The Clinical Relevance of Hypothyroidism in Patients with Solid Non-Thyroid Cancer: A Tantalizing Conundrum
Abstract
1. Introduction
2. Methods
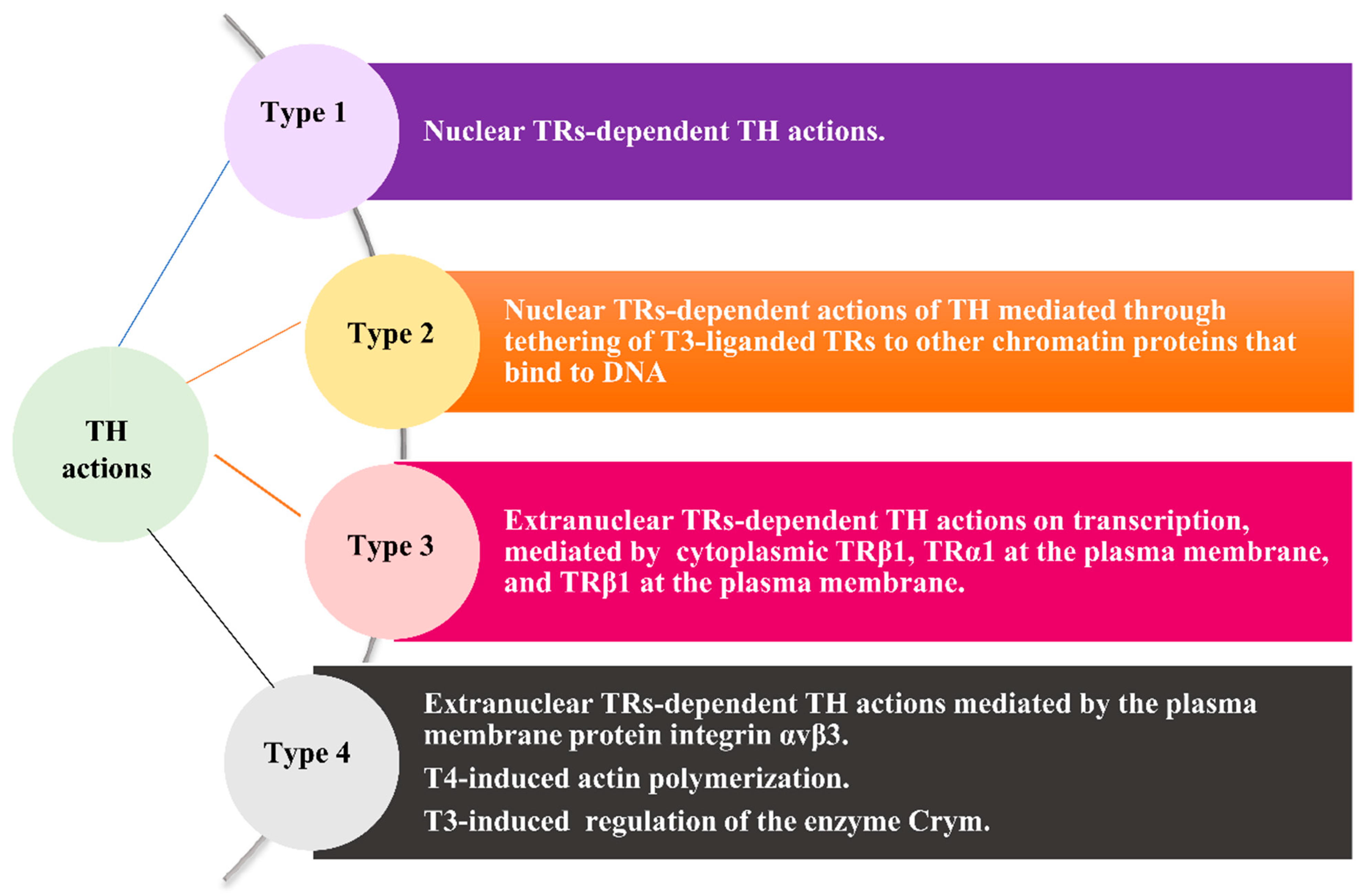
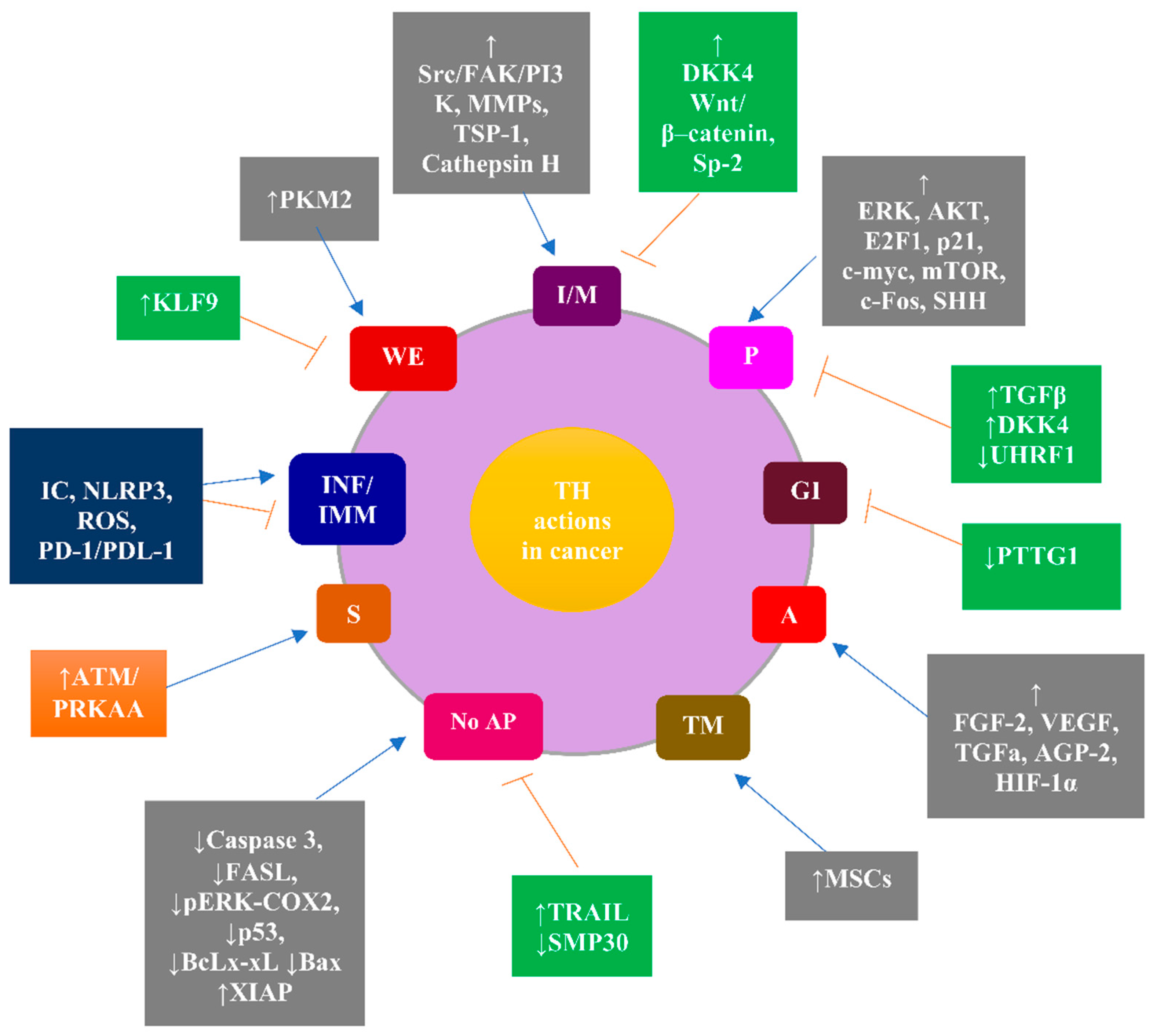
3. Molecular Aspects of the Implication of THs in Cancer as the Rationale for the Clinical Relevance of Hypothyroidism in Cancer Patients
4. Diagnosis of Hypothyroidism in Patients with Solid Non-Thyroid Cancer
5. Treatment of Hypothyroidism in Patients with Solid Non-Thyroid Cancer
6. Clinical Data on Hypothyroidism as a Potential Predictive Factor for Solid Non-Thyroid Cancer
6.1. Clinical Data Indicating an Association between Hypothyroidism or TSH Levels near the Upper Normal Range and a Decreased Risk of Solid Non-Thyroid Cancer
6.2. Clinical Data Indicating an Association between Hypothyroidism and an Increased Risk of Solid Non-Thyroid Cancer
6.3. Clinical Data Indicating the Opposing Effects of THs on the Risk of Solid Non-Thyroid Cancer or No Significant Association between Hypothyroidism and the Risk of Solid Non-Thyroid Cancer
7. Clinical Data on Hypothyroidism as a Potential Prognostic Factor for Solid Non-Thyroid Cancer
7.1. Clinical Data Indicating an Association between Hypothyroidism and a Favorable Prognosis for Solid Non-Thyroid Cancer
7.2. Clinical Data Indicating an Association between Hypothyroidism or Decreased THs Levels and an Unfavorable Prognosis of Solid Non-Thyroid Cancer
7.3. Clinical Data Indicating the Absence of a Significant Association between Hypothyroidism, THs Levels, or TSH Levels and the Prognosis of Solid Non-Thyroid Cancer
8. Clinical Data Indicating an Association between Hypothyroidism Induced by Anticancer Treatment and Favorable Prognoses
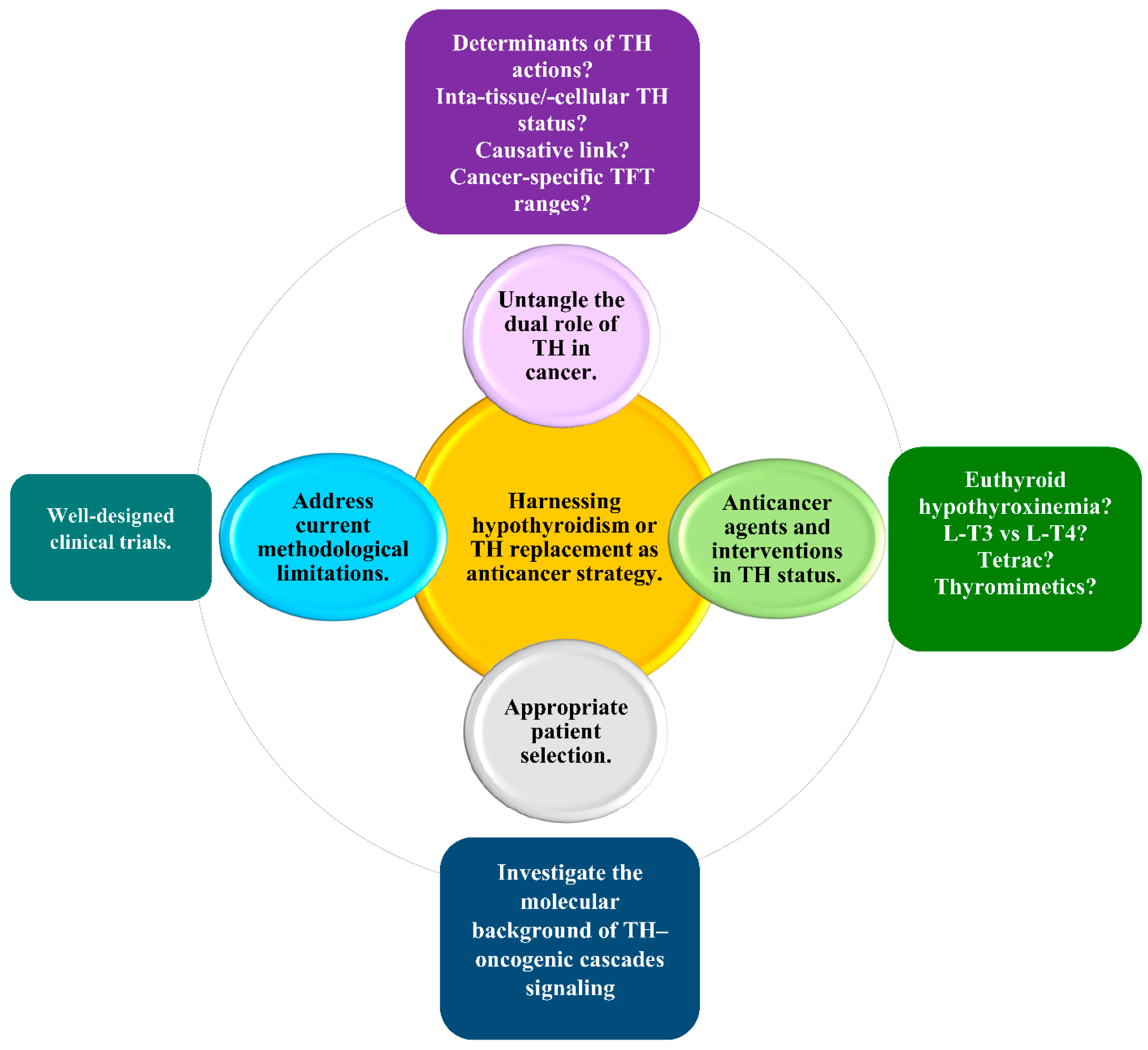
9. Harnessing Hypothyroidism in the Treatment of Solid Non-Thyroid Cancer: Current Challenges and Future Perspectives for a Personalized Approach
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kalra, S.; Priya, G.; Bhattacharya, S.; Saha, Y.R. Oncocrinology. J. Pak. Med. Assoc. 2020, 70, 757–761. [Google Scholar] [PubMed]
- Salvatore, D.; Davies, T.F.; Sohlumberger, M.J.; Hay, I.D.; Reed Larsen, P. Thyroid Physiology and Diagnostic Evaluation of Patients with Thyroid Disorders. In Williams Textbook of Endocrinology, 12th ed.; Melmed, S., Koenig, R., Rosen, C., Auchus, R., Goldfine, A.A., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2011; Chapter 11; pp. 327–361. [Google Scholar]
- Madariaga, A.G.; Palacios, S.S.; Guillén-Grima, F.; Galofré, J.C. The incidence and prevalence of thyroid dysfunction in Europe: A meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Biondi, B.; Cappola, A.R.; Cooper, D.S. Subclinical Hypothyroidism: A Review. JAMA 2019, 9, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Mateo, R.C.I.; Hennessey, J.V. Thyroxine and treatment of hypothyroidism: Seven decades of experience. Endocrine 2019, 66, 10–17. [Google Scholar] [CrossRef]
- Lindholm, J.; Laurberg, P. Hypothyroidism and thyroid substitution: Historical aspects. J. Thyroid Res. 2011, 2011, 809341. [Google Scholar] [CrossRef]
- Beatson, G.T. On the treatment of inoperable cases of carcinoma of the mamma: Suggestions for a new method of treatment, with illustrative cases. Trans. Med. Chir. Soc. Edinb. 1896, 15, 153–179. [Google Scholar]
- Sap, J.; Muñoz, A.; Damm, K.; Goldberg, Y.; Ghysdael, J.; Leutz, A.; Beug, H.; Vennström, B. Hormone binding and localization of the c-erb-A protein suggest that it is a receptor for thyroid hormone, a nuclear protein that binds to DNA and activates transcription. Nature 1986, 324, 635–640. [Google Scholar]
- Bergh, J.J.; Lin, H.Y.; Lansing, L.; Mohamed, S.N.; Davis, F.B.; Mousa, S.; Davis, P.J. Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 2005, 146, 2864–2871. [Google Scholar] [CrossRef]
- Davis, P.J.; Leonard, J.L.; Lin, H.Y.; Leinung, M.; Mousa, S.A. Molecular Basis of Nongenomic Actions of Thyroid Hormone. Vitam. Horm. 2018, 106, 67–96. [Google Scholar]
- Gauthier, B.R.; Sola-García, A.; Cáliz-Molina, M.Á.; Lorenzo, P.I.; Cobo-Vuilleumier, N.; Capilla-González, V.; Martin-Montalvo, A. Thyroid hormones in diabetes, cancer, and aging. Aging Cell 2020, 19, e13260. [Google Scholar] [CrossRef]
- Liu, Y.C.; Yeh, C.T.; Lin, K.H. Molecular Functions of Thyroid Hormone Signaling in Regulation of Cancer Progression and Anti-Apoptosis. Int. J. Mol. Sci. 2019, 20, 4986. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Goglia, F.; Leonard, J.L. Nongenomic actions of thyroid hormone. Nat. Rev. Endocrinol. 2016, 12, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Krashin, E.; Piekiełko-Witkowska, A.; Ellis, M.; Ashur-Fabian, O. Thyroid Hormones and Cancer: A Comprehensive Review of Preclinical and Clinical Studies. Front. Endocrinol. 2019, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Hercbergs, A. Clinical Implications and Impact of Discovery of the Thyroid Hormone Receptor on Integrin αvβ3—A Review. Front. Endocrinol. 2019, 10, 565. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Tang, H.Y.; Leinung, M.; Mousa, S.A.; Hercbergs, A.; Davis, P.J. Action of reverse T3 on cancer cells. Endocr. Res. 2019, 44, 148–152. [Google Scholar] [CrossRef]
- Davis, P.J.; Tang, H.Y.; Hercbergs, A.; Lin, H.Y.; Keating, K.A.; Mousa, S.A. Bioactivity of Thyroid Hormone Analogs at Cancer Cells. Front. Endocrinol. 2018, 9, 739. [Google Scholar] [CrossRef]
- Persani, L.; Brabant, G.; Dattani, M.; Bonomi, M.; Feldt-Rasmussen, U.; Fliers, E.; Gruters, A.; Maiter, D.; Schoenmakers, N.; van Trotsenburg, A.S.P. 2018 European Thyroid Association (ETA) Guidelines on the Diagnosis and Management of Central Hypothyroidism. Eur. Thyroid J. 2018, 7, 225–237. [Google Scholar] [CrossRef]
- Jonklaas, J.; Bianco, A.C.; Bauer, A.J.; Cappola, A.R.; Celi, F.S.; Cooper, D.S.; Kim, B.W.; Peeters, R.P.; Rosenthal, M.S.; Sawka, A.M.; et al. American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: Prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid 2014, 24, 1670–1751. [Google Scholar] [CrossRef]
- Torino, F.; Barnabei, A.; Paragliola, R.; Baldelli, R.; Appetecchia, M.; Corsello, S.M. Thyroid dysfunction as an unintended side effect of anticancer drugs. Thyroid 2013, 23, 1345–1366. [Google Scholar] [CrossRef]
- Torino, F.; Barnabei, A.; Baldelli, R.; Appetecchia, A. Thyroid Function Abnormalities in Patients Receiving Anticancer Agents, Thyroid Hormone; Agrawal, N.K., Ed.; IntechOpen: London, UK, 2012; Available online: https://www.intechopen.com/chapters/37926 (accessed on 21 November 2021). [CrossRef][Green Version]
- Hamnvik, O.P.; Larsen, P.R.; Marqusee, E. Thyroid Dysfunction from Antineoplastic Agents. J. Natl. Cancer Inst. 2011, 103, 1572–1587. [Google Scholar] [CrossRef]
- Carter, Y.; Sippel, R.S.; Chen, H. Hypothyroidism after a cancer diagnosis: Etiology, diagnosis, complications, and management. Oncologist 2014, 19, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Goyal, A.; Kaur, P.; Singh, R.; Kalra, S. Anticancer Drug-induced Thyroid Dysfunction. Eur. Endocrinol. 2020, 16, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Chalan, P.; Di Dalmazi, G.; Pani, F.; De Remigis, A.; Corsello, A.; Caturegli, P. Thyroid dysfunctions secondary to cancer immunotherapy. J. Endocrinol. Investig. 2018, 41, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Hercbergs, A.A.; Garfield, D.; Ashur-Fabian, O.; Davis, P.J. Re: Thyroid Dysfunction from Antineoplastic Agents. J. Natl. Cancer Inst. 2012, 104, 422–423. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, J.; Tao, C.J.; Chen, M.; Yu, Z.H.; Chen, Y.Y. Research progress of radiation-induced hypothyroidism in head and neck cancer. J. Cancer 2021, 12, 451–459. [Google Scholar] [CrossRef]
- Rizzo, L.; Mana, D.L.; Serra, H.A. Drug-induced hypothyroidism. Medicina 2017, 77, 394–404. [Google Scholar]
- Boomsma, M.J.; Bijl, H.P.; Christianen, M.E.; Beetz, I.; Chouvalova, O.; Steenbakkers, R.J.; van der Laan, B.F.; Wolffenbuttel, B.H.; Oosting, S.F.; Schilstra, C.; et al. A prospective cohort study on radiation-induced hypothyroidism: Development of an NTCP model. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e351–e356. [Google Scholar] [CrossRef]
- Vogelius, I.R.; Bentzen, S.M.; Maraldo, M.V.; Petersen, P.M.; Specht, L. Risk factors for radiation-induced hypothyroidism: A literature-based meta-analysis. Cancer 2011, 117, 5250–5260. [Google Scholar] [CrossRef]
- Makita, N.; Iiri, T. Tyrosine kinase inhibitor-induced thyroid disorders: A review and hypothesis. Thyroid 2013, 23, 151–159. [Google Scholar] [CrossRef]
- Jiao, Q.; Bi, L.; Ren, Y.; Song, S.; Wang, Q.; Wang, Y.S. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol. Cancer 2018, 17, 36. [Google Scholar] [CrossRef]
- Deligiorgi, M.V.; Siasos, G.; Vakkas, L.; Trafalis, D.T. Charting the Unknown Association of COVID-19 with Thyroid Cancer, Focusing on Differentiated Thyroid Cancer: A Call for Caution. Cancers 2021, 13, 5785. [Google Scholar] [CrossRef] [PubMed]
- Bednarczuk, T.; Brix, T.H.; Schima, W.; Zettinig, G.; Kahaly, G.J. 2021 European Thyroid Association Guidelines for the Management of Iodine-Based Contrast Media-Induced Thyroid Dysfunction. Eur. Thyroid J. 2021, 10, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Hercbergs, A.; Leith, J.T. Spontaneous remission of metastatic lung cancer following myxedema coma—An apoptosis-related phenomenon? J. Natl. Cancer Inst. 1993, 85, 1342–1343. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Flamant, F.; Cheng, S.Y.; Hollenberg, A.N.; Moeller, L.C.; Samarut, J.; Wondisford, F.E.; Yen, P.M.; Refetoff, S. Thyroid Hormone Signaling Pathways: Time for a More Precise Nomenclature. Endocrinology 2017, 158, 2052–2057. [Google Scholar] [CrossRef] [PubMed]
- Goemann, I.M.; Romitti, M.; Meyer, E.L.S.; Wajner, S.M.; Maia, A.L. Role of thyroid hormones in the neoplastic process: An overview. Endocr. Relat. Cancer 2017, 24, R367–R385. [Google Scholar] [CrossRef]
- Suhane, S.; Ramanujan, V.K. Thyroid hormone differentially modulates Warburg phenotype in breast cancer cells. Biochem. Biophys. Res. Commun. 2011, 414, 73–78. [Google Scholar] [CrossRef]
- Pappas, L.; Xu, X.L.; Abramson, D.H.; Jhanwar, S.C. Genomic instability and proliferation/survival pathways in RB1-deficient malignancies. Adv. Biol. Regul. 2017, 64, 20–32. [Google Scholar] [CrossRef]
- Kowalik, M.A.; Puliga, E.; Cabras, L.; Sulas, P.; Petrelli, A.; Perra, A.; Ledda-Columbano, G.M.; Morandi, A.; Merlin, S.; Orrù, C.; et al. Thyroid hormone inhibits hepatocellular carcinoma progression via induction of differentiation and metabolic reprogramming. J. Hepatol. 2020, 72, 1159–1169. [Google Scholar] [CrossRef]
- Liao, C.H.; Yeh, S.C.; Huang, Y.H.; Chen, R.N.; Tsai, M.M.; Chen, W.J.; Chi, H.C.; Tai, P.J.; Liao, C.J.; Wu, S.M.; et al. Positive regulation of spondin 2 by thyroid hormone is associated with cell migration and invasion. Endocr. Relat. Cancer 2010, 17, 99–111. [Google Scholar] [CrossRef][Green Version]
- Liao, C.H.; Yeh, C.T.; Huang, Y.H.; Wu, S.M.; Chi, H.C.; Tsai, M.M.; Tsai, C.Y.; Liao, C.J.; Tseng, Y.H.; Lin, Y.H.; et al. Dickkopf 4 positively regulated by the thyroid hormone receptor suppresses cell invasion in human hepatoma cells. Hepatology 2012, 55, 910–920. [Google Scholar] [CrossRef]
- De Luca, R.; Davis, P.J.; Lin, H.Y.; Gionfra, F.; Percario, Z.A.; Affabris, E.; Pedersen, J.Z.; Marchese, C.; Trivedi, P.; Anastasiadou, E.; et al. Thyroid Hormones Interaction with Immune Response, Inflammation and Non-thyroidal Illness Syndrome. Front. Cell Dev. Biol. 2021, 8, 614030. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, M.; Pellizas, C.G. Thyroid Hormone Action on Innate Immunity. Front. Endocrinol. 2019, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, C.; Buldorini, M.; Assi, E.; Cazzato, D.; De Palma, C.; Clementi, E.; Cervia, D. The thyroid hormone triiodothyronine controls macrophage maturation and functions: Protective role during inflammation. Am. J. Pathol. 2014, 184, 230–247. [Google Scholar] [CrossRef] [PubMed]
- Szabo, J.; Foris, G.; Mezosi, E.; Nagy, E.V.; Paragh, G.; Sztojka, I.; Leövey, A. Parameters of respiratory burst and arachidonic acid metabolism in polymorphonuclear granulocytes from patients with various thyroid diseases. Exp. Clin. Endocrinol. Diabetes 1996, 104, 172–176. [Google Scholar] [CrossRef]
- Wyld, L.; Bellantuono, I.; Tchkonia, T.; Morgan, J.; Turner, O.; Foss, F.; George, J.; Danson, S.; Kirkland, J.L. Senescence and Cancer: A Review of Clinical Implications of Senescence and Senotherapies. Cancers 2020, 12, 2134. [Google Scholar] [CrossRef]
- Hoare, M.; Narita, M. The Power behind the Throne: Senescence and the Hallmarks of Cancer. Annu. Rev. Cancer Biol. 2018, 2, 175–194. [Google Scholar] [CrossRef]
- Schmohl, K.A.; Müller, A.M.; Nelson, P.J.; Spitzweg, C. Thyroid Hormone Effects on Mesenchymal Stem Cell Biology in the Tumour Microenvironment. Exp. Clin. Endocrinol. Diabetes 2020, 128, 462–468. [Google Scholar] [CrossRef]
- Garber, J.R.; Cobin, R.H.; Gharib, H.; Hennessey, J.V.; Klein, I.; Mechanick, J.I.; Pessah- Pollack, R.; Singer, P.A.; Woeber, K.A.; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr. Pract. 2012, 18, 988–1028. [Google Scholar] [CrossRef]
- Jonklaas, J.; Razvi, S. Reference intervals in the diagnosis of thyroid dysfunction: Treating patients not numbers. Lancet Diabetes Endocrinol. 2019, 7, 473–483. [Google Scholar] [CrossRef]
- Keestra, S.; Högqvist Tabor, V.; Alvergne, A. Reinterpreting patterns of variation in human thyroid function: An evolutionary ecology perspective. Evol. Med. Public Health 2020, 9, 93–112. [Google Scholar] [CrossRef]
- Vasileiou, M.; Gilbert, J.; Fishburn, S.; Boelaert, K.; Guideline Committee. Thyroid disease assessment and management: Summary of NICE guidance. BMJ 2020, 368, m41, Erratum in BMJ 2020, 368, m437. [Google Scholar] [CrossRef] [PubMed]
- Burch, H.B. Drug Effects on the Thyroid. N. Engl. J. Med. 2019, 381, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S. L-T4 Therapy in the Presence of Pharmacological Interferents. Front. Endocrinol. 2019, 11, 607446. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.M.; Duntas, L.; Fadeyev, V.; Nygaard, B.; Vanderpump, M.P. 2012 ETA guidelines: The use of L-T4 + L-T3 in the treatment of hypothyroidism. Eur. Thyroid J. 2012, 1, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Jonklaas, J.; Bianco, A.C.; Cappola, A.R.; Celi, F.S.; Fliers, E.; Heuer, H.; McAninch, E.A.; Moeller, L.C.; Nygaard, B.; Sawka, A.M.; et al. Evidence-Based Use of Levothyroxine/Liothyronine Combinations in Treating Hypothyroidism: A Consensus Document. Thyroid 2021, 31, 156–182. [Google Scholar] [CrossRef]
- Ettleson, M.D.; Bianco, A.C. Individualized Therapy for Hypothyroidism: Is T4 Enough for Everyone? J. Clin. Endocrinol. Metab. 2020, 105, e3090–e3104. [Google Scholar] [CrossRef]
- Azizi, F.; Amouzegar, A.; Mehran, L.; Abdi, H. LT4 and Slow Release T3 Combination: Optimum Therapy for Hypothyroidism? Int. J. Endocrinol. Metab. 2020, 18, e100870. [Google Scholar] [CrossRef]
- Calissendorff, J.; Falhammar, H. To Treat or Not to Treat Subclinical Hypothyroidism, What Is the Evidence? Medicina 2020, 56, 40. [Google Scholar] [CrossRef]
- Sawka, A.M.; Cappola, A.R.; Peeters, R.P.; Kopp, P.A.; Bianco, A.C.; Jonklaas, J. Patient Context and Thyrotropin Levels Are Important When Considering Treatment of Subclinical Hypothyroidism. Thyroid 2019, 29, 1359–1363. [Google Scholar] [CrossRef]
- Janett-Pellegri, C.; Moutzouri, E.; Farhoumand, P.D.; Rodondi, N.; Agoritsas, T. Traitement de l’hypothyroïdie infraclinique: Mise à jour des connaissances et nouvelles «Rapid Recommendations» dans le BMJ [Treatment of subclinical hypothyroidism: An update of the evidence and the new Rapid Recommendations]. Rev. Med. Suisse 2020, 16, 455–458. [Google Scholar]
- Bekkering, G.E.; Agoritsas, T.; Lytvyn, L.; Heen, A.F.; Feller, M.; Moutzouri, E.; Abdulazeem, H.; Aertgeerts, B.; Beecher, D.; Brito, J.P.; et al. Thyroid hormones treatment for subclinical hypothyroidism: A clinical practice guideline. BMJ 2019, 365, l2006. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.; Bianco, A.C. Urgent need for further research in subclinical hypothyroidism. Nat. Rev. Endocrinol. 2019, 15, 503–504. [Google Scholar] [CrossRef] [PubMed]
- Evron, J.M.; Papaleontiou, M. Decision Making in Subclinical Thyroid Disease. Med. Clin. N. Am. 2021, 105, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Cornelli, U.; Belcaro, G.; Recchia, M.; Finco, A. Levothyroxine and lung cancer in females: The importance of oxidative stress. Reprod. Biol. Endocrinol. 2013, 11, 75. [Google Scholar] [CrossRef]
- Sarosiek, K.; Gandhi, A.V.; Saxena, S.; Kang, C.Y.; Chipitsyna, G.I.; Yeo, C.J.; Arafat, H.A. Hypothyroidism in pancreatic cancer: Role of exogenous thyroid hormone in tumor invasion-preliminary observations. J. Thyroid Res. 2016, 2016, 2454989. [Google Scholar] [CrossRef]
- Wu, C.C.; Islam, M.M.; Nguyen, P.A.; Poly, T.N.; Wang, C.H.; Iqbal, U.; Li, Y.J.; Yang, H.C. Risk of cancer in long-term levothyroxine users: Retrospective population-based study. Cancer Sci. 2021, 112, 2533–2541. [Google Scholar] [CrossRef]
- Boursi, B.; Haynes, K.; Mamtani, R.; Yang, Y.X. Thyroid dysfunction, thyroid hormone replacement and colorectal cancer risk. J. Natl. Cancer Inst. 2015, 107, djv084. [Google Scholar] [CrossRef]
- Rennert, G.; Rennert, H.S.; Pinchev, M.; Gruber, S.B. A case-control study of levothyroxine and the risk of colorectal cancer. J. Natl. Cancer Inst. 2010, 102, 568–572. [Google Scholar] [CrossRef]
- Friedman, G.D.; Schwalbe, J.S.; Habel, L.A. Re: A case-control study of levothyroxine and the risk of colorectal cancer. J. Natl. Cancer Inst. 2011, 103, 1637–1639. [Google Scholar] [CrossRef][Green Version]
- Søgaard, M.; Farkas, D.K.; Ehrenstein, V.; Jørgensen, J.O.; Dekkers, O.M.; Sørensen, H.T. Hypothyroidism and hyperthyroidism and breast cancer risk: A nationwide cohort study. Eur. J. Endocrinol. 2016, 174, 409–414. [Google Scholar] [CrossRef]
- Kim, E.Y.; Chang, Y.; Lee, K.H.; Yun, J.S.; Park, Y.L.; Park, C.H.; Ahn, J.; Shin, H.; Ryu, S. Serum concentration of thyroid hormones in abnormal and euthyroid ranges and breast cancer risk: A cohort study. Int. J. Cancer 2019, 145, 3257–3266. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, F.; Hai, R.; You, Q.; Xie, L.; Shu, L.; Zhou, X. Thyroid disease is associated with an increased risk of breast cancer: A systematic review and meta-analysis. Gland Surg. 2021, 10, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.H.; Okawa, E.R.; Roberts, M.B.; Park, S.K.; Umbricht, C.B.; Manson, J.E.; Eaton, C.B. Breast Cancer Risk in Postmenopausal Women with Medical History of Thyroid Disorder in the Women’s Health Initiative. Thyroid 2020, 30, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.X.; Knuiman, M.W.; Divitini, M.L.; Brown, S.J.; Walsh, J.; Yeap, B.B. Lower TSH and higher free thyroxine predict incidence of prostate but not breast, colorectal or lung cancer. Eur. J. Endocrinol. 2017, 177, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Mondul, A.M.; Weinstein, S.J.; Bosworth, T.; Remaley, A.T.; Virtamo, J.; Albanes, D. Circulating thyroxine, thyroid-stimulating hormone, and hypothyroid status and the risk of prostate cancer. PLoS ONE 2012, 7, e47730. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Kueck, A.S.; Stevens, R.; Curhan, G.; De Vivo, I.; Rosner, B.; Alexander, E.; Tworoger, S.S. A large cohort study of hypothyroidism and hyperthyroidism in relation to gynecologic cancers. Obstet. Gynecol. Int. 2013, 2013, 743721. [Google Scholar] [CrossRef] [PubMed]
- L’Heureux, A.; Wieland, D.R.; Weng, C.-H.; Chen, Y.-H.; Lin, C.-H.; Lin, T.-H. Association between Thyroid Disorders and Colorectal Cancer Risk in Adult Patients in Taiwan. JAMA Netw. Open 2019, 2, e193755. [Google Scholar] [CrossRef]
- Wang, B.; Lu, Z.; Huang, Y.; Li, R.; Lin, T. Does hypothyroidism increase the risk of breast cancer: Evidence from a meta-analysis. BMC Cancer 2020, 20, 733. [Google Scholar] [CrossRef]
- Chan, Y.X.; Alfonso, H.; Chubb, S.A.; Fegan, P.G.; Hankey, G.J.; Golledge, J.; Flicker, L.; Yeap, B.B. Higher thyrotropin concentration is associated with increased incidence of colorectal cancer in older men. Clin. Endocrinol. 2017, 86, 278–285. [Google Scholar] [CrossRef]
- Mu, G.; Mu, X.; Xing, H.; Xu, R.; Sun, G.; Dong, C.; Pan, Q.; Xu, C. Subclinical hypothyroidism as an independent risk factor for colorectal neoplasm. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 261–266. [Google Scholar] [CrossRef]
- Hassan, M.M.; Kaseb, A.; Li, D.; Patt, Y.Z.; Vauthey, J.N.; Thomas, M.B.; Curley, S.A.; Spitz, M.R.; Sherman, S.I.; Abdalla, E.K.; et al. Association between hypothyroidism and hepatocellular carcinoma: A case-control study in the United States. Hepatology 2009, 49, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.; Dash, C.; Leerapun, A.; Mettler, T.A.; Stadheim, L.M.; Lazaridis, K.N.; Roberts, R.; Roberts, L.R. Hypothyroidism: A possible risk factor for liver cancer in patients with no known underlying cause of liver disease. Clin. Gastroenterol. Hepatol. 2007, 5, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cao, P.; Guo, Y.; Lu, H.; Hu, Y. Exploring the Causality between Hypothyroidism and Non-alcoholic Fatty Liver: A Mendelian Randomization Study. Front. Cell Dev. Biol. 2021, 9, 643582. [Google Scholar] [CrossRef] [PubMed]
- Kizivat, T.; Maric, I.; Mudri, D.; Curcic, I.B.; Primorac, D.; Smolic, M.J. Hypothyroidism and Nonalcoholic Fatty Liver Disease: Pathophysiological Associations and Therapeutic Implications. Clin. Transl. Hepatol. 2020, 8, 347–353. [Google Scholar] [CrossRef]
- Escudé, A.M.; Pera, G.; Arteaga, I.; Expósito, C.; Rodríguez, L.; Torán, P.; Caballeria, L. Relationship between hypothyroidism and non-alcoholic fatty liver disease in the Spanish population. Med. Clin. 2020, 154, 347–353. [Google Scholar]
- Lee, K.W.; Bang, K.B.; Rhee, E.J.; Kwon, H.J.; Lee, M.Y.; Cho, Y.K. Impact of hypothyroidism on the development of non-alcoholic fatty liver disease: A 4-year retrospective cohort study. Clin. Mol. Hepatol. 2015, 21, 372–378. [Google Scholar] [CrossRef]
- Italian Association for the Study of the Liver (AISF). AISF position paper on nonalcoholic fatty liver disease (NAFLD): Updates and future directions. Dig. Liver Dis. 2017, 49, 471–483. [Google Scholar] [CrossRef]
- Tseng, F.Y.; Lin, W.Y.; Li, C.I.; Li, T.C.; Lin, C.C.; Huang, K.C. Subclinical hypothyroidism is associated with increased risk for cancer mortality in adult Taiwanese—A 10 years population-based cohort. PLoS ONE 2015, 10, e0122955. [Google Scholar] [CrossRef]
- Krashin, E.; Silverman, B.; Steinberg, D.M.; Yekutieli, D.; Giveon, S.; Fabian, O.; Hercbergs, A.; Davis, P.J.; Ellis, M.; Ashur-Fabian, O. Opposing effects of thyroid hormones on cancer risk: A population-based study. Eur. J. Endocrinol. 2021, 184, 477–486. [Google Scholar] [CrossRef]
- Fang, Y.; Yao, L.; Sun, J.; Yang, R.; Chen, Y.; Tian, J.; Yang, K.; Tian, L. Does thyroid dysfunction increase the risk of breast cancer? A systematic review and meta-analysis. J. Endocrinol. Investig. 2017, 40, 1035–1047. [Google Scholar] [CrossRef]
- Hercbergs, A.H.; Ashur-Fabian, O.; Garfield, D. Thyroid hormones and cancer: Clinical studies of hypothyroidism in oncology. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Pinter, M.; Haupt, L.; Hucke, F.; Bota, S.; Bucsics, T.; Trauner, M.; Peck-Radosavljevic, M.; Sieghart, W. The impact of thyroid hormones on patients with hepatocellular carcinoma. PLoS ONE 2017, 12, e0181878. [Google Scholar] [CrossRef] [PubMed]
- Hercbergs, A.; Mason, J.; Reddy, C.; Elson, P. Thyroid hormones and lung cancer: Primary hypothyroidism is prognostically significant for survival in lung cancer. Cancer Res. 2004, 64, 1024. [Google Scholar]
- Ashur-Fabian, O.; Blumenthal, D.T.; Bakon, M.; Nass, D.; Davis, P.J.; Hercbergs, A. Long-term response in high-grade optic glioma treated with medically induced hypothyroidism and carboplatin: A case report and review of the literature. Anticancer Drugs 2013, 24, 315–323. [Google Scholar] [CrossRef]
- Hercbergs, A.A.; Goyal, L.K.; Suh, J.H.; Lee, S.; Reddy, C.A.; Cohen, B.H.; Stevens, G.H.; Reddy, S.K.; Peereboom, D.M.; Elson, P.J.; et al. Propylthiouracil-induced chemical hypothyroidism with high-dose tamoxifen prolongs survival in recurrent high grade glioma: A phase I/II study. Anticancer Res. 2003, 23, 617–626. [Google Scholar]
- Hercbergs, A.; Johnson, R.E.; Ashur-Fabian, O.; Garfield, D.H.; Davis, P.J. Medically induced euthyroid hypothyroxinemia may extend survival in compassionate need cancer patients: An observational study. Oncologist 2015, 20, 72–76. [Google Scholar] [CrossRef]
- Rodríguez-Molinero, A.; Hercbergs, A.; Sarrias, M.; Yuste, A. Plasma 3,3′,5-Triiodo-L-thyronine [T3] level mirrors changes in tumor markers in two cases of metastatic cancer of the breast and pancreas treated with exogenous L-T3. Cancer Biomark. 2018, 21, 433–438. [Google Scholar] [CrossRef]
- Barr, C.E.; Njoku, K.; Hotchkies, L.; Ryan, N.; Wan, Y.L.; Davies, D.A.; Razvi, S.; Crosbie, E.J. Does Clinical and Biochemical Thyroid Dysfunction Impact on Endometrial Cancer Survival Outcomes? A Prospective Database Study. Cancers 2021, 13, 5444. [Google Scholar] [CrossRef]
- Berghoff, A.S.; Wippel, C.; Starzer, A.M.; Ballarini, N.; Wolpert, F.; Bergen, E.; Wolf, P.; Steindl, A.; Widhalm, G.; Gatterbauer, B.; et al. Hypothyroidism correlates with favourable survival prognosis in patients with brain metastatic cancer. Eur. J. Cancer 2020, 135, 150–158. [Google Scholar] [CrossRef]
- Hou, J.; Xiong, S.S.; Huang, Z.Q.; Cai, X.D. Decelerated tumor growth due to hypothyroidism with prolongation of survival in a patient with lung adenocarcinoma: A case report. J. Int. Med. Res. 2020, 48, 300060519885302. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, Y.; Ryu, S.; Cho, J.; Lee, W.Y.; Rhee, E.J.; Kwon, M.J.; Pastor-Barriuso, R.; Rampal, S.; Han, W.K.; et al. Thyroid hormones and mortality risk in euthyroid individuals: The Kangbuk Samsung health study. J. Clin. Endocrinol. Metab. 2014, 99, 2467–2476. [Google Scholar] [CrossRef] [PubMed]
- Seebacher, V.; Hofstetter, G.; Polterauer, S.; Reinthaller, A.; Grimm, C.; Schwameis, R.; Taucher, S.; Wagener, A.; Marth, C.; Concin, N. Does thyroid-stimulating hormone influence the prognosis of patients with endometrial cancer? A multicentre trial. Br. J. Cancer. 2013, 109, 215–218. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, L.; Liu, X.; Jiang, Y.; Wang, X.; Wang, X.; Yang, Z. Use of a Novel Thyroid-Stimulating Hormone Model for Predicting the Progression of Hepatocellular Carcinoma. Onco Targets Ther. 2020, 13, 11421–11431. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Izquierdo, J.; Filion, K.B.; Boivin, J.F.; Azoulay, L.; Pollak, M.; Yu, O.H.Y. Subclinical hypothyroidism and the risk of cancer incidence and cancer mortality: A systematic review. BMC Endocr. Disord. 2020, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- Waring, A.C.; Harrison, S.; Samuels, M.H.; Ensrud, K.E.; LeBLanc, E.S.; Hoffman, A.R.; Orwoll, E.; Fink, H.A.; Barrett-Connor, E.; Bauer, D.C.; et al. Thyroid function and mortality in older men: A prospective study. J Clin. Endocrinol. Metab. 2012, 97, 862–870. [Google Scholar] [CrossRef]
- Journy, N.M.Y.; Bernier, M.O.; Doody, M.M.; Alexander, B.H.; Linet, M.S.; Kitahara, C.M. Hyperthyroidism, Hypothyroidism, and Cause-Specific Mortality in a Large Cohort of Women. Thyroid 2017, 27, 1001–1010. [Google Scholar] [CrossRef]
- Tosovic, A.; Bondeson, A.G.; Bondeson, L.; Ericsson, U.B.; Manjer, J. T3 levels in relation to prognostic factors in breast cancer: A population-based prospective cohort study. BMC Cancer 2014, 14, 536. [Google Scholar] [CrossRef]
- Villa, N.M.; Li, N.; Yeh, M.W.; Hurvitz, S.A.; Dawson, N.A.; Leung, A.M. Serum Thyrotropin Concentrations are not Predictive of Aggressive Breast Cancer Biology in Euthyroid Individuals. Endocr. Pract. 2015, 21, 1040–1045. [Google Scholar] [CrossRef]
- Falstie-Jensen, A.M.; Kjærsgaard, A.; Lorenzen, E.L.; Jensen, J.D.; Reinertsen, K.V.; Dekkers, O.M.; Ewertz, M.; Cronin-Fenton, D.P. Hypothyroidism and the risk of breast cancer recurrence and all-cause mortality—A Danish population-based study. Breast Cancer Res. 2019, 21, 44. [Google Scholar] [CrossRef]
- Patil, V.M.; Noronha, V.; Joshi, A.; Bhattacharjee, A.; Goel, A.; Talreja, V.; Chandrasekharan, A.; Pande, N.; Mandal, T.; Ramaswamy, A.; et al. Influence of hypothyroidism after chemoradiation on outcomes in head and neck cancer. Clin. Oncol. 2018, 30, 675. [Google Scholar] [CrossRef]
- Osorio, J.C.; Ni, A.; Chaft, J.E.; Pollina, R.; Kasler, M.K.; Stephens, D.; Rodriguez, C.; Cambridge, L.; Rizvi, H.; Wolchok, J.D.; et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann. Oncol. 2017, 28, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Thuillier, P.; Joly, C.; Alavi, Z.; Crouzeix, G.; Descourt, R.; Quere, G.; Kerlan, V.; Roudaut, N. Thyroid dysfunction induced by immune checkpoint inhibitors is associated with a better progression-free survival and overall survival in non-small cell lung cancer: An original cohort study. Cancer Immunol. Immunother. 2021, 70, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Funazo, T.Y.; Nomizo, T.; Ozasa, H.; Tsuji, T.; Yasuda, Y.; Yoshida, H.; Sakamori, Y.; Nagai, H.; Hirai, T.; Kim, Y.H. Clinical impact of low serum free T4 in patients with non-small cell lung cancer treated with nivolumab. Sci. Rep. 2019, 9, 17085. [Google Scholar] [CrossRef] [PubMed]
- Sbardella, E.; Tenuta, M.; Sirgiovanni, G.; Gianfrilli, D.; Pozza, C.; Venneri, M.A.; Cortesi, E.; Marchetti, P.; Lenzi, A.; Gelibter, A.J.; et al. Thyroid disorders in programmed death 1 inhibitor-treated patients: Is previous therapy with tyrosine kinase inhibitors a predisposing factor? Clin. Endocrinol. 2020, 92, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Deligiorgi, M.V.; Sagredou, S.; Vakkas, L.; Trafalis, D.T. The Continuum of Thyroid Disorders Related to Immune Checkpoint Inhibitors: Still Many Pending Queries. Cancers 2021, 13, 5277. [Google Scholar] [CrossRef]
- Nearchou, A.; Valachis, A.; Lind, P.; Akre, O.; Sandström, P. Acquired Hypothyroidism as a Predictive Marker of Outcome in Patients with Metastatic Renal Cell Carcinoma Treated with Tyrosine Kinase Inhibitors: A Literature-Based Meta-Analysis. Clin. Genitourin. Cancer 2015, 13, 280–286. [Google Scholar] [CrossRef]
- Soni, S.; Rastogi, A.; Prasad, K.T.; Behera, D.; Singh, N. Thyroid dysfunction in non-small cell lung cancer patients treated with epidermal growth factor receptor and anaplastic lymphoma kinase inhibitors: Results of a prospective cohort. Lung Cancer 2021, 151, 16–19. [Google Scholar] [CrossRef]
- Bilen, M.A.; Patel, A.; Hess, K.R.; Munoz, J.; Busaidy, N.L.; Wheler, J.J.; Janku, F.; Falchook, G.S.; Hong, D.S.; Meric-Bernstam, F.; et al. Association between new-onset hypothyroidism and clinical response in patients treated with tyrosine kinase inhibitor therapy in phase I clinical trials. Cancer Chemother. Pharmacol. 2016, 78, 167–171. [Google Scholar] [CrossRef]
- Si, X.; Zhang, L.; Wang, H.; Zhang, X.; Wang, M.; Han, B.; Li, K.; Wang, Q.; Shi, J.; Wang, Z.; et al. Management of anlotinib-related adverse events in patients with advanced non-small cell lung cancer: Experiences in ALTER-0303. Thorac. Cancer 2019, 10, 551–556. [Google Scholar] [CrossRef]
- Schirripa, M.; Dochy, E.; Fassan, M.; Ziranu, P.; Puzzoni, M.; Scartozzi, M.; Alberti, G.; Lonardi, S.; Zagonel, V.; Monzani, F.; et al. Thyroid hormones ratio is a major prognostic marker in advanced metastatic colorectal cancer: Results from the phase III randomised CORRECT trial. Eur. J. Cancer 2020, 133, 66–73. [Google Scholar]
- Curti, B.; Daniels, G.A.; McDermott, D.F.; Clark, J.I.; Kaufman, H.L.; Logan, T.F.; Singh, J.; Kaur, M.; Luna, T.L.; Gregory, N.; et al. Improved survival and tumor control with Interleukin-2 is associated with the development of immune-related adverse events: Data from the PROCLAIMSM registry. J. Immunother. Cancer 2017, 5, 102. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Kuhn, C.; Mayr, D.; Ditsch, N.; Kailuweit, M.; Wolf, V.; Harbeck, N.; Mahner, S.; Jeschke, U.; Cavaillès, V.; et al. Cytoplasmic and Nuclear Forms of Thyroid Hormone Receptor β1 Are Inversely Associated with Survival in Primary Breast Cancer. Int. J. Mol. Sci. 2020, 21, 330. [Google Scholar] [CrossRef] [PubMed]
- Frau, C.; Loi, R.; Petrelli, A.; Perra, A.; Menegon, S.; Kowalik, M.A.; Pinna, S.; Leoni, V.P.; Fornari, F.; Gramantieri, L.; et al. Local hypothyroidism favors the progression of preneoplastic lesions to hepatocellular carcinoma in rats. Hepatology 2015, 61, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.H.; Chen, C.Y.; Lin, Y.H.; Chi, H.C.; Huang, Y.H.; Tai, P.J.; Liao, C.J.; Tsai, C.Y.; Lin, S.L.; Wu, M.H.; et al. Thyroid hormone-mediated regulation of lipocalin 2 through the Met/FAK pathway in liver cancer. Oncotarget 2015, 6, 15050–15064. [Google Scholar] [CrossRef] [PubMed]
- Zehni, A.Z.; Batz, F.; Vattai, A.; Kaltofen, T.; Schrader, S.; Jacob, S.N.; Mumm, J.N.; Heidegger, H.H.; Ditsch, N.; Mahner, S.; et al. The Prognostic Impact of Retinoid X Receptor and Thyroid Hormone Receptor alpha in Unifocal vs. Multifocal/Multicentric Breast Cancer. Int. J. Mol. Sci. 2021, 22, 957. [Google Scholar] [CrossRef]
- Brandt, J.; Borgquist, S.; Almgren, P.; Försti, A.; Huss, L.; Melander, O.; Manjer, J. Thyroid-associated genetic polymorphisms in relation to breast cancer risk in the Malmö Diet and Cancer Study. Int. J. Cancer 2018, 142, 1309–1321. [Google Scholar] [CrossRef]
- Moriggi, G.; Verga Falzacappa, C.; Mangialardo, C.; Michienzi, S.; Stigliano, A.; Brunetti, E.; Toscano, V.; Misiti, S. Thyroid hormones (T3 and T4): Dual effect on human cancer cell proliferation. Anticancer Res. 2011, 31, 89–96. [Google Scholar]
- Schiera, G.; Di Liegro, C.M.; Di Liegro, I. Involvement of Thyroid Hormones in Brain Development and Cancer. Cancers 2021, 13, 2693. [Google Scholar] [CrossRef]
- Metabolomics of Thyroid Hormones (MATcH). Available online: https://clinicaltrials.gov/ct2/show/NCT03823859 (accessed on 14 September 2021).
- Dobrinja, C.; Scomersi, S.; Giudici, F.; Vallon, G.; Lanzaro, A.; Troian, M.; Bonazza, D.; Romano, A.; Zanconati, F.; de Manzini, N.; et al. Association between benign thyroid disease and breast cancer: A single center experience. BMC Endocr. Disord. 2019, 19, 104. [Google Scholar] [CrossRef]
- Rappaport, J. Changes in Dietary Iodine Explains Increasing Incidence of Breast Cancer with Distant Involvement in Young Women. J. Cancer 2017, 8, 174–177. [Google Scholar] [CrossRef]
- Shinkov, A.; Borissova, A.M.; Kovatcheva, R.; Atanassova, I.; Vlahov, J.; Dakovska, L. The prevalence of the metabolic syndrome increases through the quartiles of thyroid stimulating hormone in a population-based sample of euthyroid subjects. Arq. Bras. Endocrinol. Metabol. 2014, 58, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Chiodini, P.; Colao, A.; Lenzi, A.; Giugliano, D. Metabolic syndrome and risk of cancer: A systematic review and meta-analysis. Diabetes Care 2012, 35, 2402–2411. [Google Scholar] [CrossRef] [PubMed]
- Cestari, S.H.; Figueiredo, N.B.; Conde, S.J.; Clara, S.; Katayama, M.L.; Padovani, C.R.; Brentani, M.M.; Nogueira, C.R. Influence of estradiol and triiodothyronine on breast cancer cell lines proliferation and expression of estrogen and thyroid hormone receptors. Arq. Bras. Endocrinol. Metabol. 2009, 53, 859–864. [Google Scholar] [CrossRef][Green Version]
- Sar, P.; Peter, R.; Rath, B.; Das Mohapatra, A.; Mishra, S.K. 3,3′5 triiodo L thyronine induces apoptosis in human breast cancer MCF-7cells, repressing SMP30 expression through negative thyroid response elements. PLoS ONE 2011, 6, e20861. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, X.; Du, J.; Wang, H.; Chen, C.; Jiao, L.; Cheng, X.; Zhou, X.; Chen, S.; Gou, S.; Zhao, W.; et al. Repositioning liothyronine for cancer immunotherapy by blocking the interaction of immune checkpoint TIGIT/PVR. Cell Commun. Signal. 2020, 18, 142. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.S.; Park, Y. Hitting the complexity of the TIGIT-CD96-CD112R-CD226 axis for next-generation cancer immunotherapy. BMB Rep. 2021, 54, 2–11. [Google Scholar] [CrossRef]
- Han, Y.; Zhu, L.; Wu, W.; Zhang, H.; Hu, W.; Dai, L.; Yang, Y. Small Molecular Immune Modulators as Anticancer Agents. Adv. Exp. Med. Biol. 2020, 1248, 547–618. [Google Scholar]
- Calmeiro, J.; Carrascal, M.A.; Tavares, A.R.; Ferreira, D.A.; Gomes, C.; Falcão, A.; Cruz, M.T.; Neves, B.M. Dendritic Cell Vaccines for Cancer Immunotherapy: The Role of Human Conventional Type 1 Dendritic Cells. Pharmaceutics 2020, 12, 158. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chung, I.H.; Tsai, M.M.; Tseng, Y.H.; Chi, H.C.; Tsai, C.Y.; Lin, Y.H.; Wang, Y.C.; Chen, C.P.; Wu, T.I.; et al. Thyroid hormone enhanced human hepatoma cell motility involves brain-specific serine protease 4 activation via ERK signaling. Mol. Cancer 2014, 13, 162. [Google Scholar] [CrossRef]
- Gnoni, G.V.; Rochira, A.; Leone, A.; Damiano, F.; Marsigliante, S.; Siculella, L. 3,5,3′ triiodo-L-thyronine induces SREBP-1 expression by non-genomic actions in human HEP G2 cells. J. Cell Physiol. 2012, 227, 2388–2397. [Google Scholar] [CrossRef]
- Lin, Y.H.; Liao, C.J.; Huang, Y.H.; Wu, M.H.; Chi, H.C.; Wu, S.M.; Chen, C.Y.; Tseng, Y.H.; Tsai, C.Y.; Chung, I.H.; et al. Thyroid hormone receptor represses miR-17 expression to enhance tumor metastasis in human hepatoma cells. Oncogene 2013, 32, 4509–4518. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Davis, F.B.; Mousa, S.A.; Luidens, M.K.; Lin, H.Y. Membrane receptor for thyroid hormone: Physiologic and pharmacologic implications. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Mousa, S.A.; Lin, H.Y. Nongenomic Actions of Thyroid Hormone: The Integrin Component. Physiol. Rev. 2021, 101, 319–352. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.; Chang, T.C.; Chin, Y.T.; Pan, Y.S.; Chang, W.J.; Liu, F.C.; Hastuti, E.D.; Chiu, S.J.; Wang, S.H.; Changou, C.A.; et al. NDAT Targets PI3K-Mediated PD-L1 Upregulation to Reduce Proliferation in Gefitinib-Resistant Colorectal Cancer. Cells 2020, 9, 1830. [Google Scholar] [CrossRef]
- Ashur-Fabian, O.; Zloto, O.; Fabian, I.; Tsarfaty, G.; Ellis, M.; Steinberg, D.M.; Hercbergs, A.; Davis, P.J.; Fabian, I.D. Tetrac Delayed the Onset of Ocular Melanoma in an Orthotopic Mouse Model. Front. Endocrinol. 2019, 9, 775. [Google Scholar] [CrossRef]
- Yang, Y.; Ko, P.J.; Pan, Y.S.; Lin, H.Y.; Whang-Peng, J.; Davis, P.J.; Wang, K. Role of thyroid hormone-integrin αvβ3-signal and therapeutic strategies in colorectal cancers. J. Biomed. Sci. 2021, 28, 24. [Google Scholar] [CrossRef]
- Leith, J.T.; Mousa, S.A.; Hercbergs, A.; Lin, H.Y.; Davis, P.J. Radioresistance of cancer cells, integrin αvβ3 and thyroid hormone. Oncotarget 2018, 9, 37069–37075. [Google Scholar] [CrossRef]
- Davis, P.J.; Glinsky, G.V.; Lin, H.Y.; Leith, J.T.; Hercbergs, A.; Tang, H.Y.; Ashur-Fabian, O.; Incerpi, S.; Mousa, S.A. Cancer Cell Gene Expression Modulated from Plasma Membrane Integrin αvβ3 by Thyroid Hormone and Nanoparticulate Tetrac. Front. Endocrinol. 2015, 5, 240. [Google Scholar] [CrossRef]
- Chu, Y.D.; Yeh, C.T. The Molecular Function and Clinical Role of Thyroid Stimulating Hormone Receptor in Cancer Cells. Cells 2020, 9, 1730. [Google Scholar] [CrossRef]
- Rowe, C.W.; Paul, J.W.; Gedye, C.; Tolosa, J.M.; Bendinelli, C.; McGrath, S.; Smith, R. Targeting the TSH receptor in thyroid cancer. Endocr. Relat. Cancer 2017, 24, R191–R202. [Google Scholar] [CrossRef]
- Columbano, A.; Chiellini, G.; Kowalik, M.A. GC-1: A Thyromimetic with Multiple Therapeutic Applications in Liver Disease. Gene Expr. 2017, 17, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, F.; Sestito, S.; Runfola, M.; Rapposelli, S.; Chiellini, G. Selective Thyroid Hormone Receptor-Beta (TRβ) Agonists: New Perspectives for the Treatment of Metabolic and Neurodegenerative Disorders. Front. Med. 2020, 7, 331. [Google Scholar] [CrossRef] [PubMed]
- Köhrle, J. The Colorful Diversity of Thyroid Hormone Metabolites. Eur. Thyroid J. 2019, 8, 115–129. [Google Scholar] [CrossRef] [PubMed]
| Anticancer Treatment | Incidence | Suggested Underlying Mechanisms | Screening Tests |
|---|---|---|---|
| Radiotherapy | 20–60% |
| NTCP models are currently under evaluation. |
| Chemotherapy | NA due tofew cases |
| No specific recommendations. |
| IFN-α | 2.4–31% |
| Baseline antithyroid antibody test. |
| HD IL-2 | 10–60% |
| Evaluation of TSH levels at baseline and Q 2–3 months during therapy. |
| Bexarotene | 29–100% |
|
|
| ICPi |
|
| Evaluation of TSH and fT4 levels at baseline, at each course for 6 months, Q 2 courses for the next 6 months, and afterwards in case of clinical signs. |
| TKIs |
|
| TSH evaluation at baseline and monitoring of patients treated with TKIs. |
| • NA |
| It is advisable to rule out thyroid pathology before contrast studies, especially in children, elderly patients, and patients with renal insufficiency. |
| Cancer Type | Study Type and Population | Results | Ref |
|---|---|---|---|
| Metastatic NSCLC | Case report. |
| [35] |
| Nonsurgical HCC | Retrospective study on 667 patients diagnosed at the Division of Gastroenterology and Hepatology/Medical University of Vienna between 1992 and 2012. |
| [94] |
| NSCLC and SCLC (stages I–IV) | Retrospective case-control study assessing the occurrence of primary hypothyroidism among 1979 patients. |
| [95] |
| High-grade optic glioma | Case report. |
| [96] |
| Recurrent glioma treated with TMX | Phase I/II study on 22 patients in the US. |
| [97] |
| End-stage solid cancer (brain, ovary, lung, pancreas, salivary gland, breast cancer, mesothelioma, soft-tissue sarcoma) | Uncontrolled observational study on 23 patients. |
| [98] |
| Metastatic triple negative breast cancer and pancreatic cancer | Case report. |
| [99] |
| EC | A prospective study on 333 patients.Median FU: 35 months. |
| [100] |
| Solid cancers (lung cancer (69.9%), breast cancer (24.3%), melanoma (5.8%)) with newly diagnosed BMs | Evaluation of thyroid function in patients with BMs in a discovery cohort of 1692 patients and an independent validation cohort of 191 patients. |
| [101] |
| Stage IV lung adenocarcinoma | Case report. |
| [102] |
| Cancer Type | Study Type and Population | Results | Ref |
|---|---|---|---|
| Colorectal cancer | Case–control study of 273 cases. |
| [82] |
| Various cancertypes |
|
| [90] |
| Nonsurgical HCC |
|
| [94] |
| Various cancer types |
|
| [103] |
| EC |
|
| [104] |
| HCC |
|
| [105] |
| Various cancer types. |
|
| [106] |
| Cancer Type | Study Type and Population | Results | Ref |
|---|---|---|---|
| Breast cancer |
|
| [75] |
| Various cancer types |
|
| [103] |
| Various cancer types |
|
| [106,107] |
| Various cancer types |
|
| [107] |
| Various cancer types |
|
| [108] |
| Breast cancer |
|
| [109] |
| Breast cancer |
|
| [110] |
| Breast cancer |
|
| [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deligiorgi, M.V.; Trafalis, D.T. The Clinical Relevance of Hypothyroidism in Patients with Solid Non-Thyroid Cancer: A Tantalizing Conundrum. J. Clin. Med. 2022, 11, 3417. https://doi.org/10.3390/jcm11123417
Deligiorgi MV, Trafalis DT. The Clinical Relevance of Hypothyroidism in Patients with Solid Non-Thyroid Cancer: A Tantalizing Conundrum. Journal of Clinical Medicine. 2022; 11(12):3417. https://doi.org/10.3390/jcm11123417
Chicago/Turabian StyleDeligiorgi, Maria V., and Dimitrios T. Trafalis. 2022. "The Clinical Relevance of Hypothyroidism in Patients with Solid Non-Thyroid Cancer: A Tantalizing Conundrum" Journal of Clinical Medicine 11, no. 12: 3417. https://doi.org/10.3390/jcm11123417
APA StyleDeligiorgi, M. V., & Trafalis, D. T. (2022). The Clinical Relevance of Hypothyroidism in Patients with Solid Non-Thyroid Cancer: A Tantalizing Conundrum. Journal of Clinical Medicine, 11(12), 3417. https://doi.org/10.3390/jcm11123417





