Comparison of Postoperative Stability of Intraocular Lenses after Phacovitrectomy for Rhegmatogenous Retinal Detachment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. IOLs
2.3. Operative Procedures
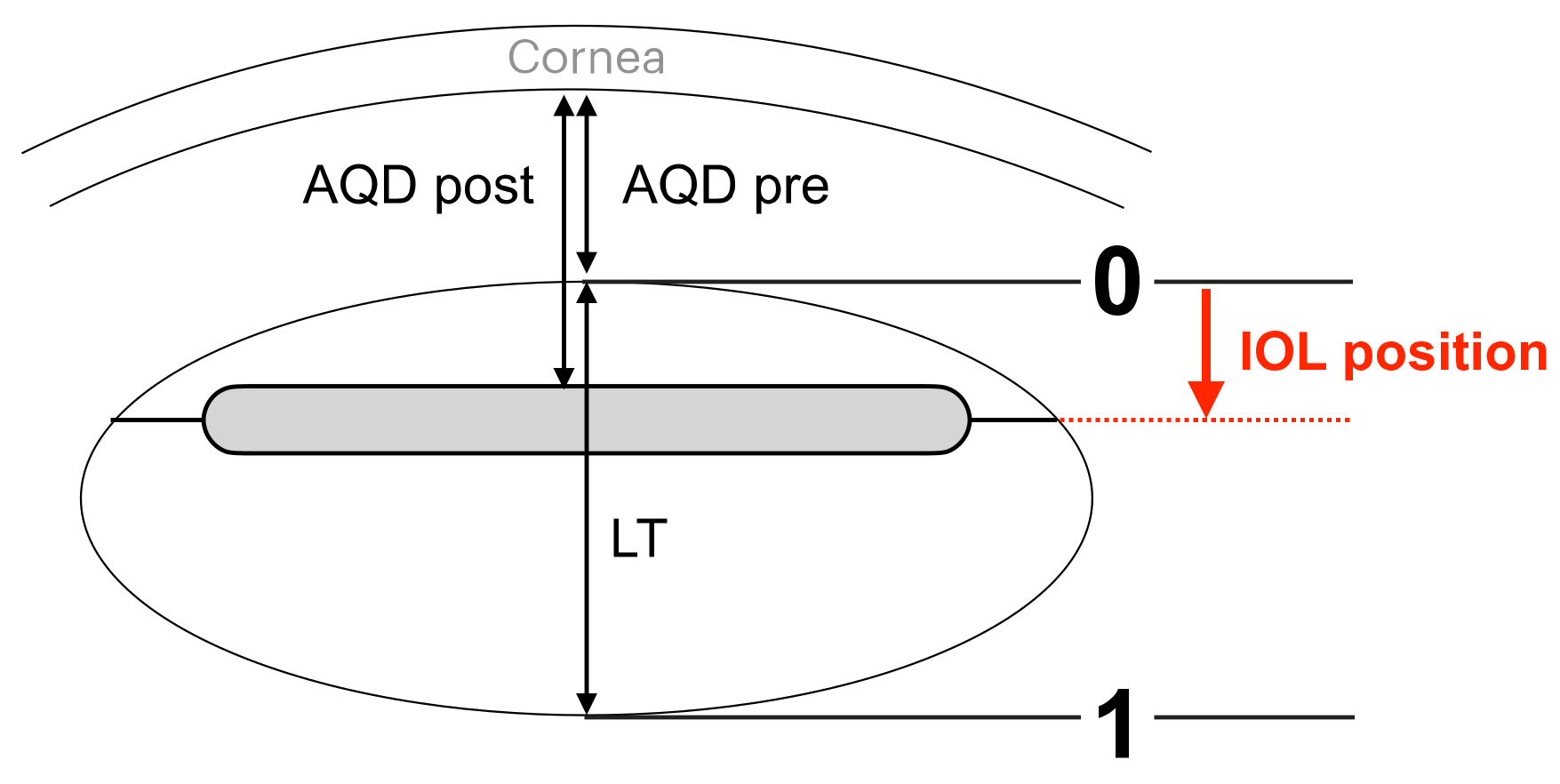
2.4. Assessments
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Postoperative Refractive Error
3.3. Swept-Source Anterior Segment Optical Coherence Tomography Parameters and Intraocular Lens Position
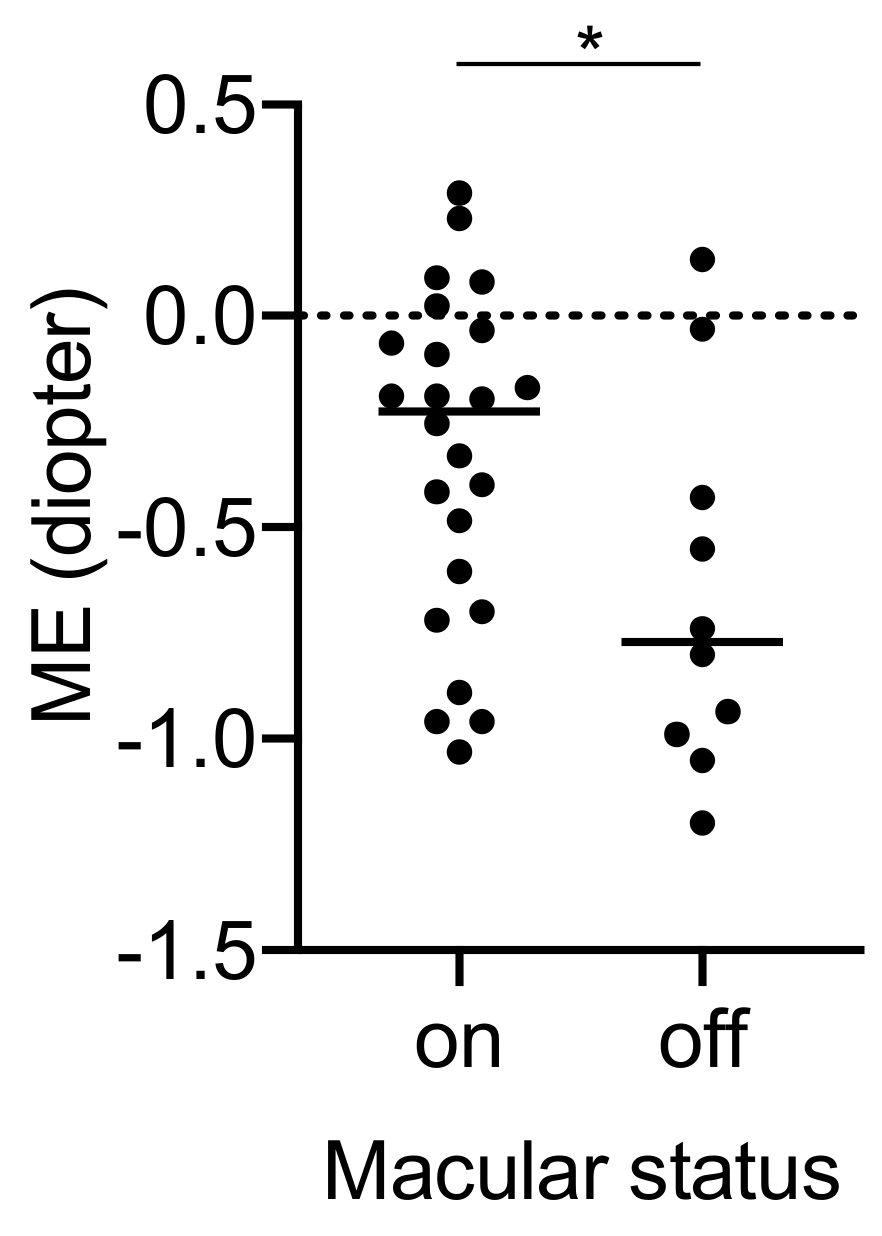
3.4. Simple Regression Analysis between ME and Variables
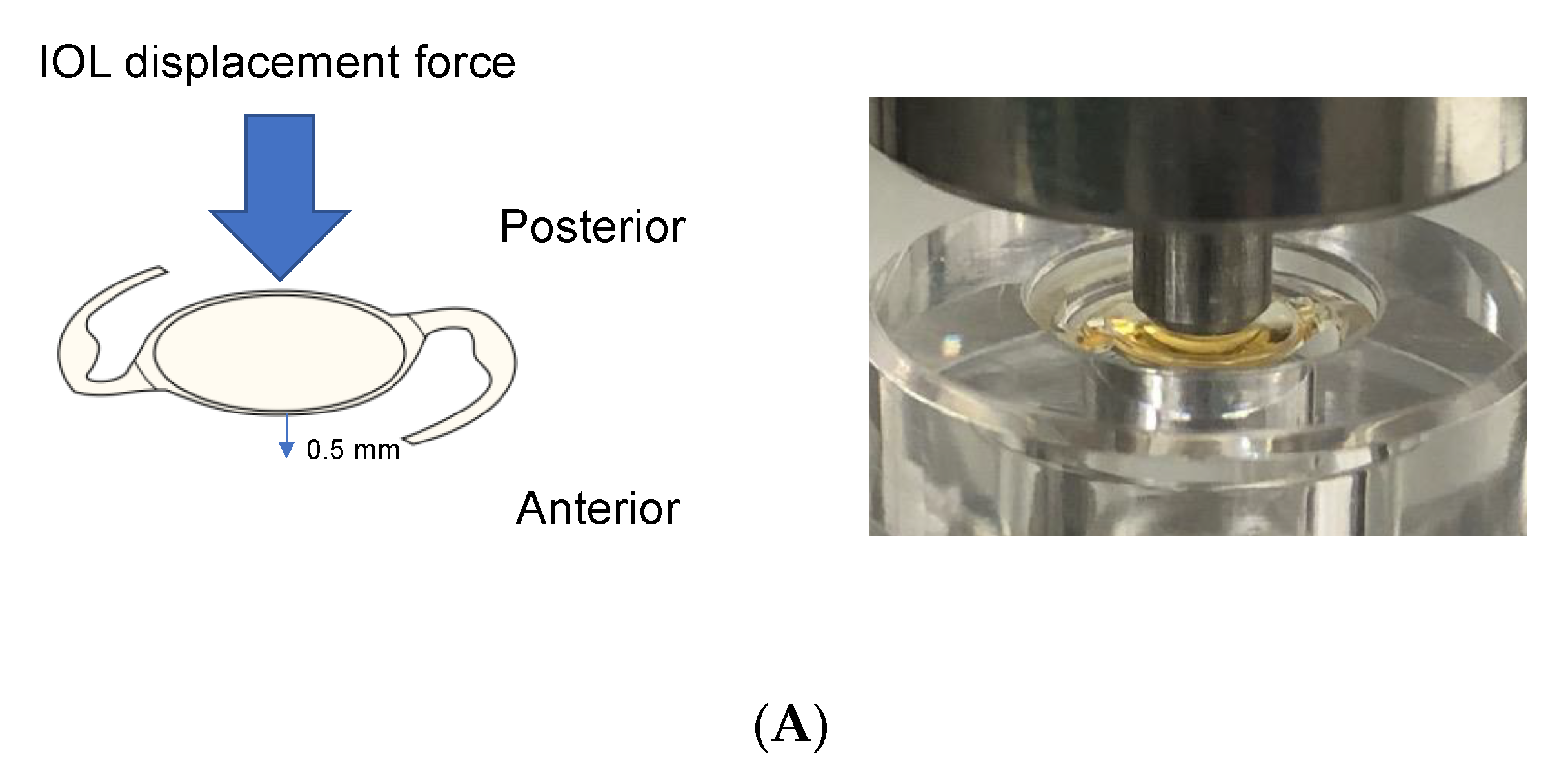
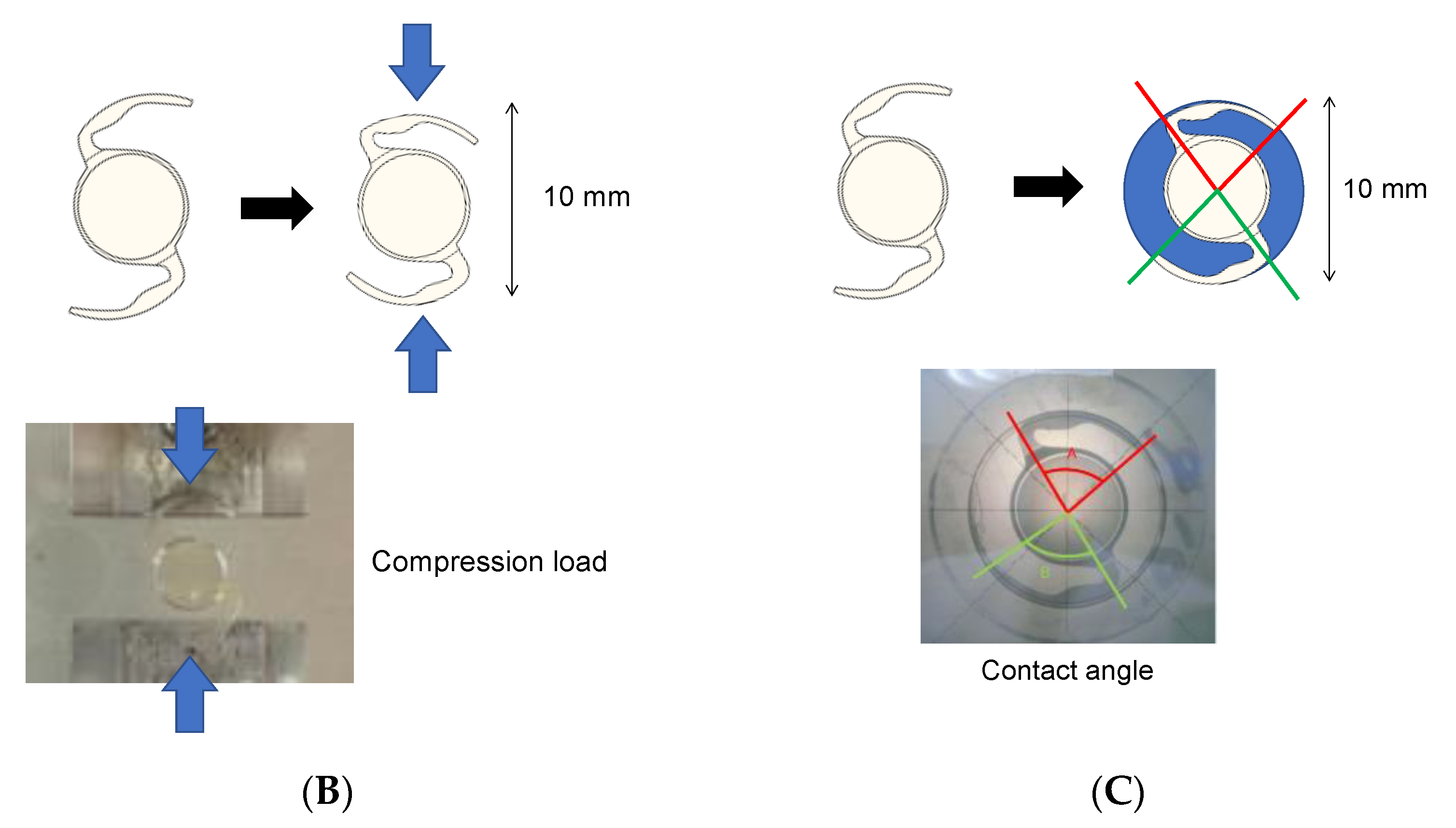
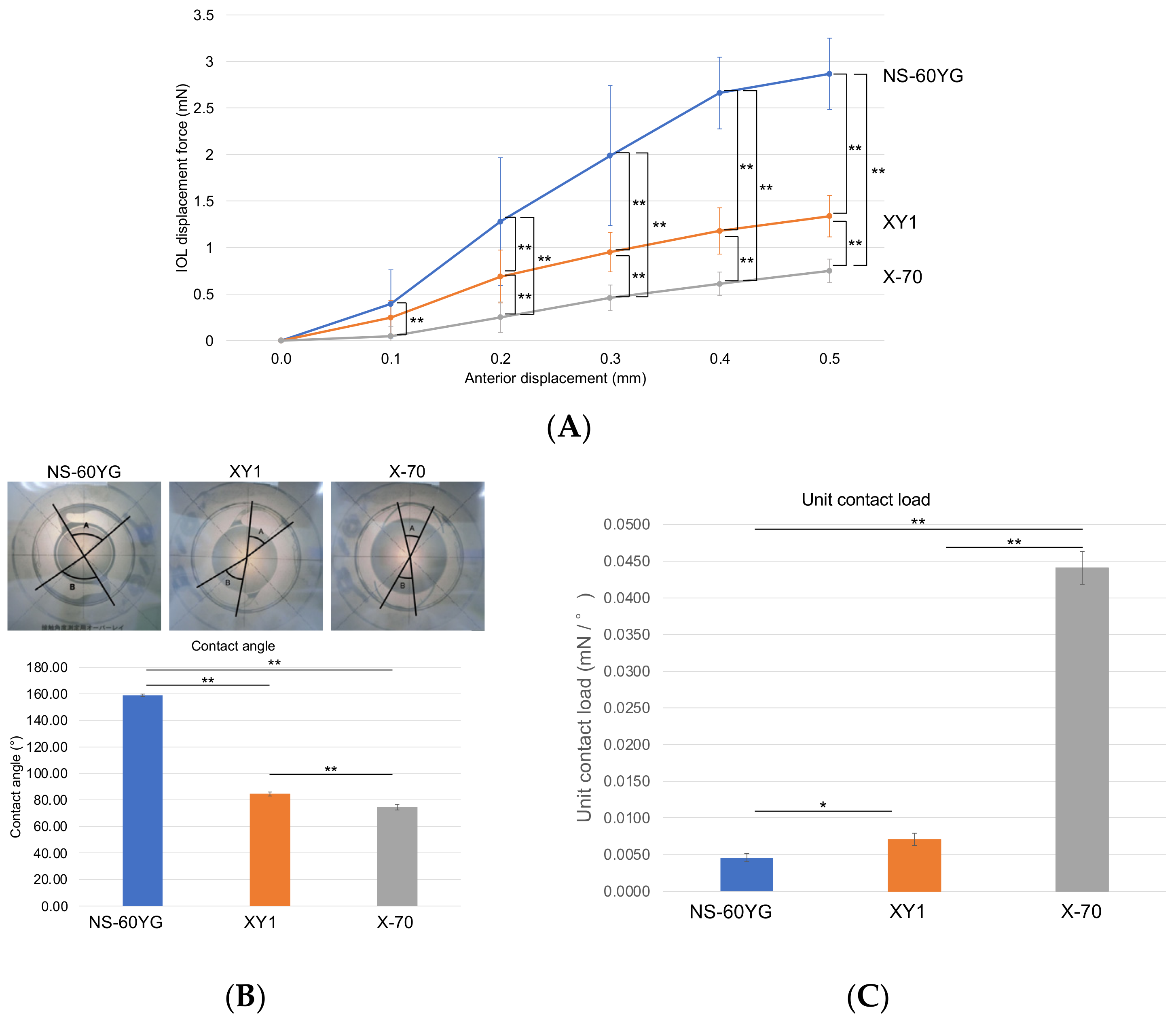
3.5. IOL Displacement Force, Contact Angle, and Compression Load
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heimann, H.; Bartz-Schmidt, K.U.; Bornfeld, N.; Weiss, C.; Hilgers, R.D.; Foerster, M.H.; Scleral Buckling versus Primary Vitrectomy in Rhegmatogenous Retinal Detachment Study Group. Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment: A prospective randomized multicenter clinical study. Ophthalmology 2007, 114, 2142–2154. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, N.; Wakabayashi, T.; Sakaguchi, H.; Nishida, K. Effect of Gas Tamponade on the Intraocular Lens Position and Refractive Error after Phacovitrectomy: A Swept-Source Anterior Segment OCT Analysis. Ophthalmology 2020, 127, 511–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demetriades, A.M.; Gottsch, J.D.; Thomsen, R.; Azab, A.; Stark, W.J.; Campochiaro, P.A.; de Juan, E., Jr.; Haller, J.A. Combined phacoemulsification, intraocular lens implantation, and vitrectomy for eyes with coexisting cataract and vitreoretinal pathology. Am. J. Ophthalmol. 2003, 135, 291–296. [Google Scholar] [CrossRef]
- Tan, A.; Bertrand-Boiche, M.; Angioi-Duprez, K.; Berrod, J.P.; Conart, J.B. Outcomes of Combined Phacoemulsification and Pars Plana Vitrectomy for Rhegmatogenous Retinal Detachment: A Comparative Study. Retina 2021, 41, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Raman, S.V.; Pappas, G.; Simcock, P.; Ling, R.; Shaw, S. Phacovitrectomy for primary retinal detachment repair in presbyopes. Retina 2007, 27, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Hotte, G.J.; de Bruyn, D.P.; de Hoog, J. Post-operative Refractive Prediction Error after Phacovitrectomy: A Retrospective Study. Ophthalmol. Ther. 2018, 7, 83–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mochiji, M.; Kaidzu, S.; Ishiba, Y.; Matsuda, Y.; Tanito, M. Measurement of Force Required for Anterior Displacement of Intraocular Lenses and Its Defining Parameters. Materials 2020, 13, 4593. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, N.; Wakabayashi, T.; Sakaguchi, H.; Nishida, K. Optical Biometry-Based Intraocular Lens Calculation and Refractive Outcomes after Phacovitrectomy for Rhegmatogenous Retinal Detachment and Epiretinal Membrane. Sci. Rep. 2018, 8, 11319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nejima, R.; Miyai, T.; Kataoka, Y.; Miyata, K.; Honbou, M.; Tokunaga, T.; Kawana, K.; Kiuchi, T.; Oshika, T. Prospective intrapatient comparison of 6.0-millimeter optic single-piece and 3-piece hydrophobic acrylic foldable intraocular lenses. Ophthalmology 2006, 113, 585–590. [Google Scholar] [CrossRef]
- Cho, K.H.; Park, I.W.; Kwon, S.I. Changes in postoperative refractive outcomes following combined phacoemulsification and pars plana vitrectomy for rhegmatogenous retinal detachment. Am. J. Ophthalmol. 2014, 158, 251–256.e2. [Google Scholar] [CrossRef] [PubMed]
- Cabeza-Gil, I.; Ariza-Gracia, M.A.; Remon, L.; Calvo, B. Systematic Study on the Biomechanical Stability of C-Loop Intraocular Lenses: Approach to an Optimal Design of the Haptics. Ann. Biomed. Eng. 2020, 48, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
| NS-60YG | XY1 | X-70 | |
|---|---|---|---|
| Design | Aspherical, 1 piece | Aspherical, 1 piece | Spherical, 3 piece |
| Material | Acrylic resin | Acrylic resin | Optic, acrylic resinhaptic, polyvinylidene difluoride |
| Optic diameter, mm | 6.0 | 6.0 | 7.0 |
| Total length, mm | 13.0 | 13.0 | 13.2 |
| Haptics angle, ° | 0 | 0 | 7 |
| Appearance |  |  |  |
| Characteristics | Overall | Intraocular Lens Group | p-Value | ||
|---|---|---|---|---|---|
| NS-60YG | XY1 | X-70 | |||
| Eyes/patients | 37/36 | 15/14 | 11/11 | 11/11 | - |
| Age, y | 60.6 ± 8.3 | 60.0 ± 7.4 | 60.5 ± 8.1 | 61.6 ± 9.5 | 0.89 |
| Men/women | 26/11 | 14/1 | 7/4 | 5/6 | <0.05 |
| Macular status (on/off) | 27/10 | 11/4 | 8/3 | 8/3 | 0.99 |
| Preoperative BCVA, logMAR | 0.30 ± 0.60 | 0.39 ± 0.71 | 0.21 ± 0.39 | 0.31 ± 0.61 | 0.77 |
| Postoperative BCVA, logMAR | −0.02 ± 0.18 | 0.02 ± 0.24 | −0.05 ± 0.12 | −0.02 ± 0.10 | 0.85 |
| Axial length, mm | 25.6 ± 1.4 | 25.7 ± 1.2 | 25.6 ± 1.2 | 25.5 ± 1.8 | 0.97 |
| AQD, mm | 2.79 ± 0.33 | 2.69 ± 0.29 | 2.91 ± 0.37 | 2.82 ± 0.28 | 0.25 |
| ACW, mm | 11.69 ± 0.34 | 11.68 ± 0.40 | 11.71 ± 0.38 | 11.66 ± 0.34 | 0.95 |
| CCT, µm | 552.31 ± 37.99 | 562.86 ± 25.28 | 546.00 ± 42.77 | 544.50 ± 43.41 | 0.43 |
| Lens thickness, mm | 4.51 ± 0.28 | 4.51 ± 0.28 | 4.51 ± 0.28 | 4.51 ± 0.28 | 0.94 |
| Overall | Intraocular Lens Group | p-Value | |||
|---|---|---|---|---|---|
| NS-60YG (n = 15 Eyes) | XY1 (n = 11 Eyes) | X-70 (n = 11 Eyes) | |||
| MedAE | 0.44 ± 0.33 | 0.31 ± 0.26 | 0.44 ± 0.36 | 0.58 ± 0.32 | 0.14 |
| ME | −0.39 ± 0.39 | −0.23 ± 0.34 | −0.37 ± 0.44 | −0.58 ± 0.32 | 0.09 |
| Overall | Intraocular Lens Group | p-Value | |||
|---|---|---|---|---|---|
| NS-60YG (n = 15 Eyes) | XY1 (n = 11 Eyes) | X-70 (n = 11 Eyes) | |||
| 1 week postoperatively | |||||
| AQD, mm | 4.06 ± 0.33 | 4.13 ± 0.29 | 3.99 ± 0.37 | 4.02 ± 0.28 | 0.56 |
| IOL position, a.u | 0.27 ± 0.07 | 0.32 ± 0.07 | 0.24 ± 0.05 | 0.26 ± 0.04 | <0.05 |
| IOL tilt, degree ° | 4.51 ± 1.95 | 4.35 ± 2.61 | 4.61 ± 1.32 | 4.64 ± 1.37 | 0.93 |
| IOL decentration, mm | 0.26 ± 0.18 | 0.29 ± 0.18 | 0.38 ± 0.14 | 0.13 ± 0.11 | <0.01 |
| 1 month postoperatively | |||||
| AQD, mm | 4.19 ± 0.26 | 4.26 ± 0.26 | 4.13 ± 0.25 | 4.13 ± 0.23 | 0.40 |
| IOL position, a.u | 0.30 ± 0.05 | 0.35 ± 0.05 | 0.27 ± 0.03 | 0.28 ± 0.03 | <0.01 |
| IOL tilt, degree ° | 3.94 ± 1.39 | 3.68 ± 1.74 | 3.96 ± 1.39 | 4.37 ± 1.37 | 0.67 |
| IOL decentration, mm | 0.25 ± 0.15 | 0.27 ± 0.13 | 0.30 ± 0.17 | 0.15 ± 0.09 | 0.13 |
| Mean Prediction Refractive Error | ||
|---|---|---|
| Variable | r | p-Value |
| IOL | −0.037 | 0.72 |
| Axial length | −0.054 | 0.35 |
| Preoperative AQD | −0.15 | 0.64 |
| AQD 1 month postoperatively | 0.40 | 0.35 |
| IOL position 1 month postoperatively | −1.04 | 0.068 |
| Macular status (on/off) | 0.34 | 0.048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akiyama, A.; Yokota, H.; Aso, H.; Hanazaki, H.; Iwasaki, M.; Yamagami, S.; Nagaoka, T. Comparison of Postoperative Stability of Intraocular Lenses after Phacovitrectomy for Rhegmatogenous Retinal Detachment. J. Clin. Med. 2022, 11, 3438. https://doi.org/10.3390/jcm11123438
Akiyama A, Yokota H, Aso H, Hanazaki H, Iwasaki M, Yamagami S, Nagaoka T. Comparison of Postoperative Stability of Intraocular Lenses after Phacovitrectomy for Rhegmatogenous Retinal Detachment. Journal of Clinical Medicine. 2022; 11(12):3438. https://doi.org/10.3390/jcm11123438
Chicago/Turabian StyleAkiyama, Ayaka, Harumasa Yokota, Hiroshi Aso, Hirotsugu Hanazaki, Masanori Iwasaki, Satoru Yamagami, and Taiji Nagaoka. 2022. "Comparison of Postoperative Stability of Intraocular Lenses after Phacovitrectomy for Rhegmatogenous Retinal Detachment" Journal of Clinical Medicine 11, no. 12: 3438. https://doi.org/10.3390/jcm11123438
APA StyleAkiyama, A., Yokota, H., Aso, H., Hanazaki, H., Iwasaki, M., Yamagami, S., & Nagaoka, T. (2022). Comparison of Postoperative Stability of Intraocular Lenses after Phacovitrectomy for Rhegmatogenous Retinal Detachment. Journal of Clinical Medicine, 11(12), 3438. https://doi.org/10.3390/jcm11123438






