Rapid Response of Refractory Systemic Lupus Erythematosus Skin Manifestations to Anifrolumab—A Case-Based Review of Clinical Trial Data Suggesting a Domain-Based Therapeutic Approach
Abstract
:1. Introduction
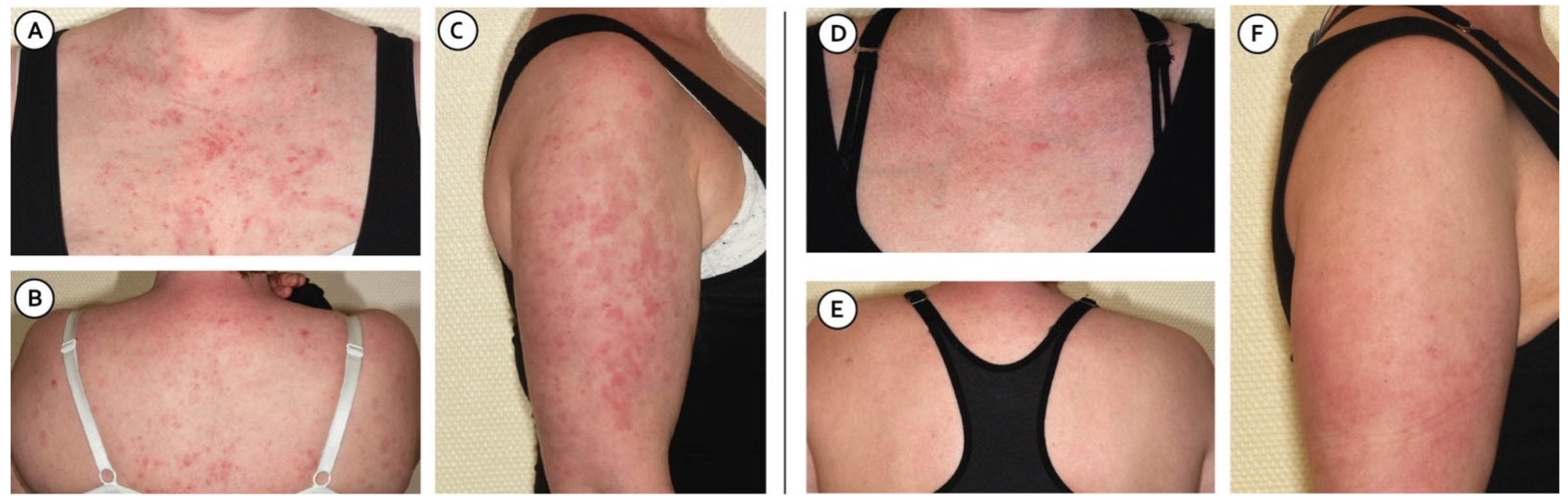
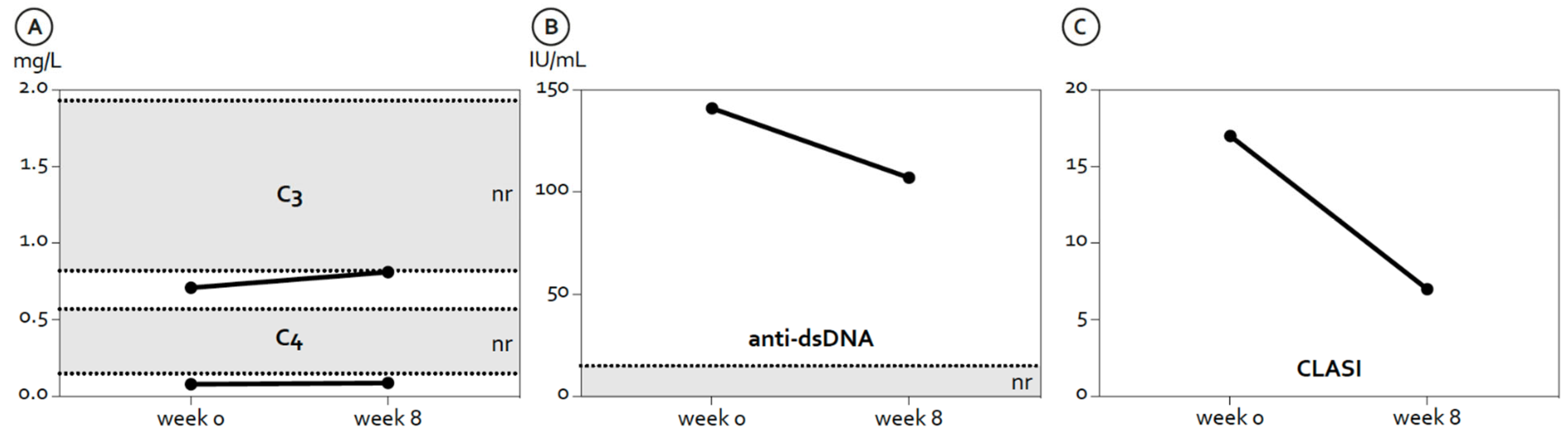
2. Case Description
3. Review of Anifrolumab Mechanism of Action and Clinical Trials
3.1. Development and Mechanism of Action of Anifrolumab
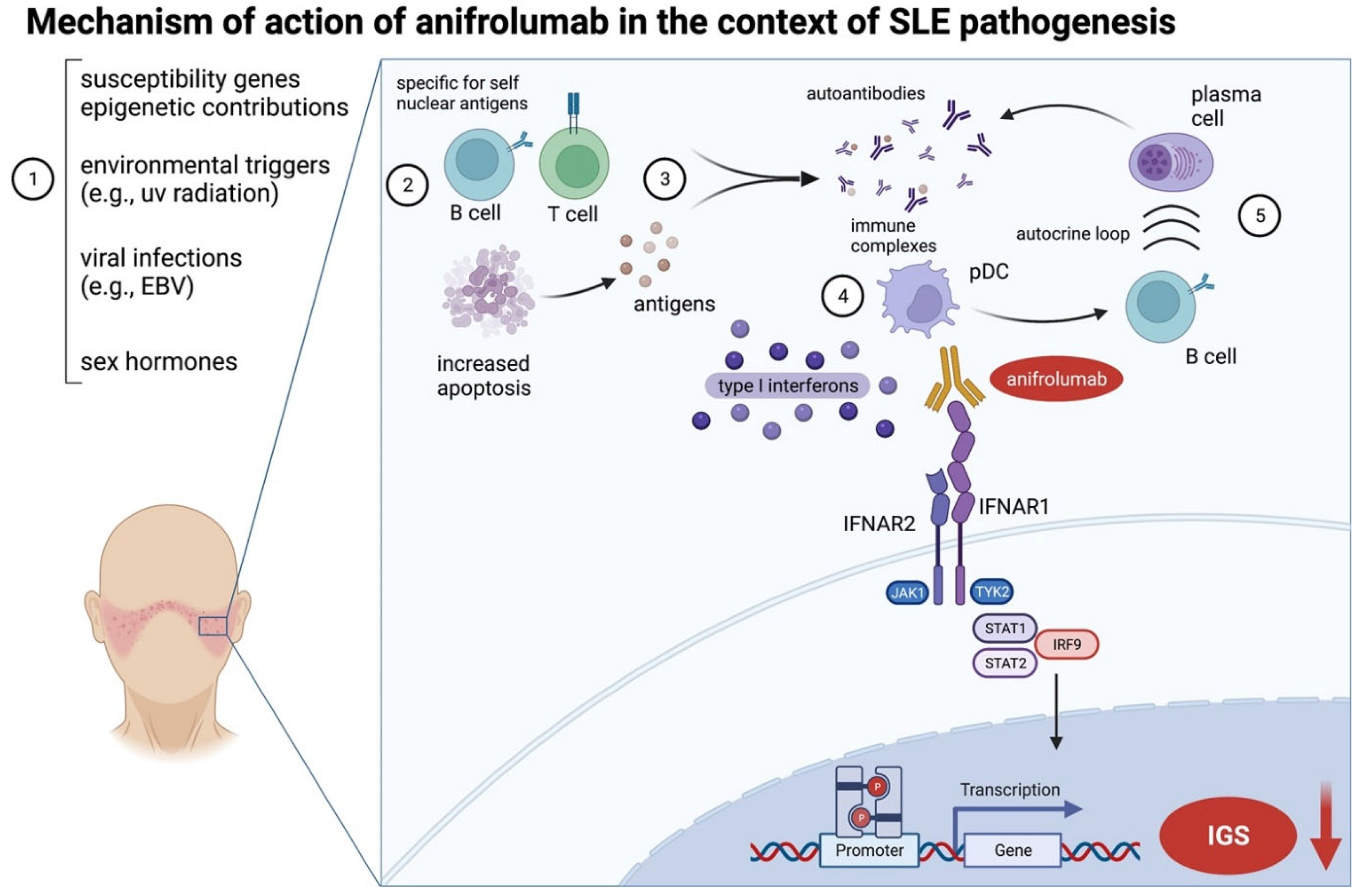
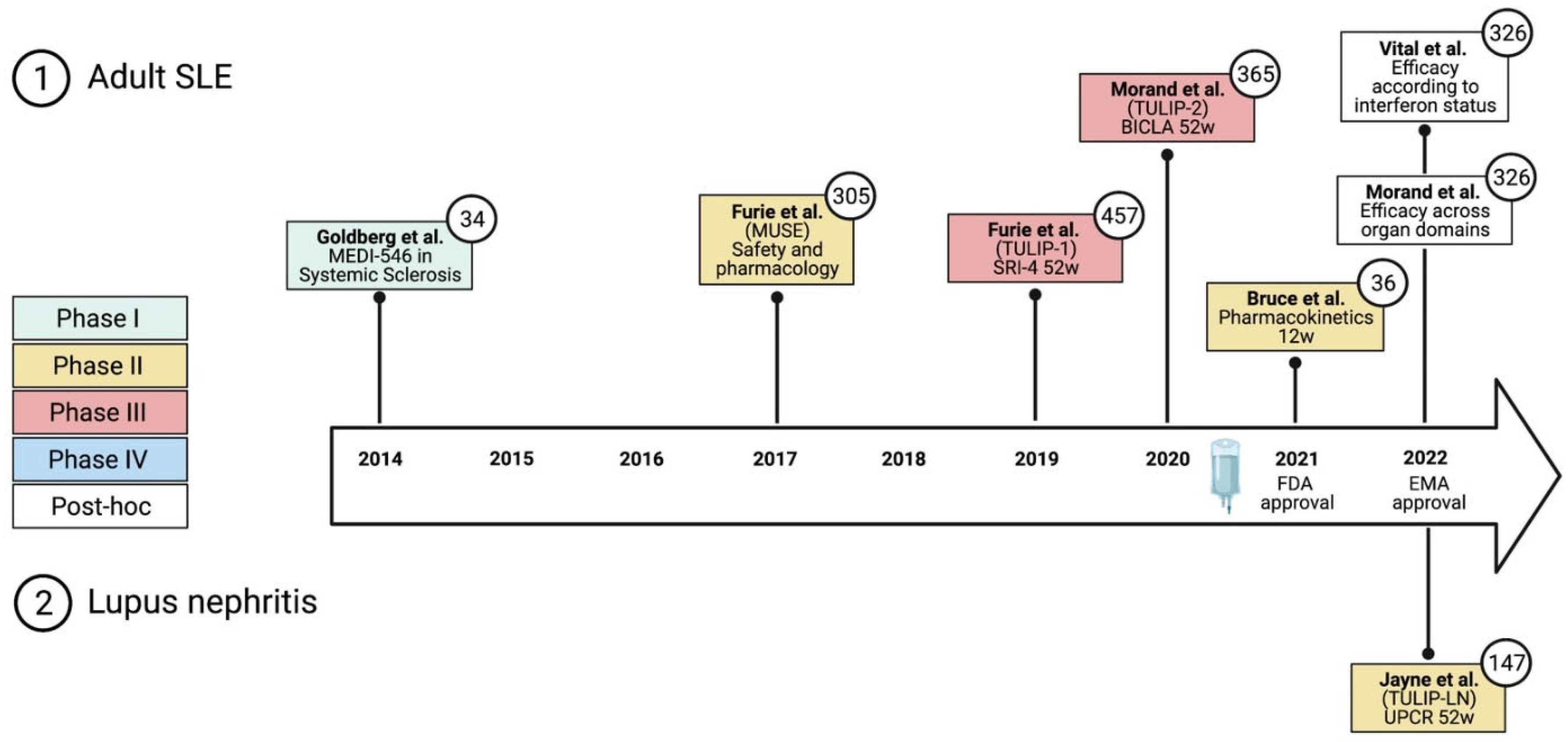
3.2. Clinical Trial Data
3.2.1. Early Phase I and Phase II Trials
3.2.2. Phase III Trials—TULIP-1 and TULIP-2
| First Author, Year | Trial Acronym | Phase | N of Participants | Primary Endpoint Assessment | Outcome Measures (% Responders) § (Anifrolumab 300 mg/Placebo) | ||
|---|---|---|---|---|---|---|---|
| Intravenous administration | SRI-4 | BICLA | CLASI | ||||
| Furie, 2017 [17] | MUSE | IIb | 305 | 24 weeks | 34.3/17.6 | 53.5/25.7 | 63/30.8 |
| Furie, 2019 [2] | TULIP-1 | III | 457 | 52 weeks | 36/40 | 37/27 | 42/25 * |
| Morand, 2020 [1] | TULIP-2 | III | 365 | 52 weeks | 55.5/37.3 | 47.8/31.5 | 49/25 * |
| Subcutaneous administration | |||||||
| Bruce, 2021 [21] | - | II | 36 | 12 weeks | -/- | -/- | 45/44 * |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morand, E.F.; Furie, R.; Tanaka, Y.; Bruce, I.N.; Askanase, A.D.; Richez, C.; Bae, S.-C.; Brohawn, P.Z.; Pineda, L.; Berglind, A.; et al. Trial of Anifrolumab in Active Systemic Lupus Erythematosus. N. Engl. J. Med. 2020, 382, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Furie, R.A.; Morand, E.F.; Bruce, I.N.; Manzi, S.; Kalunian, K.C.; Vital, E.M.; Ford, T.L.; Gupta, R.; Hiepe, F.; Santiago, M.; et al. Type I Interferon Inhibitor Anifrolumab in Active Systemic Lupus Erythematosus (TULIP-1): A Randomised, Controlled, Phase 3 Trial. Lancet Rheumatol. 2019, 1, e208–e219. [Google Scholar] [CrossRef]
- Tsokos, G.C.; Lo, M.S.; Costa Reis, P.; Sullivan, K.E. New Insights into the Immunopathogenesis of Systemic Lupus Erythematosus. Nat. Rev. Rheumatol. 2016, 12, 716–730. [Google Scholar] [CrossRef] [PubMed]
- Kaul, A.; Gordon, C.; Crow, M.K.; Touma, Z.; Urowitz, M.B.; van Vollenhoven, R.; Ruiz-Irastorza, G.; Hughes, G. Systemic Lupus Erythematosus. Nat. Rev. Dis. Primers 2016, 2, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Niewold, T.B. Interferon Alpha as a Primary Pathogenic Factor in Human Lupus. J. Interferon Cytokine Res. 2011, 31, 887–892. [Google Scholar] [CrossRef] [Green Version]
- Rönnblom, L.; Leonard, D. Interferon Pathway in SLE: One Key to Unlocking the Mystery of the Disease. Lupus Sci. Med. 2019, 6, e000270. [Google Scholar] [CrossRef] [Green Version]
- Cooles, F.A.H.; Isaacs, J.D. The Interferon Gene Signature as a Clinically Relevant Biomarker in Autoimmune Rheumatic Disease. Lancet Rheumatol. 2022, 4, e61–e72. [Google Scholar] [CrossRef]
- Khamashta, M.; Merrill, J.T.; Werth, V.P.; Furie, R.; Kalunian, K.; Illei, G.G.; Drappa, J.; Wang, L.; Greth, W. Sifalimumab, an Anti-Interferon-α Monoclonal Antibody, in Moderate to Severe Systemic Lupus Erythematosus: A Randomised, Double-Blind, Placebo-Controlled Study. Ann. Rheum. Dis. 2016, 75, 1909–1916. [Google Scholar] [CrossRef] [Green Version]
- Kalunian, K.C.; Merrill, J.T.; Maciuca, R.; McBride, J.M.; Townsend, M.J.; Wei, X.; Davis, J.C.; Kennedy, W.P. A Phase II Study of the Efficacy and Safety of Rontalizumab (RhuMAb Interferon-α) in Patients with Systemic Lupus Erythematosus (ROSE). Ann. Rheum. Dis. 2016, 75, 196–202. [Google Scholar] [CrossRef]
- Riggs, J.M.; Hanna, R.N.; Rajan, B.; Zerrouki, K.; Karnell, J.L.; Sagar, D.; Vainshtein, I.; Farmer, E.; Rosenthal, K.; Morehouse, C.; et al. Characterisation of Anifrolumab, a Fully Human Anti-Interferon Receptor Antagonist Antibody for the Treatment of Systemic Lupus Erythematosus. Lupus Sci. Med. 2018, 5, e000261. [Google Scholar] [CrossRef]
- Oganesyan, V.; Gao, C.; Shirinian, L.; Wu, H.; Dall’Acqua, W.F. Structural Characterization of a Human Fc Fragment Engineered for Lack of Effector Functions. Acta Cryst. D Biol. Cryst. 2008, 64, 700–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.S.W.; Rojas, O.L.; Gommerman, J.L. B Cell Depletion Therapies in Autoimmune Disease: Advances and Mechanistic Insights. Nat. Rev. Drug Discov. 2021, 20, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Chasset, F.; Dayer, J.-M.; Chizzolini, C. Type I Interferons in Systemic Autoimmune Diseases: Distinguishing Between Afferent and Efferent Functions for Precision Medicine and Individualized Treatment. Front. Pharmacol. 2021, 12, 633821. [Google Scholar] [CrossRef] [PubMed]
- Jayne, D.; Rovin, B.; Mysler, E.F.; Furie, R.A.; Houssiau, F.A.; Trasieva, T.; Knagenhjelm, J.; Schwetje, E.; Chia, Y.L.; Tummala, R.; et al. Phase II Randomised Trial of Type I Interferon Inhibitor Anifrolumab in Patients with Active Lupus Nephritis. Ann. Rheum. Dis. 2022, 81, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.; Geppert, T.; Schiopu, E.; Frech, T.; Hsu, V.; Simms, R.W.; Peng, S.L.; Yao, Y.; Elgeioushi, N.; Chang, L.; et al. Dose-Escalation of Human Anti-Interferon-α Receptor Monoclonal Antibody MEDI-546 in Subjects with Systemic Sclerosis: A Phase 1, Multicenter, Open Label Study. Arthritis Res. Ther. 2014, 16, R57. [Google Scholar] [CrossRef] [Green Version]
- Muskardin, T.L.W.; Niewold, T.B. Type I Interferon in Rheumatic Diseases. Nat. Rev. Rheumatol. 2018, 14, 214–228. [Google Scholar] [CrossRef]
- Furie, R.; Khamashta, M.; Merrill, J.T.; Werth, V.P.; Kalunian, K.; Brohawn, P.; Illei, G.G.; Drappa, J.; Wang, L.; Yoo, S.; et al. Anifrolumab, an Anti-Interferon-α Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus. Arthritis Rheumatol. 2017, 69, 376–386. [Google Scholar] [CrossRef] [Green Version]
- Salmon, J.E.; Niewold, T.B. A Successful Trial for Lupus—How Good Is Good Enough? N. Engl. J. Med. 2020, 382, 287–288. [Google Scholar] [CrossRef]
- Ohmura, K. Which Is the Best SLE Activity Index for Clinical Trials? Mod. Rheumatol. 2021, 31, 20–28. [Google Scholar] [CrossRef]
- Merrill, J.T. For Lupus Trials, the Answer Might Depend on the Question. Lancet Rheumatol. 2019, 1, e196–e197. [Google Scholar] [CrossRef]
- Bruce, I.N.; Nami, A.; Schwetje, E.; Pierson, M.E.; Rouse, T.; Chia, Y.L.; Kuruvilla, D.; Abreu, G.; Tummala, R.; Lindholm, C. Pharmacokinetics, Pharmacodynamics, and Safety of Subcutaneous Anifrolumab in Patients with Systemic Lupus Erythematosus, Active Skin Disease, and High Type I Interferon Gene Signature: A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 2 Study. Lancet Rheumatol. 2021, 3, e101–e110. [Google Scholar] [CrossRef]
- Morand, E.F.; Furie, R.A.; Bruce, I.N.; Vital, E.M.; Dall’Era, M.; Maho, E.; Pineda, L.; Tummala, R. Efficacy of Anifrolumab across Organ Domains in Patients with Moderate-to-Severe Systemic Lupus Erythematosus: A Post-Hoc Analysis of Pooled Data from the TULIP-1 and TULIP-2 Trials. Lancet Rheumatol. 2022, 4, e282–e292. [Google Scholar] [CrossRef]
- Klein, R.; Moghadam-Kia, S.; LoMonico, J.; Okawa, J.; Coley, C.; Taylor, L.; Troxel, A.B.; Werth, V.P. Development of the CLASI as a Tool to Measure Disease Severity and Responsiveness to Therapy in Cutaneous Lupus Erythematosus. Arch. Dermatol. 2011, 147, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, I.; Klein, R.; Okawa, J.; Werth, V.P. The Interferon-Regulated Gene Signature Is Elevated in Subacute Cutaneous Lupus Erythematosus and Discoid Lupus Erythematosus and Correlates with the Cutaneous Lupus Area and Severity Index Score. Br. J. Derm. 2012, 166, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Shipman, W.D.; Chyou, S.; Ramanathan, A.; Izmirly, P.M.; Sharma, S.; Pannellini, T.; Dasoveanu, D.C.; Qing, X.; Magro, C.M.; Granstein, R.D.; et al. A Protective Langerhans Cell-Keratinocyte Axis That Is Dysfunctional in Photosensitivity. Sci. Transl. Med. 2018, 10, eaap9527. [Google Scholar] [CrossRef] [Green Version]
- Billi, A.C.; Ma, F.; Plazyo, O.; Gharaee-Kermani, M.; Wasikowski, R.; Hile, G.A.; Xing, X.; Yee, C.M.; Rizvi, S.M.; Maz, M.P.; et al. Nonlesional Lupus Skin Contributes to Inflammatory Education of Myeloid Cells and Primes for Cutaneous Inflammation. Sci. Transl. Med. 2022, 14, eabn2263. [Google Scholar] [CrossRef]
- Plüß, M.; Piantoni, S.; Tampe, B.; Kim, A.; Korsten, P. Product Review: Belimumab for Systemic Lupus Erythematosus–Focus on Lupus Nephritis. Hum. Vaccines Immunother. 2022, 2072143. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plüß, M.; Piantoni, S.; Wincup, C.; Korsten, P. Rapid Response of Refractory Systemic Lupus Erythematosus Skin Manifestations to Anifrolumab—A Case-Based Review of Clinical Trial Data Suggesting a Domain-Based Therapeutic Approach. J. Clin. Med. 2022, 11, 3449. https://doi.org/10.3390/jcm11123449
Plüß M, Piantoni S, Wincup C, Korsten P. Rapid Response of Refractory Systemic Lupus Erythematosus Skin Manifestations to Anifrolumab—A Case-Based Review of Clinical Trial Data Suggesting a Domain-Based Therapeutic Approach. Journal of Clinical Medicine. 2022; 11(12):3449. https://doi.org/10.3390/jcm11123449
Chicago/Turabian StylePlüß, Marlene, Silvia Piantoni, Chris Wincup, and Peter Korsten. 2022. "Rapid Response of Refractory Systemic Lupus Erythematosus Skin Manifestations to Anifrolumab—A Case-Based Review of Clinical Trial Data Suggesting a Domain-Based Therapeutic Approach" Journal of Clinical Medicine 11, no. 12: 3449. https://doi.org/10.3390/jcm11123449
APA StylePlüß, M., Piantoni, S., Wincup, C., & Korsten, P. (2022). Rapid Response of Refractory Systemic Lupus Erythematosus Skin Manifestations to Anifrolumab—A Case-Based Review of Clinical Trial Data Suggesting a Domain-Based Therapeutic Approach. Journal of Clinical Medicine, 11(12), 3449. https://doi.org/10.3390/jcm11123449








