Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Summary of Key Clinical Trials
Abstract
:1. Introduction
2. Clinical Trials Evaluating the Efficacy of CRS-HIPEC for Colorectal Cancer
2.1. Netherlands Trial
2.2. PRODIGE 7
2.3. Conclusion to Efficacy of CRS-HIPEC
3. Clinical Trials Evaluating Early Interventions in Patients at High-Risk for Colorectal Peritoneal Metastases
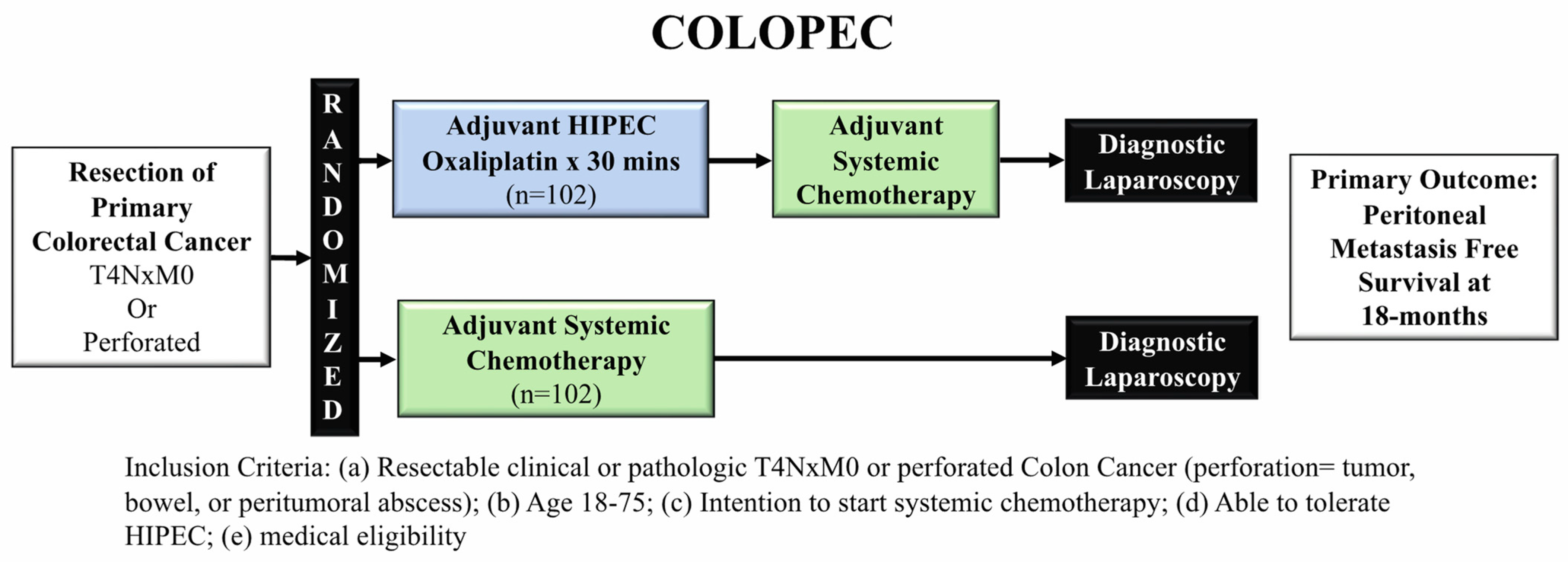
3.1. COLOPEC
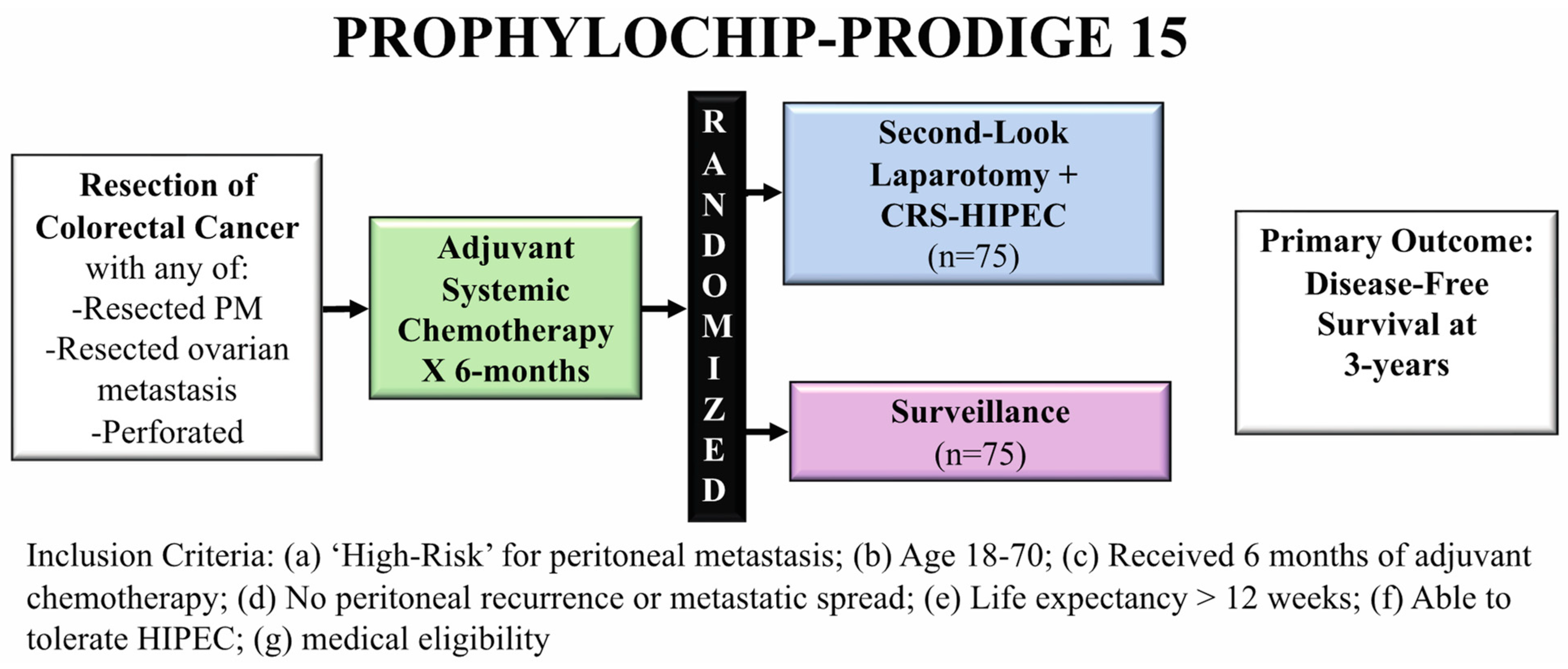
3.2. PROPHYLOCHIP-PRODIGE 15
3.3. Conclusion to Early Interventions in High-Risk Patients for Peritoneal Metastases
4. Future Directions
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- SEER Cancer Stat Facts: Colorectal Cancer. National Cancer Institute. Bethesda, MD. Available online: https://seer.cancer.gov/statfacts/html/colorect.html (accessed on 24 November 2021).
- Thomassen, I.; van Gestel, Y.R.; Lemmens, V.E.; de Hingh, I.H. Incidence, prognosis, and treatment options for patients with synchronous peritoneal carcinomatosis and liver metastases from colorectal origin. Dis. Colon Rectum 2013, 56, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Koppe, M.J.; Boerman, O.C.; Oyen, W.J.; Bleichrodt, R.P. Peritoneal carcinomatosis of colorectal origin: Incidence and current treatment strategies. Ann. Surg. 2006, 243, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Quere, P.; Facy, O.; Manfredi, S.; Jooste, V.; Faivre, J.; Lepage, C.; Bouvier, A.M. Epidemiology, Management, and Survival of Peritoneal Carcinomatosis from Colorectal Cancer: A Population-Based Study. Dis. Colon Rectum 2015, 58, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Segelman, J.; Granath, F.; Holm, T.; Machado, M.; Mahteme, H.; Martling, A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br. J. Surg. 2012, 99, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Riihimaki, M.; Hemminki, A.; Sundquist, J.; Hemminki, K. Patterns of metastasis in colon and rectal cancer. Sci. Rep. 2016, 6, 29765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spratt, J.S.; Adcock, R.A.; Sherrill, W.; Travathen, S. Hyperthermic peritoneal perfusion system in canines. Cancer Res. 1980, 40, 253–255. [Google Scholar]
- Sugarbaker, P.H. Peritoneal carcinomatosis: Natural history and rational therapeutic interventions using intraperitoneal chemotherapy. Cancer Treat. Res. 1996, 81, 149–168. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Management of peritoneal-surface malignancy: The surgeon’s role. Langenbecks Arch. Surg. 1999, 384, 576–587. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Laboratory and clinical basis for hyperthermia as a component of intracavitary chemotherapy. Int. J. Hyperth. 2007, 23, 431–442. [Google Scholar] [CrossRef]
- Dodson, R.M.; Kuncewitch, M.; Votanopoulos, K.I.; Shen, P.; Levine, E.A. Techniques for Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2018, 25, 2152–2158. [Google Scholar] [CrossRef]
- Verwaal, V.J.; van Ruth, S.; de Bree, E.; van Sloothen, G.W.; van Tinteren, H.; Boot, H.; Zoetmulder, F.A. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J. Clin. Oncol. 2003, 21, 3737–3743. [Google Scholar] [CrossRef] [PubMed]
- Verwaal, V.J.; Bruin, S.; Boot, H.; van Slooten, G.; van Tinteren, H. 8-year follow-up of randomized trial: Cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann. Surg. Oncol. 2008, 15, 2426–2432. [Google Scholar] [CrossRef] [PubMed]
- Quenet, F.; Elias, D.; Roca, L.; Goere, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Cashin, P.; Sugarbaker, P.H. Hyperthermic intraperitoneal chemotherapy (HIPEC) for colorectal and appendiceal peritoneal metastases: Lessons learned from PRODIGE 7. J. Gastrointest. Oncol. 2021, 12, S120–S128. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; Mehta, S.; Kammar, P.; Saklani, A. Limitations of the PRODIGE 7 trial. Lancet Oncol. 2021, 22, e175. [Google Scholar] [CrossRef]
- Hompes, D.; D’Hoore, A.; Wolthuis, A.; Fieuws, S.; Mirck, B.; Bruin, S.; Verwaal, V. The use of Oxaliplatin or Mitomycin C in HIPEC treatment for peritoneal carcinomatosis from colorectal cancer: A comparative study. J. Surg. Oncol. 2014, 109, 527–532. [Google Scholar] [CrossRef]
- Prabhu, A.; Brandl, A.; Wakama, S.; Sako, S.; Ishibashi, H.; Mizumoto, A.; Takao, N.; Ichinose, M.; Motoi, S.; Liu, Y.; et al. Effect of oxaliplatin-based chemotherapy on chemosensitivity in patients with peritoneal metastasis from colorectal cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: Proof-of-concept study. BJS Open 2021, 5, zraa075. [Google Scholar] [CrossRef]
- Nagourney, R.A.; Evans, S.; Tran, P.H.; Nagourney, A.J.; Sugarbaker, P.H. Colorectal cancer cells from patients treated with FOLFOX or CAPOX are resistant to oxaliplatin. Eur. J. Surg. Oncol. 2021, 47, 738–742. [Google Scholar] [CrossRef]
- Kirstein, M.N.; Root, S.A.; Moore, M.M.; Wieman, K.M.; Williams, B.W.; Jacobson, P.A.; Marker, P.H.; Tuttle, T.M. Exposure-response relationships for oxaliplatin-treated colon cancer cells. Anticancer Drugs 2008, 19, 37–44. [Google Scholar] [CrossRef]
- Becouarn, Y.; Ychou, M.; Ducreux, M.; Borel, C.; Bertheault-Cvitkovic, F.; Seitz, J.F.; Nasca, S.; Nguyen, T.D.; Paillot, B.; Raoul, J.L.; et al. Phase II trial of oxaliplatin as first-line chemotherapy in metastatic colorectal cancer patients. Digestive Group of French Federation of Cancer Centers. J. Clin. Oncol. 1998, 16, 2739–2744. [Google Scholar] [CrossRef]
- de Gramont, A.; Vignoud, J.; Tournigand, C.; Louvet, C.; Andre, T.; Varette, C.; Raymond, E.; Moreau, S.; Le Bail, N.; Krulik, M. Oxaliplatin with high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer. Eur. J. Cancer 1997, 33, 214–219. [Google Scholar] [CrossRef]
- Strohlein, M.A.; Heiss, M.M. Limitations of the PRODIGE 7 trial. Lancet Oncol. 2021, 22, e178. [Google Scholar] [CrossRef]
- Klaver, C.E.L.; Wisselink, D.D.; Punt, C.J.A.; Snaebjornsson, P.; Crezee, J.; Aalbers, A.G.J.; Brandt, A.; Bremers, A.J.A.; Burger, J.W.A.; Fabry, H.F.J.; et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): A multicentre, open-label, randomised trial. Lancet Gastroenterol. Hepatol. 2019, 4, 761–770. [Google Scholar] [CrossRef]
- Goere, D.; Glehen, O.; Quenet, F.; Guilloit, J.M.; Bereder, J.M.; Lorimier, G.; Thibaudeau, E.; Ghouti, L.; Pinto, A.; Tuech, J.J.; et al. Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-PRODIGE 15): A randomised, phase 3 study. Lancet Oncol. 2020, 21, 1147–1154. [Google Scholar] [CrossRef]
- Moran, B.J.; Guerra, G.R. Randomized controlled trials in surgical resection of colorectal peritoneal metastases: Disentangling negativity in PRODIGE 7 and PROPHYLOCHIP. Colorectal Dis. 2021, 23, 1303–1305. [Google Scholar] [CrossRef]
- de Bree, E.; Koops, W.; Kroger, R.; van Ruth, S.; Witkamp, A.J.; Zoetmulder, F.A. Peritoneal carcinomatosis from colorectal or appendiceal origin: Correlation of preoperative CT with intraoperative findings and evaluation of interobserver agreement. J. Surg. Oncol. 2004, 86, 64–73. [Google Scholar] [CrossRef]
- Patel, S.; Pandey, D.; Saklani, A. Results from the PROPHYLOCHIP-PRODIGE 15 trial. Lancet Oncol. 2020, 21, e497. [Google Scholar] [CrossRef]
- Rovers, K.P.; Bakkers, C.; Nienhuijs, S.W.; Burger, J.W.A.; Creemers, G.M.; Thijs, A.M.J.; Brandt-Kerkhof, A.R.M.; Madsen, E.V.E.; van Meerten, E.; Tuynman, J.B.; et al. Perioperative Systemic Therapy vs Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Alone for Resectable Colorectal Peritoneal Metastases: A Phase 2 Randomized Clinical Trial. JAMA Surg. 2021, 156, 710–720. [Google Scholar] [CrossRef]
- Rovers, K.P.; Bakkers, C.; Simkens, G.; Burger, J.W.A.; Nienhuijs, S.W.; Creemers, G.M.; Thijs, A.M.J.; Brandt-Kerkhof, A.R.M.; Madsen, E.V.E.; Ayez, N.; et al. Perioperative systemic therapy and cytoreductive surgery with HIPEC versus upfront cytoreductive surgery with HIPEC alone for isolated resectable colorectal peritoneal metastases: Protocol of a multicentre, open-label, parallel-group, phase II-III, randomised, superiority study (CAIRO6). BMC Cancer 2019, 19, 390. [Google Scholar] [CrossRef] [Green Version]
- Pereira, F.; Serrano, A.; Manzanedo, I.; Perez-Viejo, E.; Gonzalez-Moreno, S.; Gonzalez-Bayon, L.; Arjona-Sanchez, A.; Torres, J.; Ramos, I.; Barrios, M.E.; et al. GECOP-MMC: Phase IV randomized clinical trial to evaluate the efficacy of hyperthermic intraperitoneal chemotherapy (HIPEC) with mytomicin-C after complete surgical cytoreduction in patients with colon cancer peritoneal metastases. BMC Cancer 2022, 22, 536. [Google Scholar] [CrossRef]
- Zongyuan, L.; Xiaolin, P.; Hua, J. Ascites and serial plasm circulating tumor DNA as a prognostic factor in peritoneal carcinomatosis after hyperthermic intraperitoneal chemotherapy. In Proceedings of the ASCO GastroIntestinal Cancer Symposium, San Francisco, CA, USA, 20–22 January 2022; p. 661. [Google Scholar]
- Beagan, J.J.; Sluiter, N.R.; Bach, S.; Eijk, P.P.; Vlek, S.L.; Heideman, D.A.M.; Kusters, M.; Pegtel, D.M.; Kazemier, G.; van Grieken, N.C.T.; et al. Circulating Tumor DNA as a Preoperative Marker of Recurrence in Patients with Peritoneal Metastases of Colorectal Cancer: A Clinical Feasibility Study. J. Clin. Med. 2020, 9, 1738. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, J.M.; Riviere, P.; Lanman, R.B.; Kelly, K.J.; Veerapong, J.; Lowy, A.M.; Kurzrock, R. Prognostic Utility of Pre- and Postoperative Circulating Tumor DNA Liquid Biopsies in Patients with Peritoneal Metastases. Ann. Surg. Oncol. 2020, 27, 3259–3267. [Google Scholar] [CrossRef] [PubMed]
- Chicago Consensus Working Group. The Chicago Consensus on Peritoneal Surface Malignancies: Management of Colorectal Metastases. Ann. Surg. Oncol. 2020, 27, 1761–1767. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner, K.M.; Morris, M.C.; Sohal, D.; Sussman, J.J.; Wilson, G.C.; Ahmad, S.A.; Patel, S.H. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Summary of Key Clinical Trials. J. Clin. Med. 2022, 11, 3406. https://doi.org/10.3390/jcm11123406
Turner KM, Morris MC, Sohal D, Sussman JJ, Wilson GC, Ahmad SA, Patel SH. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Summary of Key Clinical Trials. Journal of Clinical Medicine. 2022; 11(12):3406. https://doi.org/10.3390/jcm11123406
Chicago/Turabian StyleTurner, Kevin M., Mackenzie C. Morris, Davendra Sohal, Jeffrey J. Sussman, Gregory C. Wilson, Syed A. Ahmad, and Sameer H. Patel. 2022. "Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Summary of Key Clinical Trials" Journal of Clinical Medicine 11, no. 12: 3406. https://doi.org/10.3390/jcm11123406
APA StyleTurner, K. M., Morris, M. C., Sohal, D., Sussman, J. J., Wilson, G. C., Ahmad, S. A., & Patel, S. H. (2022). Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Summary of Key Clinical Trials. Journal of Clinical Medicine, 11(12), 3406. https://doi.org/10.3390/jcm11123406





