Long-Term Exposure to Air Pollution and Incidence of Venous Thromboembolism in the General Population: A Population-Based Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Database
2.2. Study Subjects and Definition of Clinical Outcomes
2.3. Baseline Demographic and Clinical Characteristics
2.4. Measurements of Air Pollutants
2.5. Statistical Analyses
3. Results
3.1. Baseline and Clinical Characteristics of Incident Cases of VTE
3.2. Laboratory Abnormalities
3.3. Physical Activity, Alcohol Consumption, and Smoking Status
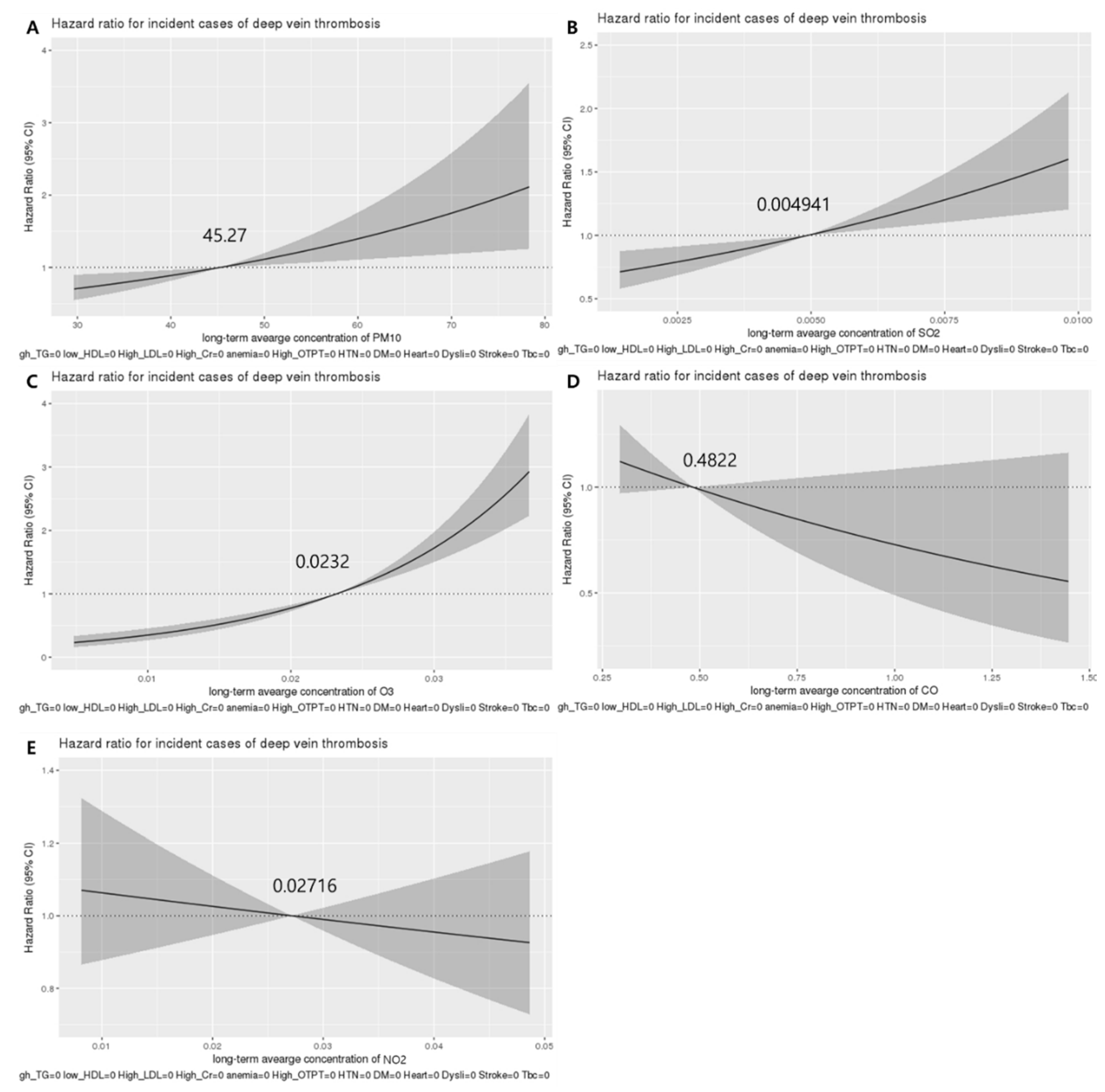
3.4. Association between the Long-Term Average Concentration of Air Pollutants and the Incidence of VTE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brook, R.D.; Rajagopalan, S.; Pope, C.A.; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate Matter Air Pollution and Cardiovascular Disease. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emmerechts, J.; Alfaro-Moreno, E.; Vanaudenaerde, B.M.; Nemery, B.; Hoylaerts, M.F. Short-term exposure to particulate matter induces arterial but not venous thrombosis in healthy mice. J. Thromb. Haemost. 2010, 8, 2651–2661. [Google Scholar] [CrossRef] [PubMed]
- Baccarelli, A.; Martinelli, I.; Zanobetti, A.; Grillo, P.; Hou, L.-F.; Bertazzi, P.A.; Mannucci, P.M.; Schwartz, J. Exposure to Particulate Air Pollution and Risk of Deep Vein Thrombosis. Arch. Intern. Med. 2008, 168, 920. [Google Scholar] [CrossRef] [PubMed]
- Kloog, I.; Zanobetti, A.; Nordio, F.; Coull, B.A.; Baccarelli, A.A.; Schwartz, J. Effects of airborne fine particles (PM2.5) on deep vein thrombosis admissions in the northeastern United States. J. Thromb. Haemost. 2015, 13, 768–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, L.; Wang, Q.-Y.; Cheng, Z.-P.; Hu, B.; Liu, J.-D.; Hu, Y. Air pollution and venous thrombosis: A meta-analysis. Sci. Rep. 2016, 6, 32794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ageno, W.; Casella, I.B.; Han, C.K.; Raskob, G.E.; Schellong, S.; Schulman, S.; Singer, D.E.; Kimura, K.; Tang, W.; Desch, M.; et al. RE-COVERY DVT/PE: Rationale and design of a prospective observational study of acute venous thromboembolism with a focus on dabigatran etexilate. Thromb. Haemost. 2017, 117, 415–421. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Health Effects of Particulate Matter: Policy Implications for Countries in Eastern Europe, Caucasus and Central Asia; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- World Health Organization. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Robertson, S.; Miller, M.R. Ambient air pollution and thrombosis. Part. Fibre Toxicol. 2018, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- World Health Orgarnization. WHO Air Quality Database. 2022. Available online: https://www.who.int/publications/m/item/who-air-quality-database-2022 (accessed on 18 April 2022).
- Renzi, M.; Stafoggia, M.; Michelozzi, P.; Davoli, M.; Forastiere, F.; Solimini, A.G. Long-term exposure to air pollution and risk of venous thromboembolism in a large administrative cohort. Environ. Health 2022, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Renzi, M.; Stafoggia, M.; Michelozzi, P.; Davoli, M.; Forastiere, F.; Solimini, A.G. Short-term exposure to PM(2.5) and risk of venous thromboembolism: A case-crossover study. Thromb. Res. 2020, 190, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Franklin, B.; Cascio, W.; Hong, Y.; Howard, G.; Lipsett, M.; Luepker, R.; Mittleman, M.; Samet, J.; Smith, S.C., Jr.; et al. Air pollution and cardiovascular disease: A statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 2004, 109, 2655–2671. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S.S.; Ferrante, M.; Gaudio, A.; Fiore, V. Deep vein thrombosis related to environment (Review). Mol. Med. Rep. 2017, 15, 3445–3448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilmour, P.S.; Morrison, E.R.; Vickers, M.A.; Ford, I.; Ludlam, C.A.; Greaves, M.; Donaldson, K.; MacNee, W. The procoagulant potential of environmental particles (PM10). Occup. Environ. Med. 2005, 62, 164–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pekkanen, J.; Brunner, E.J.; Anderson, H.R.; Tiittanen, P.; Atkinson, R.W. Daily concentrations of air pollution and plasma fibrinogen in London. Occup. Environ. Med. 2000, 57, 818–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baccarelli, A.; Zanobetti, A.; Martinelli, I.; Grillo, P.; Hou, L.; Giacomini, S.; Bonzini, M.; Lanzani, G.; Mannucci, P.M.; Bertazzi, P.A.; et al. Effects of exposure to air pollution on blood coagulation. J. Thromb. Haemost. 2007, 5, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Poredoš, P. Interrelationship between venous and arterial thrombosis. Int. Angiol. A J. Int. Union Angiol. 2017, 36, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Conde, I.; Lopez, J.A. Classification of venous thromboembolism (VTE). Role of acute inflammatory stress in venous thromboembolism. J. Thromb. Haemost. 2005, 3, 2573–2575. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, G.M.; Green, D.; Bellmeyer, A.; Baker, C.M.; Burgess, Z.; Rajamannan, N.; Christman, J.W.; Foiles, N.; Kamp, D.W.; Ghio, A.J.; et al. Ambient particulate matter accelerates coagulation via an IL-6-dependent pathway. J. Clin. Investig. 2007, 117, 2952–2961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristics | Subjects with VTE (N = 3158) | General Population without VTE (N = 335,420) | p-Value |
|---|---|---|---|
| Age, mean ± SD | 56.97 ± 9.97 | 51.35 ± 8.78 | <0.001 |
| Gender, N (%) | 0.024 | ||
| Male | 1650 (52.25) | 182,012 (54.26) | |
| Female | 1508 (47.75) | 153,408 (45.74) | |
| Economic status, N (%) | 0.009 | ||
| 0–2 decile (the lowest level) | 510 (16.15) | 50,039 (14.92) | |
| 3–4 decile | 476 (15.07) | 44,219 (13.18) | |
| 5–6 decile | 429 (13.58) | 51,451 (15.34) | |
| 7–8 decile | 647 (20.49) | 69,750 (20.79) | |
| 9–10 decile (the highest level) | 1096 (34.71) | 119,961 (35.76) | |
| Body mass index, mean ± SD | 24.36 ± 3.44 | 23.99 ± 3.02 | <0.001 |
| Obesity, N (%) * | 1289 (40.83) | 116,513 (34.75) | <0.001 |
| Waist circumference, cm | 83.88 ± 9.06 | 82.38 ± 8.46 | <0.001 |
| High blood pressure | 2385 (74.62) | 236,336 (70.47) | <0.001 |
| Systolic blood pressure, mmHg | 128.10 ± 16.67 | 125.60 ± 14.93 | <0.001 |
| Diastolic blood pressure, mmHg | 78.25 ± 10.81 | 76.81 ± 9.71 | <0.001 |
| Hypertension | 641 (52.71) | 114,347 (44.46) | <0.001 |
| Diabetes | 225 (19.93) | 42,706 (18.48) | 0.211 |
| Heart diseases | 136 (12.35) | 16,196 (7.34) | <0.001 |
| Stroke | 58 (5.32) | 6252 (2.87) | <0.001 |
| Tuberculosis | 24 (2.68) | 5399 (2.55) | 0.679 |
| Increased total cholesterol | 1265 (39.63) | 146,589 (43.71) | <0.001 |
| Increased triglyceride | 762 (28.01) | 94,584 (28.71) | 0.428 |
| Decreased high-density lipoprotein | 1940 (71.27) | 230,580 (69.94) | 0.132 |
| Increased low-density lipoprotein | 782 (28.78) | 108,376 (32.99) | <0.001 |
| Increased creatinine | 61 (2.24) | 4411 (1.34) | <0.001 |
| Anemia | 619 (19.39) | 41,478 (12.37) | <0.001 |
| Increased liver enzyme | 466 (14.6) | 53,009 (15.81) | 0.063 |
| Increased γ-glutamyl transpeptidase | 513 (16.07) | 52,084 (15.53) | 0.401 |
| Physical activity, min/week | <0.001 | ||
| <500 MET | 795 (52.79) | 101660 (35.26) | |
| 500–1000 MET | 416 (27.62) | 102351 (35.50) | |
| 1000–1500 MET | 179 (11.89) | 55708 (19.32) | |
| ≥1500 MET | 116 (7.70) | 28629 (9.93) | |
| MET, mean ± SD | 564.4 ± 561.3 | 775.3 ± 530.1 | <0.001 |
| Alcohol consumption, N (%) | <0.001 | ||
| Non-drinker | 1877 (76.21) | 56,545 (40.97) | |
| Social drinker | 449 (18.23) | 71,673 (51.93) | |
| Heavy drinker | 137 (5.56) | 9789 (7.09) | |
| Smoking status, N (%) | <0.001 | ||
| Non-smoker | 2168 (70.87) | 212,620 (63.61) | |
| Ex-smoker | 448 (14.65) | 73,562 (22.01) | |
| Current smoker | 443 (14.48) | 48,064 (14.38) | |
| Pack years | 23.63 ± 20.60 | 20.96 ± 16.17 | 0.004 |
| Air Pollutant | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted Model 1 * | Adjusted Model 2 ** | ||||
| PM10 † | 1.059 (1.052–1.066) | <0.001 | 1.024 (1.008–1.040) | 0.003 | 1.026 (1.004–1.048) | 0.018 |
| SO2 ‡ | 1.203 (1.178–1.229) | <0.001 | 1.097 (1.036–1.162) | 0.002 | 1.088 (1.005–1.179) | 0.038 |
| NO2 ‡ | 0.993 (0.989–0.997) | <0.001 | 0.997 (0.986–1.008) | 0.587 | 0.999 (0.984–1.014) | 0.892 |
| O3 ‡ | 1.064 (1.058–1.069) | <0.001 | 1.075 (1.054–1.097) | <0.001 | 1.074 (1.046–1.103) | <0.001 |
| CO § | 0.892 (0.866–0.919) | <0.001 | 0.947 (0.877–1.022) | 0.159 | 0.949 (0.856–1.053) | 0.328 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwon, J.G.; Lee, S.A.; Park, K.-Y.; Oh, S.U.; Kim, J.S.; Seo, H.-M. Long-Term Exposure to Air Pollution and Incidence of Venous Thromboembolism in the General Population: A Population-Based Retrospective Cohort Study. J. Clin. Med. 2022, 11, 3517. https://doi.org/10.3390/jcm11123517
Gwon JG, Lee SA, Park K-Y, Oh SU, Kim JS, Seo H-M. Long-Term Exposure to Air Pollution and Incidence of Venous Thromboembolism in the General Population: A Population-Based Retrospective Cohort Study. Journal of Clinical Medicine. 2022; 11(12):3517. https://doi.org/10.3390/jcm11123517
Chicago/Turabian StyleGwon, Jun Gyo, Sang Ah Lee, Kye-Yeung Park, Se Uk Oh, Joung Soo Kim, and Hyun-Min Seo. 2022. "Long-Term Exposure to Air Pollution and Incidence of Venous Thromboembolism in the General Population: A Population-Based Retrospective Cohort Study" Journal of Clinical Medicine 11, no. 12: 3517. https://doi.org/10.3390/jcm11123517
APA StyleGwon, J. G., Lee, S. A., Park, K.-Y., Oh, S. U., Kim, J. S., & Seo, H.-M. (2022). Long-Term Exposure to Air Pollution and Incidence of Venous Thromboembolism in the General Population: A Population-Based Retrospective Cohort Study. Journal of Clinical Medicine, 11(12), 3517. https://doi.org/10.3390/jcm11123517






