Antibiotic Cement Utilization for the Prophylaxis and Treatment of Infections in Spine Surgery: Basic Science Principles and Rationale for Clinical Use
Abstract
1. Introduction
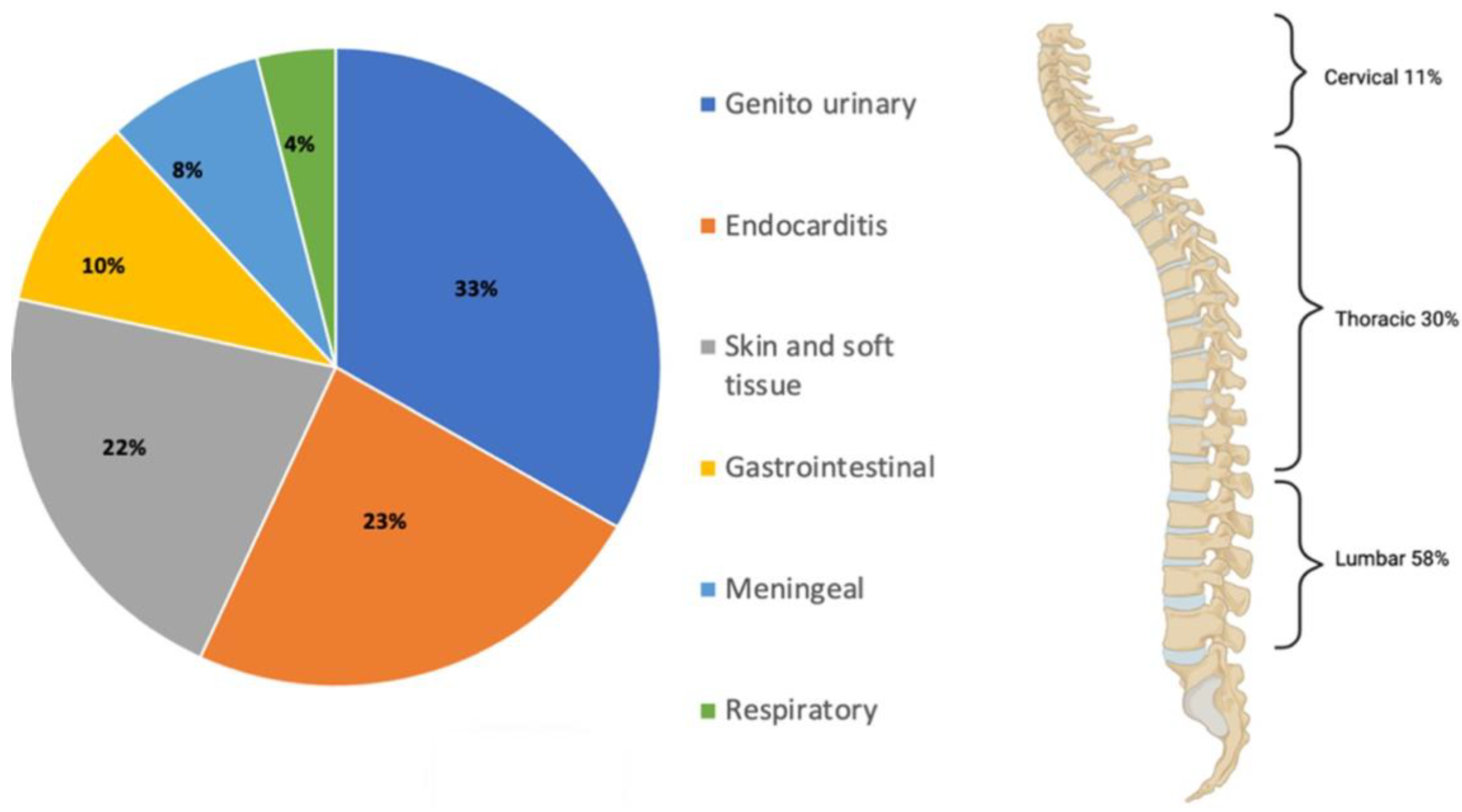
2. Microbiology of Spinal Infections
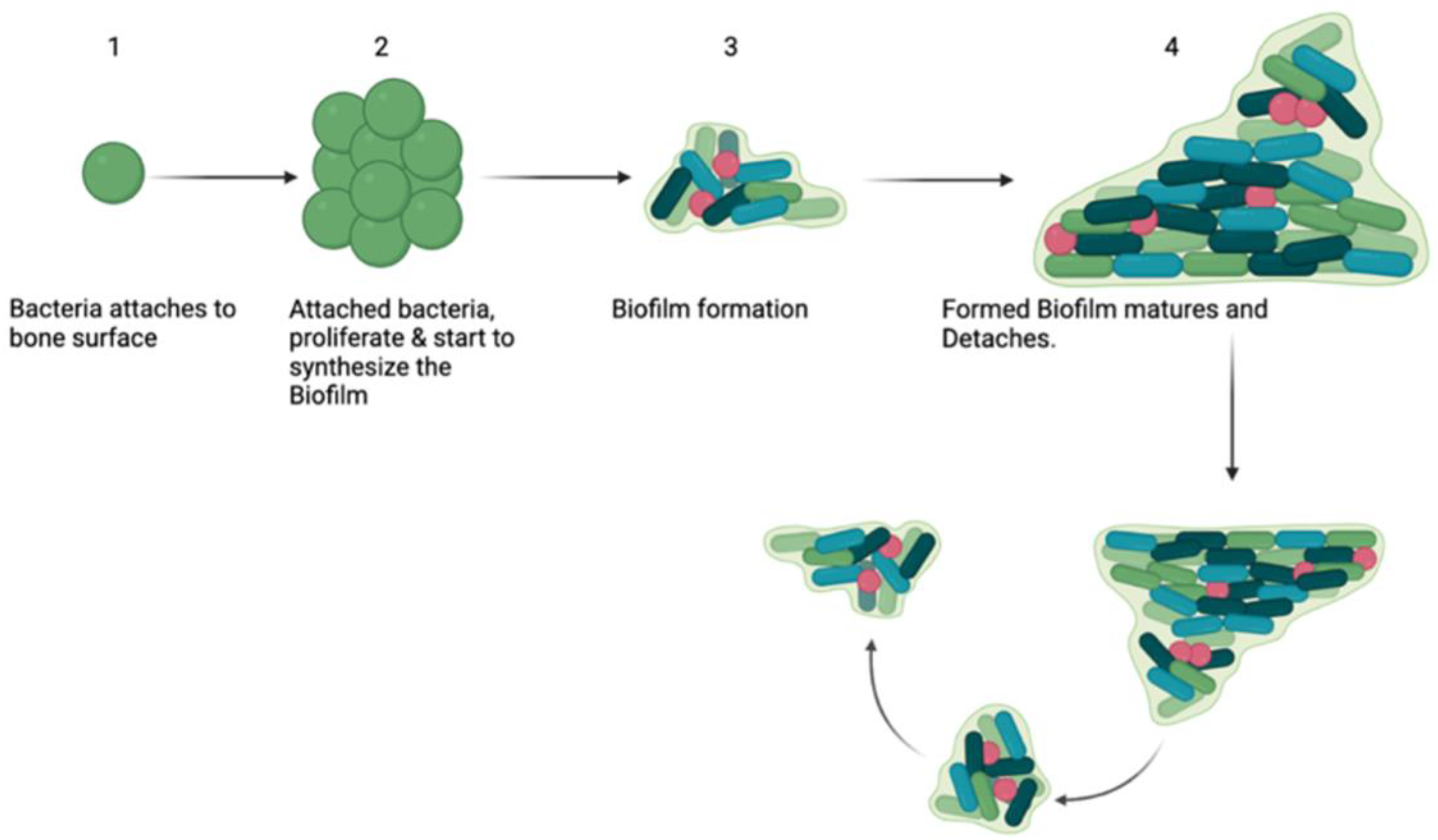
2.1. Biofilms
2.2. Bacteria
2.3. Fungi
2.4. Parasites
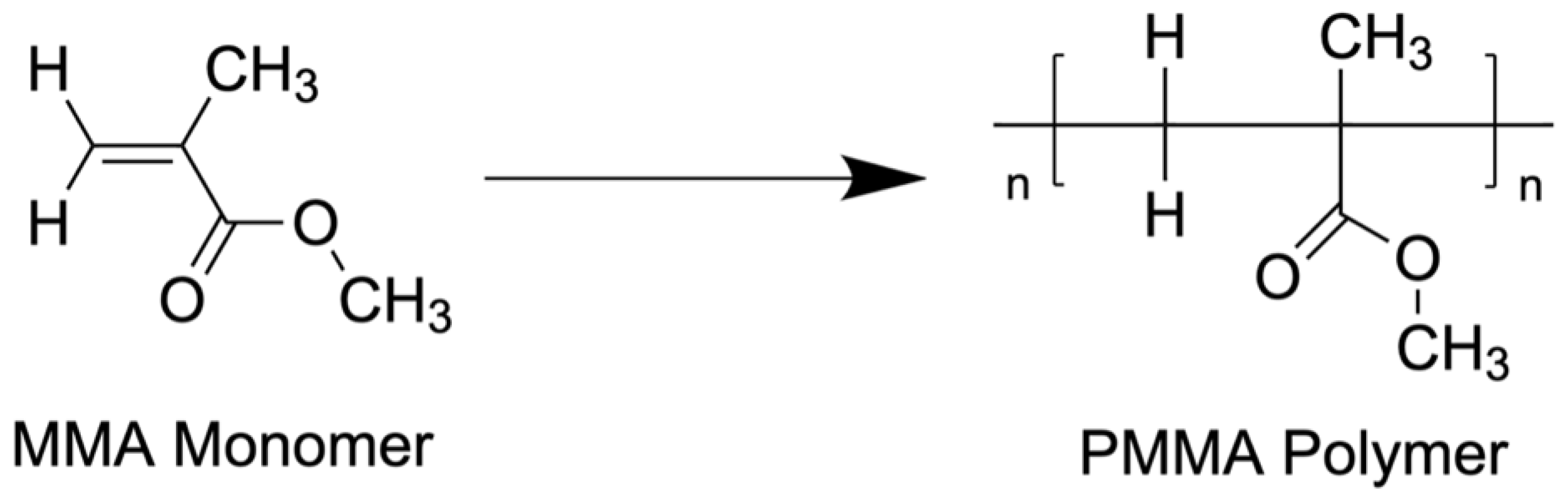
3. Fundamentals of Antibiotic Cement
4. Safety Concerns and Hazards
5. Antibiotic Cement Use and Outcomes in Spine Surgery
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salvati, E.A.; Callaghan, J.J.; Brause, B.D.; Klein, R.F.; Small, R.D. Reimplantation in infection. Elution of gentamicin from cement and beads. Clin. Orthop. Relat. Res. 1986, 207, 83–93. [Google Scholar] [CrossRef]
- Parvizi, J.; Saleh, K.J.; Ragland, P.S.; Pour, A.E.; Mont, M.A. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop. 2008, 79, 335–341. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, C.; Cheng, T.; Peng, X.; Zhang, W.; Qin, H.; Zhang, X. A systematic review and meta-analysis of antibiotic-impregnated bone cement use in primary total hip or knee arthroplasty. PLoS ONE 2013, 8, e82745. [Google Scholar] [CrossRef] [PubMed]
- Kasha, S.; Rathore, S.; Kumar, H. Antibiotic cement spacer and induced membrane bone grafting in open fractures with bone loss: A case series. Indian J. Orthop. 2019, 53, 237. [Google Scholar] [CrossRef]
- Cui, Q.; Mihalko, W.M.; Shields, J.S.; Ries, M.; Saleh, K.J. Antibiotic-Impregnated Cement Spacers for the Treatment of Infection Associated with Total Hip or Knee Arthroplasty. J. Bone Jt. Surg. 2007, 89, 871–882. [Google Scholar] [CrossRef]
- Josefsson, G.; Lindberg, L.; Wiklander, B. Systemic antibiotics and gentamicin-containing bone cement in the prophylaxis of postoperative infections in total hip arthroplasty. Clin. Orthop. Relat. Res. 1981, 159, 194–200. [Google Scholar] [CrossRef]
- Engesaeter, L.B.; Lie, S.A.; Espehaug, B.; Furnes, O.; Vollset, S.E.; Havelin, L.I. Antibiotic prophylaxis in total hip arthroplasty: Effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop. Scand. 2003, 74, 644–651. [Google Scholar] [CrossRef]
- Careri, S.; Vitiello, R.; Oliva, M.S.; Ziranu, A.; Maccauro, G.; Perisano, C. Masquelet technique and osteomyelitis: Innovations and literature review. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 210–216. [Google Scholar] [CrossRef]
- Kremers, H.M.; Nwojo, M.E.; Ransom, J.E.; Wood-Wentz, C.M.; Melton, L.J.; Huddleston, P.M. Trends in the Epidemiology of Osteomyelitis: A Population-Based Study, 1969 to 2009. J. Bone Jt. Surg. 2015, 97, 837–845. [Google Scholar] [CrossRef]
- Grammatico, L.; Baron, S.; Rusch, E.; Lepage, B.; Surer, N.; Desenclos, J.C.; Besnier, J.M. Epidemiology of vertebral osteomyelitis (VO) in France: Analysis of hospital-discharge data 2002–2003. Epidemiol. Infect. 2008, 136, 653–660. [Google Scholar] [CrossRef]
- Joshi, S.M.; Hatfield, R.H.; Martin, J.; Taylor, W. Spinal epidural abscess: A diagnostic challenge. Br. J. Neurosurg. 2003, 17, 160–163. [Google Scholar] [CrossRef] [PubMed]
- McHenry, M.C.; Easley, K.A.; Locker, G.A. Vertebral Osteomyelitis: Long-Term Outcome for 253 Patients from 7 Cleveland-Area Hospitals. Clin. Infect. Dis. 2002, 34, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Mylona, E.; Samarkos, M.; Kakalou, E.; Fanourgiakis, P.; Skoutelis, A. Pyogenic Vertebral Osteomyelitis: A Systematic Review of Clinical Characteristics. Semin. Arthritis Rheum. 2009, 39, 10–17. [Google Scholar] [CrossRef]
- Tsantes, A.; Papadopoulos, D.; Vrioni, G.; Sioutis, S.; Sapkas, G.; Benzakour, A.; Benzakour, T.; Angelini, A.; Ruggieri, P.; Mavrogenis, A.; et al. Spinal Infections: An Update. Microorganisms 2020, 8, 476. [Google Scholar] [CrossRef] [PubMed]
- Chahoud, J.; Kanafani, Z.; Kanj, S.S. Surgical Site Infections Following Spine Surgery: Eliminating the Controversies in the Diagnosis. Front. Med. 2014, 1, 7. [Google Scholar] [CrossRef]
- Anderson, P.A.; Savage, J.W.; Vaccaro, A.R.; Radcliff, K.; Arnold, P.M.; Lawrence, B.D.; Shamji, M.F. Prevention of Surgical Site Infection in Spine Surgery. Neurosurgery 2017, 80, S114–S123. [Google Scholar] [CrossRef]
- Gerometta, A.; Olaverri, J.C.R.; Bitan, F. Infections in spinal instrumentation. Int. Orthop. (SICOT) 2012, 36, 457–464. [Google Scholar] [CrossRef]
- Wiley, A.M.; Ha’eri, G.B. Routes of infection. A study of using “tracer particles” in the orthopedic operating room. Clin. Orthop. Relat. Res. 1979, 139, 150–155. [Google Scholar]
- Mah, T.-F. Biofilm-specific antibiotic resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- Masters, E.A.; Trombetta, R.P.; de Mesy Bentley, K.L.; Boyce, B.F.; Gill, A.L.; Gill, S.R.; Nishitani, K.; Ishikawa, M.; Morita, Y.; Ito, H.; et al. Evolving concepts in bone infection: Redefining “biofilm”, “acute vs. chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy”. Bone Res. 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Suh, K.T.; Kim, S.-J.; Lee, J.S. Implant Removal for the Management of Infection After Instrumented Spinal Fusion. J. Spinal Disord. Tech. 2010, 23, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Saeed, K.; Esposito, S.; Ascione, T.; Bassetti, M.; Bonnet, E.; Carnelutti, A.; Chan, M.; Lye, D.C.; Cortes, N.; Dryden, M.; et al. Hot topics on vertebral osteomyelitis from the International Society of Antimicrobial Chemotherapy. Int. J. Antimicrob. Agents 2019, 54, 125–133. [Google Scholar] [CrossRef]
- Urish, K.L.; Cassat, J.E. Staphylococcus aureus Osteomyelitis: Bone, Bugs, and Surgery. Infect. Immun. 2020, 88, e00932-19. [Google Scholar] [CrossRef]
- Garcia, D.; Gilmore, A.; Berns, E.; Spake, C.; Dockery, D.M.; Vishwanath, N.; Glasser, J.; Antoci, V.; Daniels, A.; Born, C.T. Silver carboxylate and titanium dioxide-polydimethylsiloxane coating decreases adherence of multi-drug resistant Serratia marcescens on spinal implant materials. Spine Deform. 2021, 9, 1493–1500. [Google Scholar] [CrossRef]
- Bariteau, J.T.; Waryasz, G.R.; McDonnell, M.; Fischer, S.A.; Hayda, C.R.A.; Born, C.T. Fungal Osteomyelitis and Septic Arthritis. J. Am. Acad. Orthop. Surg. 2014, 22, 390–401. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef]
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef]
- Dhawan, S.; Jain, D.; Mehta, V.S. Balantidium coli: An unrecognized cause of vertebral osteomyelitis and myelopathy: Case report. SPI 2013, 18, 310–313. [Google Scholar] [CrossRef]
- Song, X.H.; Ding, L.W.; Wen, H. Bone hydatid disease. Postgrad. Med. J. 2007, 83, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Monge-Maillo, B.; Olmedo Samperio, M.; Pérez-Molina, J.A.; Norman, F.; Mejía, C.R.; Tojeiro, S.C.; López-Vélez, R. Osseous cystic echinococcosis: A case series study at a referral unit in Spain. PLoS Negl. Trop. Dis. 2019, 13, e0007006. [Google Scholar] [CrossRef] [PubMed]
- Monzón, R.A.; Coury, J.G.; Disse, G.D.; Lum, Z.C. Bone Cement in Total Hip and Knee Arthroplasty. JBJS Rev. 2019, 7, e6. [Google Scholar] [CrossRef] [PubMed]
- van Vugt, T.A.G.; Arts, J.J.; Geurts, J.A.P. Antibiotic-Loaded Polymethylmethacrylate Beads and Spacers in Treatment of Orthopedic Infections and the Role of Biofilm Formation. Front. Microbiol. 2019, 10, 1626. [Google Scholar] [CrossRef] [PubMed]
- Masri, B.A.; Duncan, C.P.; Beauchamp, C.P. Long-term elution of antibiotics from bone-cement. J. Arthroplast. 1998, 13, 331–338. [Google Scholar] [CrossRef]
- Martínez-Moreno, J.; Merino, V.; Nácher, A.; Rodrigo, J.L.; Climente, M.; Merino-Sanjuán, M. Antibiotic-loaded Bone Cement as Prophylaxis in Total Joint Replacement: Antibiotic Loaded Bone Cement. Orthop. Surg. 2017, 9, 331–341. [Google Scholar] [CrossRef]
- Jiranek, W.A.; Hanssen, A.D.; Greenwald, A.S. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J. Bone Jt. Surg. Am. 2006, 88, 2487–2500. [Google Scholar] [CrossRef]
- Penner, M.J.; Masri, B.A.; Duncan, C.P. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J. Arthroplast. 1996, 11, 939–944. [Google Scholar] [CrossRef]
- Bertazzoni Minelli, E.; Benini, A.; Magnan, B.; Bartolozzi, P. Release of gentamicin and vancomycin from temporary human hip spacers in two-stage revision of infected arthroplasty. J. Antimicrob. Chemother. 2004, 53, 329–334. [Google Scholar] [CrossRef]
- González Della Valle, A.; Bostrom, M.; Brause, B.; Harney, C.; Salvati, E.A. Effective bactericidal activity of tobramycin and vancomycin eluted from acrylic bone cement. Acta Orthop. Scand. 2001, 72, 237–240. [Google Scholar] [CrossRef]
- Anagnostakos, K.; Meyer, C. Antibiotic Elution from Hip and Knee Acrylic Bone Cement Spacers: A Systematic Review. BioMed Res. Int. 2017, 2017, 4657874. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, J.G.E.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Backgrounds of antibiotic-loaded bone cement and prosthesis-related infection. Biomaterials 2004, 25, 545–556. [Google Scholar] [CrossRef]
- Hanssen, A.D.; Spangehl, M.J. Practical applications of antibiotic-loaded bone cement for treatment of infected joint replacements. Clin. Orthop. Relat. Res. 2004, 427, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Penner, M.J.; Duncan, C.P.; Masri, B.A. The in vitro elution characteristics of antibiotic-loaded CMW and Palacos-R bone cements. J. Arthroplast. 1999, 14, 209–214. [Google Scholar] [CrossRef]
- Edin, M.L.; Miclau, T.; Lester, G.E.; Lindsey, R.W.; Dahners, L.E. Effect of cefazolin and vancomycin on osteoblasts in vitro. Clin. Orthop. Relat. Res. 1996, 333, 245–251. [Google Scholar] [CrossRef]
- Ince, A.; Schütze, N.; Karl, N.; Löhr, J.F.; Eulert, J. Gentamicin negatively influenced osteogenic function in vitro. Int. Orthop. 2007, 31, 223–228. [Google Scholar] [CrossRef]
- Chohfi, M.; Langlais, F.; Fourastier, J.; Minet, J.; Thomazeau, H.; Cormier, M. Pharmacokinetics, uses, and limitations of vancomycin-loaded bone cement. Int. Orthop. 1998, 22, 171–177. [Google Scholar] [CrossRef]
- Kendoff, D.O.; Gehrke, T.; Stangenberg, P.; Frommelt, L.; Bösebeck, H. Bioavailability of Gentamicin and Vancomycin Released from an Antibiotic Containing Bone Cement in Patients Undergoing a Septic One-Stage Total Hip Arthroplasty (THA) Revision: A Monocentric Open Clinical Trial. HIP Int. 2016, 26, 90–96. [Google Scholar] [CrossRef]
- Tansy, M.F.; Hohenleitner, F.J.; Landin, W.E.; Kendall, F.M. Chronic biological effects of methyl methacrylate vapor. Environ. Res. 1980, 21, 108–116. [Google Scholar] [CrossRef]
- Nicholas, C.A.; Lawrence, W.H.; Autian, J. Embryotoxicity and fetotoxicity from maternal inhalation of methyl methacrylate monomer in rats. Toxicol. Appl. Pharmacol. 1979, 50, 451–458. [Google Scholar] [CrossRef]
- Darre, E.; Jergensen, L.G.; Vedel, P.; Jensen, J.S. Breathing Zone Concentrations of Methylmethacrylate Monomer During Joint Replacement Operations. Pharmacol. Toxicol. 1992, 71, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.H. Proportionate Cancer Mortality in Methyl Methacrylate-Exposed Orthopedic Surgeons Compared to General Surgeons. J. Med. Toxicol. 2011, 7, 125–132. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pre-Packed Vacuum Bone Cement Mixing Systems. A Further Step in Reducing Methylmethacrylate Exposure in Surgery. Ann. Occup. Hyg. 2010, 54, 955–961. [Google Scholar] [CrossRef][Green Version]
- Thomas, B.; Kulichova, D.; Wolf, R.; Summer, B.; Mahler, V.; Thomas, P. High frequency of contact allergy to implant and bone cement components, in particular gentamicin, in cemented arthroplasty with complications: Usefulness of late patch test reading: Gentamicin allergy in complicated arthroplasty? Contact Dermat. 2015, 73, 343–349. [Google Scholar] [CrossRef]
- Park, H.B.; Choi, J.S.; Park, S.H.; Kee, W.J.; Koh, Y.-I. Drug Fever Due to Piperacillin/Tazobactam Loaded into Bone Cement. J. Korean Med. Sci. 2011, 26, 301. [Google Scholar] [CrossRef]
- Dunne, N.J.; Hill, J.; McAfee, P.; Kirkpatrick, R.; Patrick, S.; Tunney, M. Incorporation of large amounts of gentamicin sulphate into acrylic bone cement: Effect on handling and mechanical properties, antibiotic release, and biofilm formation. Proc. Inst. Mech. Eng. Part H 2008, 222, 355–365. [Google Scholar] [CrossRef]
- Lautenschlager, E.P.; Jacobs, J.J.; Marshall, G.W.; Meyer, P.R. Mechanical properties of bone cements containing large doses of antibiotic powders. J. Biomed. Mater. Res. 1976, 10, 929–938. [Google Scholar] [CrossRef]
- Lynch, M.; Esser, M.P.; Shelley, P.; Wroblewski, B.M. Deep infection in Charnley low-friction arthroplasty. Comparison of plain and gentamicin-loaded cement. J. Bone Jt. Surg. Br. 1987, 69, 355–360. [Google Scholar] [CrossRef]
- DeLuise, M.; Scott, C.P. Addition of hand-blended generic tobramycin in bone cement: Effect on mechanical strength. Orthopedics 2004, 27, 1289–1291. [Google Scholar] [CrossRef]
- Kim, D.S.; Jang, S.Y.; Kong, M.H.; Song, K.Y.; Kang, D.S. Lumbar Nerve Root Compression due to Leakage of Bone Cement after Vertebroplasty. Korean J. Neurotrauma 2014, 10, 155–158. [Google Scholar] [CrossRef][Green Version]
- Zhang, K.; Shen, Y.; Ren, Y.; Zou, D. Prevention and treatment of bone cement-related complications in patients receiving percutaneous kyphoplasty. Int. J. Clin. Exp. Med. 2015, 8, 2371–2377. [Google Scholar] [PubMed]
- Librianto, D.; Fachrisal; Saleh, I. Gelatin sponge as a rare and forgotten cause of early-onset neurological deficit post osteotomy of thoracolumbar kyphosis—A case report and review of literature. Int. J. Surg. Case Rep. 2020, 75, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Rustagi, T.; Patel, K.; Kadrekar, S.; Jain, A. Oxidized Cellulose (Surgicel) Causing Postoperative Cauda Equine Syndrome. Cureus 2017, 9, e1500. [Google Scholar] [CrossRef] [PubMed]
- Kinnari, T.J.; Esteban, J.; Zamora, N.; Fernandez, R.; López-Santos, C.; Yubero, F.; Mariscal, D.; Puertolas, J.A.; Gomez-Barrena, E. Effect of surface roughness and sterilization on bacterial adherence to ultra-high molecular weight polyethylene. Clin. Microbiol. Infect. 2010, 16, 1036–1041. [Google Scholar] [CrossRef]
- Sanzén, L.; Walder, M. Antibiotic resistance of coagulase-negative staphylococci in an orthopaedic department. J. Hosp. Infect. 1988, 12, 103–108. [Google Scholar] [CrossRef]
- Neut, D. Biomaterial-associated infection of gentamicin-loaded PMMA beads in orthopaedic revision surgery. J. Antimicrob. Chemother. 2001, 47, 885–891. [Google Scholar] [CrossRef]
- Thomes, B.; Murray, P.; Bouchier-Hayes, D. Development of resistant strains of Staphylococcus epidermidis on gentamicin-loaded bone cement in vivo. J. Bone Jt. Surg. Br. 2002, 84, 758–760. [Google Scholar] [CrossRef]
- Opalko, M.; Bösebeck, H.; Vogt, S. Properties and clinical application safety of antibiotic-loaded bone cement in kyphoplasty. J. Orthop. Surg. Res. 2019, 14, 238. [Google Scholar] [CrossRef]
- Kim, C.H.; Ju, C.I.; Lee, S.M.; Kim, S.W. Efficacy of Antibiotic-Loaded Cement Augmentation for Correcting Low Grade Pedicle Screw Loosening. Korean J. Neurotrauma 2021, 17, 41. [Google Scholar] [CrossRef]
- Chen, L.-H.; Yang, S.-C.; Niu, C.-C.; Lai, P.-L.; Chen, W.-J. Percutaneous Drainage Followed by Antibiotic-Impregnated Cement Vertebroplasty for Pyogenic Vertebral Osteomyelitis: A Case Report. J. Trauma Inj. Infect. Crit. Care 2008, 64, E8–E11. [Google Scholar] [CrossRef]
- Masuda, S.; Fujibayashi, S.; Otsuki, B.; Kimura, H.; Matsuda, S. Efficacy of Target Drug Delivery and Dead Space Reduction Using Antibiotic-loaded Bone Cement for the Treatment of Complex Spinal Infection. Clin. Spine Surg. 2017, 30, E1246–E1250. [Google Scholar] [CrossRef] [PubMed]
- Ogihara, S.; Murase, S.; Oguchi, F.; Saita, K. Deep surgical site infection after posterior instrumented fusion for rheumatoid upper cervical subluxation treated with antibiotic-loaded bone cement: Three case reports. Medicine 2020, 99, e20892. [Google Scholar] [CrossRef] [PubMed]
- Laratta, J.L.; Lombardi, J.M.; Shillingford, J.N.; Reddy, H.P.; Gvozdyev, B.V.; Kim, Y.J. Permanent implantation of antibiotic cement over exposed instrumentation eradicates deep spinal infection. J. Spine Surg. 2018, 4, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-J.; Lee, S.-R.; Kim, S.-T.; Kim, T.-H.; Lee, S.-H. Spinal Epidural Abscess with Pyogenic Arthritis of Facet Joint Treated with Antibiotic-Bone Cement Beads—A Case Report. Asian Spine J. 2007, 1, 61. [Google Scholar] [CrossRef] [PubMed]
- Slavnic, D.; Tong, D.; Anton, G.; Bashiti, R.; Carr, D.; Hanson, C.; Lytle, E.; Richards, B.; Soo, T.-M. Efficacy and safety with the use of Antibiotic-impregnated Poly-methyl methacrylate (AI-PMMA) for thoracolumbar spinal reconstruction in pyogenic Spondylodiscitis: Retrospective cohort study. Interdiscip. Neurosurg. 2021, 26, 101324. [Google Scholar] [CrossRef]

| Brand | Radiopacifier | Color | Antibiotics Mixture | Setting Time and Temperature | Viscosity | Use |
|---|---|---|---|---|---|---|
| Palacos R + G bone cement | zirconium dioxide | green | 0.5 g of gentamicin per 40.6 g | 8 min, 45 s at 19 °C | high | arthroplasty |
| Depuy CMW1 | barium sulfate | none | Optional: 1 g gentamicin per 40 g | 12 min, 30 s at 19 °C | high | arthroplasty |
| Depuy CMW2 | barium sulfate | none | Optional: 1 g gentamicin per 40 g | 6 min, 30 s at 19 °C | high | arthroplasty |
| Depuy CMW3 | barium sulfate | none | Optional: 1 g gentamicin per 40 g | 12 min, 30 s at 19 °C | medium | arthroplasty |
| Simplex P | barium sulfate | none | Option 1 g tobramycin per 40 g | 10 min at 19 °C | medium | arthroplasty |
| Refobacin Bone Cement R | zirconium dioxide | green | 0.5 g gentamicin per 40 g | 11 min at 19 °C | high | arthroplasty |
| Cobalt HV | zirconium dioxide | blue | Optional: 0.5 g gentamicin per 40 g | 5 min at 23 °C | high | arthroplasty |
| Osteopal G | zirconium dioxide | green | 0.325 g gentamicin per 26.53 g | 23 min, 30 s at 20 °C | low | kyphoplasty and vertebroplasty |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, G.M.; Osorio, C.; Berns, E.M.; Masood, U.; Alsoof, D.; McDonald, C.L.; Zhang, A.S.; Younghein, J.A.; Kuris, E.O.; Telfeian, A.; et al. Antibiotic Cement Utilization for the Prophylaxis and Treatment of Infections in Spine Surgery: Basic Science Principles and Rationale for Clinical Use. J. Clin. Med. 2022, 11, 3481. https://doi.org/10.3390/jcm11123481
Anderson GM, Osorio C, Berns EM, Masood U, Alsoof D, McDonald CL, Zhang AS, Younghein JA, Kuris EO, Telfeian A, et al. Antibiotic Cement Utilization for the Prophylaxis and Treatment of Infections in Spine Surgery: Basic Science Principles and Rationale for Clinical Use. Journal of Clinical Medicine. 2022; 11(12):3481. https://doi.org/10.3390/jcm11123481
Chicago/Turabian StyleAnderson, George M., Camilo Osorio, Ellis M. Berns, Umar Masood, Daniel Alsoof, Christopher L. McDonald, Andrew S. Zhang, John Andrew Younghein, Eren O. Kuris, Albert Telfeian, and et al. 2022. "Antibiotic Cement Utilization for the Prophylaxis and Treatment of Infections in Spine Surgery: Basic Science Principles and Rationale for Clinical Use" Journal of Clinical Medicine 11, no. 12: 3481. https://doi.org/10.3390/jcm11123481
APA StyleAnderson, G. M., Osorio, C., Berns, E. M., Masood, U., Alsoof, D., McDonald, C. L., Zhang, A. S., Younghein, J. A., Kuris, E. O., Telfeian, A., & Daniels, A. H. (2022). Antibiotic Cement Utilization for the Prophylaxis and Treatment of Infections in Spine Surgery: Basic Science Principles and Rationale for Clinical Use. Journal of Clinical Medicine, 11(12), 3481. https://doi.org/10.3390/jcm11123481






