramillo, J.; Jatem, E.; Salcedo, M.T.; Agraz, I.; Ramos, N.; Carnicer, C.; Valtierra, N.; Ostos, E. Efficacy and safety of bortezomib in refractory lupus nephritis: A single-center experience. Lupus 2020, 29, 118–125. [Google Scholar] [CrossRef]
- Petri, M.; Orbai, A.M.; Alarcon, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [Google Scholar] [CrossRef]
- Arbuckle, M.R.; McClain, M.T.; Rubertone, M.V.; Scofield, R.H.; Dennis, G.J.; James, J.A.; Harley, J.B. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 2003, 349, 1526–1533. [Google Scholar] [CrossRef] [PubMed]


| Case Study | Diagnosis | Comments | Ref |
|---|---|---|---|
| Ishiyama 1996 | SSc/ILD | - | [20] |
| Wakasugi 1996 | SLE/epileptic seizure/chorea | - | [19] |
| Oide 2001 | Pulmonary-renal syndrome | - | [18] |
| Miyachi 2002 | SSc/DM overlap | anti-Ku and anti-Ki/SL | [17] |
| Disease | Tojo et al. 1981 | Bernstein et al. 1986 | Riboldi et al. 1987 | Boey et al. 1988 | Sakamoto et al. 1989 | Yamanaka et al. 1992 | Fredi et al. 2014 |
|---|---|---|---|---|---|---|---|
| Method | DID | CIE | CIE | DID | ELISA | ELISA | CIE |
| SLE | 30/255 (11.8%) | 20/300 (6.7%) | 27/217 (12.4%) | 8/94 (8.5%) | 30/140 (21.4%) | 21/111 (18.9%) | 31/540 (5.8%) |
| SjS | 1/38 (2.6%) | 2/25 (8.0%) | |||||
| SS | 2/60 (3.3%) | ||||||
| SSc | 0/90 (0.0%) | 0/119 (0.0%) | 3/25 (12.0%) | 2/30 (6.7%) | |||
| PM/DM | 0/29 (0.0%) | 0/14 (0.0%) | (0.0%) | 1/30 (3.3%) | |||
| RA | 0/33 (0.0%) | 2/70 (2.9%) | 0/37 (0.0%) | (1.4%) | 2/50 (4.0%) | ||
| OS | 7/36 (19.4%) | ||||||
| PN | 0/6 (0.0%) | ||||||
| MCTD | 1/50 (2.0%) | 3/21 (14.3%) | 1/12 (8.3%) | ||||
| HI | 0/28 (0.0%) | (0.0%) | |||||
| PBC | 1/135 (0.7%) | ||||||
| ITP | 1/110 (0.9%) | ||||||
| VAS | 2/11 (18.2%) | ||||||
| pRP | 0/59 (0.0%) | ||||||
| Demographics | |||||||
| Male sex | yes | yes | |||||
| Other associations | Arthritis/pericarditis, Sm | White SLE, Ro(SS-A), PCNA | PCNA | CNS, Sm | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahler, M.; Bentow, C.; Aure, M.-A.; Fritzler, M.J.; Satoh, M. Significance of Autoantibodies to Ki/SL as Biomarkers for Systemic Lupus Erythematosus and Sicca Syndrome. J. Clin. Med. 2022, 11, 3529. https://doi.org/10.3390/jcm11123529
Mahler M, Bentow C, Aure M-A, Fritzler MJ, Satoh M. Significance of Autoantibodies to Ki/SL as Biomarkers for Systemic Lupus Erythematosus and Sicca Syndrome. Journal of Clinical Medicine. 2022; 11(12):3529. https://doi.org/10.3390/jcm11123529
Chicago/Turabian StyleMahler, Michael, Chelsea Bentow, Mary-Ann Aure, Marvin J. Fritzler, and Minoru Satoh. 2022. "Significance of Autoantibodies to Ki/SL as Biomarkers for Systemic Lupus Erythematosus and Sicca Syndrome" Journal of Clinical Medicine 11, no. 12: 3529. https://doi.org/10.3390/jcm11123529
APA StyleMahler, M., Bentow, C., Aure, M.-A., Fritzler, M. J., & Satoh, M. (2022). Significance of Autoantibodies to Ki/SL as Biomarkers for Systemic Lupus Erythematosus and Sicca Syndrome. Journal of Clinical Medicine, 11(12), 3529. https://doi.org/10.3390/jcm11123529







