Abstract
In some malignant tumours, the changes in neutrophil counts in relation to other blood cells are connected with unfavourable prognosis. Nevertheless, the prognostic value of the combinations of the haematological components in glioblastoma (GBM) remains under dispute. The clinical significance of the neutrophil-to-lymphocyte ratio (NLR), systemic immune inflammation index (SII), and systemic inflammation response index (SIRI) was investigated in our study. We retrospectively studied 358 patients (males n = 195; females n = 163) aged 59.9 ± 13.5 yrs with newly diagnosed glioma and admitted to the Neurosurgery Centre. Routine blood tests and clinical characteristics were recorded within the first hour of hospital admission. The inflammatory variables: NLR, SII and SIRI exceeded the reference values and were significantly elevated in Grade 3 and Grade 4 tumour. The Cox model analysis showed that the age ≥ 63 years, NLR ≥ 4.56 × 103/µL, SII ≥ 2003 × 103/µL and SIRI ≥ 3.03 × 103/µL significantly increased the risk of death in Grade 4 tumour patients. In the inflammatory variables, NLR demonstrated the highest impact on the survival time (HR 1.56; 95% CI 1.145–2.127; p = 0.005). In the first Polish study including GBM patients, the age in relation to simple parameters derived from complete blood cell count were found to have prognostic implications in the survival rate.
1. Introduction
Glioblastoma (GBM, WHO grade IV) is the most aggressive primary malignant brain tumour in adults, with an average survival time of 12–18 months after initial diagnosis. GBM patients is based on standard treatment, including surgery, chemotherapy, and radiotherapy [1]. The incidence of GBM is much higher in the elderly individuals, while younger people are more likely to develop lower-grade gliomas. The discovery of somatic mutations in the gene encoding isocitrate dehydrogenase-1 (IDH1) initiated a new period of laboratory diagnostics of GBM [2]. The fifth edition of the World Health Organisation Classification of Tumours of the CNS, published in 2021, established new tumour types and subtypes based on profiling genome-wide DNA methylation [3]. However, disadvantages in diagnostic techniques have impeded their widespread application [4]. Therefore, it has become extremely urgent to find additional markers to predict the outcomes in patients with glioma that are easy to assay. It has long been known that chronic inflammation is an adverse factor that promotes both tumour initiation and progression, and markers of inflammation increase the proliferation and survival of tumour cells and increases the blood supply to tumours [5,6]. The increase in the number of neutrophils, lymphocytes, macrophages or platelets in the peripheral blood is related to the inflammatory response. Due to the strong overproduction of granulocyte colony factor (G-CSF) by neoplastic cells, severe neutrophilia and lymphopenia are observed in patients with gliomas. G-CSF is well recognised as a potent regulator of neutrophil production [7].
Routine blood tests to assess inflammatory processes are often useful in the early diagnosis of several diseases as well as in clinical prognosis in traumatic intracerebral haemorrhage [8]. The assessment of the basic parameters in the peripheral blood count, which is both inexpensive and easy to perform, provides us with information about changes in the numbers of lymphocytes, neutrophils, monocytes and platelets. Recently, some studies have found that the combinations of the haematological components, such as the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), the lymphocyte-to-monocyte ratio (LMR), systemic immune inflammation index (SII), fibrinogen to albumin (FAR) and C-reactive to albumin (CAR) were effective prognostic indicators in patients with a variety of cancers [9,10,11,12,13,14,15,16]. Particular attention was also paid to the role of platelets in the glioblastoma growth. Whether tumour-secreted inflammatory mediators are the underlying mechanism for tumour-platelet interaction, remains to be elucidated [17]. Haematological markers compared to molecular prognostic markers, such as IDH1 mutation [18], mRNA-profiles [19] and NMR spectroscopy-based metabolomics of blood cells [20] can be used to assess the prognosis of GBM patients in order to guide decisions on further therapeutic procedures. Recently, some meta-analyses [4,21,22,23] demonstrated that a high NLR could be considered a high-risk prognostic factor in GMB, and adjustment in chemotherapy should be recommended for high-risk patients. Therefore, we aimed to assess the prognostic values of combined ratios of blood cells in newly diagnosed glioblastoma and to demonstrate the diagnostic usefulness of the systemic inflammatory variables in prediction of survival time as well as to assess of the impact that the inflammation has on the tumour progress.
2. Materials and Methods
2.1. Study Population
A retrospective study was carried out in n = 358 patients (males n = 195 and females n = 163) with newly diagnosed glioma who had undergone an operation in Neurosurgery Centre University Hospital in Zielona Gora between August 2004 and May 2021 (Table 1). The overall survival was defined as the interval between the diagnosis and death. For the patients who had not died prior to the last follow-up, the overall survival was censored at the date of the last follow-up. All patients underwent a craniotomy on GBM with either total or subtotal resection. The pathological diagnoses were based on the classification of CNS tumours [2]. The following exclusion criteria were used: biopsy only, age below 18 years, no definite diagnosis, incomplete baseline clinical data, adjuvant therapy like chemotherapy or radiotherapy received before operation, malnutrition and perioperative mortality (survival < 20 days). For 57 patients with the Grade 4 tumour, time of death was not confirmed by (Polish) National Cancer Registry. Eventually, 178 patients with the Grade 4 tumour (males n = 91 aged 60.1 ± 13.0 years and females n = 87 aged 63.9 ± 11.2 years) and median survival rate of 217 days (the range from 20 to 2946) were included in the survival analysis and regression models. Importantly, every patient diagnosed with a primary brain tumour and enrolled in our study had a very recent diagnosis with no prior specific treatment, including glucocorticoids. The bioethics commission in Zielona Góra approved the research protocol (No. 02/131/2020) in accordance with the Helsinki Declaration.

Table 1.
The clinical characteristics of patients with glial tumours (n = 358).
2.2. Clinical Assessment
Medical records were reviewed, and the following clinical data were collected: gender, age at operation, locations and hemisphere of tumours, pathological diagnoses, and some biomarkers. Ki-67 proliferation index was expressed as the percentage of cells with Ki-67-positive immunostained nuclei using the Ventana BenchMark GX (Ventana Medical Systems Inc., Tucson, AZ, USA). The expression of Ki-67 was categorised into two groups: low and intermediate (Ki-67 < 30%), and high (Ki-67 ≥ 30%) according to the recommendations by Chen et al. [24]. The date on postoperative adjuvant therapies and survival time were collected through documentation analysis.
2.3. Blood Cells and Inflammatory Variables
Blood samples were collected once preoperatively in a volume of 9 mL for laboratory tests using S-Monovette-EDTA K2 tubes (Sarsted AG & Co. KG, Nümbrecht, Germany) within one hour of admission to hospital, and blood counts were immediately analysed. For the other biochemical analyses, blood samples were centrifuged at 3000 rpm for 10 min, and plasma (3 mL) were stored at −80 °C for further study. Haematological parameters including total white blood cell count (WBC), platelet count (PLT) and differential WBC were determined by Sysmex XN-1000 (Sysmex Europe Gmbh, Norderstedt, Germany). The complete blood count and biochemical analysis was carried out in Central Laboratory of the University Hospital in Zielona Gora. The neutrophil-to-lymphocyte ratio (NLR × 103/µL), the platelet-to-lymphocyte ratio (PLR × 103/µL), the lymphocyte-to-monocyte ratio (LMR × 103/µL), the systemic immune inflammation index (SII × 103/µL = Platelets × Neutrophils/Lymphocytes) and the systemic inflammation response index (SIRI × 103/µL = Monocytes × Neutrophils/Lymphocytes) were calculated and compared to reference values according to Luo et al. [25] and Qi et al. [26].
2.4. Statistical Analysis
Statistical analyses were performed using the RStudio, Version 4.1.2 (RStudio, Boston, MA, USA) [27]. The variables were reported as mean values ± standard deviation (SD) or median with interquartile range (iqr). The statistical significance between groups was compared by Kruskal–Wallis rank sum test. The predictive values of variables were evaluated by measuring the area under the receiver operating characteristic curve (ROC curve). The optimal threshold value for clinical stratification (cut-off value) was obtained by calculating the Youden index. Survival curves were plotted by using the Kaplan–Meier method. Cox proportional hazards regression (HR) was performed for both univariate and multivariate analyses. Statistical significance was set at p-value < 0.05.
3. Results
3.1. Study Population
Among 358 study patients 54.5% were males aged 57.4 ± 14.1 years and 45.5% were females aged 59.6 ± 13.6 years. GBM was mostly located in the supratentorial region (frontal, temporal parietal, and occipital lobes), with the highest incidence in the frontal lobe and multiple lobes. Ki-67 ≥ 30% was recorded at 33.6% for all WHO grades and increased to 44.7% in Grade 4 tumour. Patients with high-grade glioma constituted above 50% of the analysed group with a median survival of 217 days and median age of 63 years. No gender differences in the overall survival were observed in Grade 4 tumour (males 327 ± 447 days and females 344 ± 465 days, p = 0.806).
3.2. Blood Cells and Inflammatory Variables
The WBC were found to fall within the referential ranges and did not differ significantly between groups (p = 0.205). Among immune cells, neutrophils showed the greatest changes especially in patients with Grade 3 and Grade 4 tumours. The counts of lymphocytes, monocytes and platelets did not exhibit significant changes compared to reference levels or Grade 1 group (Table 2). NRL, SII and SIRI exceeded the reference values proposed by Luo et al. [21] and were significantly elevated in Grades 2–4 tumour groups. PLR and LMR were found to fall within the referential ranges and did not differ significantly between groups (Table 3).

Table 2.
White blood cells and platelets counts (n = 358).

Table 3.
Blood cell count-derived inflammation indices (n = 358).
The results of the ROC analysis of age and inflammatory variables i.e., NLR, SII and SIRI ranged between 0.6 and 0.7, indicating a potential diagnostic value for clinical prognosis for patients with high-grade glioma. The optimal threshold values corresponded to 63 years for age, 4.56 × 103/µL for NLR, 2300 × 103/µL for SII and 3.03 × 103/µL for SIRI (Table 4). Kaplan–Meyer survival curves showed that elder patients (≥63 years) and with NLR, SII or SIRI higher than the optimal threshold value had a significantly higher risk of shorter overall survival time (Figure 1a–d). With regard to the age, the survival probability decreased by 50% in patients aged ≥ 63 years (Figure 1a).

Table 4.
The statistical characteristics of the ROC curve for the univariate logistic model for Grade 4 tumour patients (n = 178).


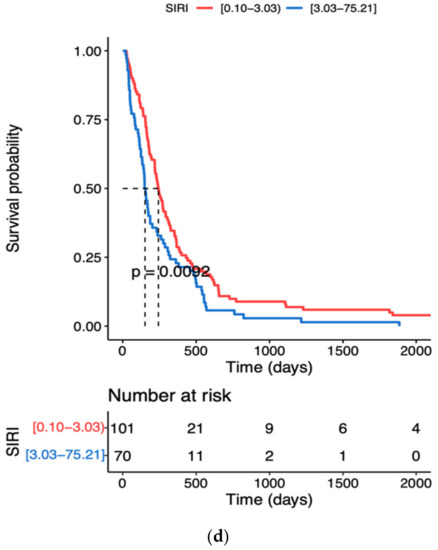
Figure 1.
Kaplan–Meier survival curves during hospitalisation of GBM patients with different cut-off values of the age and systemic inflammation markers: (a) Age, (b) NLR neutrophil to lymphocyte ratio, (c) SII systemic immune inflammation index, (d) SIRI systemic inflammation response index; the dotted line designates median survival.
The univariate Cox model analysis confirmed that the age, NLR, SII and SIRI above the optimal threshold values significantly increased the risk of death (Table 5). Among the analysed inflammatory variables, NLR demonstrated the highest impact on the survival rate (HR 1.56, 95% CI 1.145–2.127, p = 0.005).

Table 5.
Univariate and multivariate Cox model analysis for Grade 4 tumour patients (n = 178).
4. Discussion
The incidence of glioblastoma increases with age, and the median diagnosis is 64 years. The peak incidence is between the ages of 75 and 84, and after the age of 85, the risk is reduced [28]. With a growing and ageing population, the number of cases is expected to increase. Our retrospective study confirms that the survival probability decreases considerably faster in older than young patients with high-grade glioma. Kaplan–Meier survival curves using cut-off values obtained from ROC curves showed a significantly higher risk of death in patients aged 63–90 than in those aged 23–63 years (Figure 1a). Poorer survival in the older age group has already been reported and attributed to a multitude of age-associated changes including the dysregulation of the immune system. Gan et al. [16] indicated a high NLR as an unfavourable predictor of prognosis for elderly patients with high-grade glioma. We observed that patients aged ≥ 63 years (cut-off value) with NRL ≥ 4.56 × 103/µL ran a significantly higher risk of shorter overall survival time. One of the features of immunoageing is a chronic and systemic low-grade inflammation [29]. The inflammation that often accompanies the elderly is also associated with the development of all stages of tumours, both transformation and metastasis [30]. The tumour-derived molecules can condition the bone marrow to increase the release of neutrophil precursors, such as myelocytes and promyelocytes, thereby leading to an increase in circulating immune cells [31]. The greatest changes in neutrophil counts were observed especially in Grade 3 and 4 tumour patients (Table 2). The function of neutrophils remains controversial as they were shown to have both tumour promoting and limiting properties [32]. Reduced lymphocytes activity and T-cell responses to suppress the immune system may enhance tumour progression and metastasis [33,34]. Neutrophils are also involved in tumour development and the formation of a microinflammatory environment, thereby contributing to tumour growth and angiogenesis [32]. Moreover, neutrophils belong to the main cytokine sources, such as interleukin 6, hepatocyte growth factor, transforming growth factor-β, interleukin 8, and matrix metalloproteinases and they are the factors which play an important role in different stages of tumour development [30,31,32]. Neutrophils release the factors of tumour-related angiogenesis, such as vascular endothelial growth factor, angiopoietin-1 and fibroblast growth factor-2 [35]. A growing body of evidence indicates that the neutrophil count is positively related to GBM grade and is an early predictor of tumour progression in patients with glioblastoma [36]. Lymphocytes are responsible for immune surveillance, and they were reported to be protective prognostic factors for cancer patients [37]. Changes in the T-cell population are associated with immune disorders. It has been observed that a reduction in the number of CD4+ lymphocytes in patients with breast and cervical cancer may be associated with faster tumour growth and lymph node infiltration, but, on the other hand, high levels of CD4+ T lymphocytes can reduce the risk of tumour. Reduction in the CD3+, CD4+, CD8+ and CD56+ T cell population in patients with advanced neoplasms reduces the lymphocyte-dependent antitumor cellular response which results in a worse prognosis [38]. Ding et al. [39] also showed that circulating lymphocytes show a cytotoxic effect, which contributes to the inhibition of tumour proliferation and metastasis. Tumour-activated macrophages can promote tumour growth, invasion and migration. They also induce the apoptosis of CD8+ T cells [40], affect tumour angiogenesis and can be associated with a poor prognosis [41]. By interacting with neoplastic cells in the tumour microenvironment through receptors or downstream effectors, activated platelets can promote proliferation, growth and cell survival [17,42,43]. Our study demonstrated significant changes only in neutrophil count; whereas lymphocytes, monocytes and platelets did not exhibit significant changes compared to reference levels (Table 2). The lymphopenia and thrombocytosis are frequent events during GBM disease progression observed after chemoradiation therapy or adjuvant temozolomide [44,45]. However, chemotherapy or radiotherapy received before the operation were included in the exclusion criteria in our study. Therefore, NLR, SII and SIRI, which are based on neutrophil count, could be taken advantage of to assess pro-tumour immune status, and to predict the survival time in newly diagnosed GBM patients.
Kaplan–Meier survival curves using cut-off values showed that increased levels of NLR, SII and SIRI in Grade 4 tumour patients were significantly associated with the survival rate (Figure 1b–d). However, after multivariate Cox regression analysis, only the NLR remained significantly associated with overall survival. A poorer prognosis was observed in the patients with NLR ≥ 4.56 × 103/µL when compared with the patients with NLR < 4.56 × 103/µL. We also evaluated the PLR and LMR, but neither of these variables correlated with the overall survival. NLR showed the greatest potential in the assessment of survival rate in GBM patients. Meta-analysis by Wang et al. [22] indicated that NLR compared to other inflammatory markers was the independent index for prediction of the overall survival in gliomas, and its preoperative assessment can help to evaluate disease progression, optimise treatment and follow-up in glioma patients. Inflammation markers such as the acute phase positive protein CRP appear insufficient for preoperative prognostic assessment. According to Schnaider et al. [46], the ineffectiveness of CRP may result from a lack of specificity as well as to the possible correction prior to elective surgery related to the potential infection and glucocorticoids administration in GBM patients.
The neutrophilia inhibits the immune system response by suppressing the cytolytic activity of cytotoxic T cells and natural killer cells, which is expressed in further changes in NLR [47,48]. The available studies have shown that neutrophils with intrinsic anti-tumour activity are recruited to the tumour where they are reprogrammed to an immunosuppressive pro-tumour phenotype. The tumour-associated neutrophils (TAN) are capable of supporting tumour progression by promoting the angiogenic switch, by stimulating tumour cell motility, migration and invasion, and by modulating other immune cells as part of the ‘immunosuppressive switch’ [49]. However, neutrophil reprogramming depends on the exposure to various inflammatory mediators found in the tumour microenvironment. For example, the presence of transforming growth factor-β (TGFβ) has been demonstrated to promote a pro-tumour phenotype, whereas the presence of interferon-β or the inhibition of TGFβ signalling has been found to result in TAN of an anti-tumour phenotype [49,50]. GBM has been shown to exhibit the highest neutrophil infiltration among all gliomas, with increased neutrophils recruitment being associated with promoted tumour progression and resistance to treatment [51]. Our study identified the new immune-related prognostic peripheral signals, which are associated with increased inflammation, immune infiltration and activation with a shorter overall survival in GBM patients. Between low-grade (1st and 2nd grade) and high-grade glioma (3rd and 4th grade) the differences were significant mainly with regard to neutrophils and NLR.
Inflammatory indices SII and SIRI above the optimal threshold values significantly increased the risk of death (Figure 1c,d). Taking into account the similarities between the formulas for SII and SIRI, the compatibility between these two inflammatory indices is not surprising, as both indicators have been shown to enhance tumour growth, immune escape and cell survival [51]. By means of multivariate Cox analysis, Topkan et al. [52] demonstrated a significant alliance between a low SIRI and longer progression-free and overall survival durations. The authors indicated SIRI as a novel and independent predictor of survival outcomes in newly diagnosed GBM patients intended to undergo postoperative Stupp protocol. Our study focused on the preoperative period and demonstrated significant changes in the counts of neutrophils and insignificant differences in monocyte and platelet counts. Therefore, we adhere to the concept of a crucial role of neutrophils in GBM progression and/or survival probability for Grade 4 tumour patients, which was confirmed by multivariate Cox regression analysis (Table 5). In this respect, a systemic review of 204 meta-analyses has been carried out [23]. These meta-analyses included individual studies which presented neutrophil counts, NLR or tumour-associated neutrophils categorically as either a high or low value in various types of cancers. In total, 29% of the meta-analyses presented strong or highly suggestive evidence for associations between NLR and overall survival [23]. Although NLR holds a clinical promise in its association with poor GBM prognosis, further observations are required to provide evidence whether NLR may aid clinical diagnostics.
Despite many efforts, glioblastoma patients still pose an interdisciplinary challenge in selecting the right treatment [46]. Therefore, an early and reliable prognostication of a patient’s survival, preferably prior to surgery, has a huge relevance. Currently used imaging methods, such as positron emission tomography or magnetic resonance imaging techniques, NMR spectroscopy-based metabolomics are still extremely valuable both in the diagnosis of malignant brain tumours and in the therapeutic evaluation of GBM. Still though, the differentiation between malignant and inflamed brain tissue remains a challenge with imaging protocols [53]. Moreover, GBM differs from other solid tumours in the low to nearly absent frequency of systemically circulating tumour cells, which enhances the argumentation for the remark of NRL based on the neutrophil count.
5. Conclusions
In the first Polish study of the kind, we demonstrated that some parameters derived from complete blood cell analysis, mainly NLR, had prognostic implications in the course of glioblastoma, which may enable neurosurgeons to identify patients with poorer prognosis, and augment personalised approach to surgical treatment strategy.
6. Limitations
The limitations of this study include its retrospective nature and the relatively small sample size of Grade 1 and 2 tumour patients, which may have impacted the statistical analysis and affect the final results as a consequence.
Author Contributions
P.J. and A.Z.-L.: conception and design, analysis and interpretation of the data, critical review, and approval of the final version submitted for publication. M.K., P.D., J.S., J.K. and K.N.: blood sample collection. A.T., M.K. and P.D.: statistical analysis and drafting of the paper, critical review, and approval of the final version submitted for publication. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funds from the University of Zielona Gora (No. 222267/E-545/S/2019).
Institutional Review Board Statement
The study protocol was approved by the Bioethics Commission at Regional Medical Chamber Zielona Gora, Poland (No. 02/131/2020), in accordance with the Helsinki Declaration.
Informed Consent Statement
Informed consent was obtained from all patients admitted to Neurosurgery Center University Hospital in Zielona Gora.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chang, K.; Zhang, B.; Guo, X.; Zong, M.; Rahman, R.; Sanchez, D.; Winder, N.; Reardon, D.A.; Zhao, B.; Wen, P.Y.; et al. Multimodal imaging patterns predict survival in recurrent glioblastoma patients treated with bevacizumab. Neuro. Oncol. 2016, 18, 1680–1687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, D.N.; Perry, A.; Weaselling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neur. Oncol. 2021, 2, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wen, H.-B.; Zhao, Y.-H.; Huang, W.-H.; Wang, Z.-F.; Li, Z.-Q. Systemic inflammatory indicators as prognosticators in glioblastoma patients: A comprehensive meta-analysis. Front Neurol. 2020, 7, 580101. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 4, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Basheer, A.S.; Abas, F.; Othman, I.; Naidu, R. Role of inflammatory mediators, macrophages, and neutrophils in glioma maintenance and progression: Mechanistic understanding and potential therapeutic applications. Cancers 2021, 23, 4226. [Google Scholar] [CrossRef]
- Massara, M.; Persico, P.; Bonavita, O.; Poeta, V.M.; Locati, M.; Simonelli, M.; Bonecchi, R. Neutrophils in gliomas. Front Immunol. 2017, 26, 1349. [Google Scholar] [CrossRef] [Green Version]
- Defort, P.; Retkowska-Tomaszewska, N.; Kot, M.; Jarmużek, P.; Tylutka, A.; Zembroń-Lacny, A. Inflammatory predictors of prognosis in patients with traumatic cerebral haemorrhage: Retrospective study. J. Clin. Med. 2022, 28, 705. [Google Scholar] [CrossRef]
- Kim, E.Y.; Lee, J.W.; Yoo, H.M. The platelet-to-lymphocyte ratio versus neutrophil-to-lymphocyte ratio: Which is better as a prognostic factor in gastric cancer? Ann. Surg. Oncol. 2015, 22, 4363–4370. [Google Scholar]
- Li, J.; Zhou, X.; Xiang, Y.; Zhang, S.; Feng, W.; Yuan, Y.; Liu, Y.; Yin, S. Clinical significance of preoperative fibrinogen to albumin ratio in patients with glioblastoma: A Singe Center Experience. Cancer Manag. Res. 2021, 14, 3259–3269. [Google Scholar] [CrossRef]
- Sahin, F.; Yildiz, P. Serum platelet, MPV, PCT and PDW values, neutrophil to lymphocyte and platelet to lymphocyte ratios in lung cancer diagnosis. Eur. Respir. J. 2015, 46, PA4279. [Google Scholar]
- Sun, P.; Chen, C.; Xia, Y.; Bi, X.; Liu, P.; Zhang, F.; Yang, H.; An, X.; Jiang, W.; Wang, F. The ratio of c-reactive protein/albumin is a novel inflammatory predictor of overall survival in cisplatin-based treated patients with metastatic nasopharyngeal carcinoma. Dis. Markers 2017, 2017, 6570808. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.-N.; Goyal, H.; Yu, S.; Luo, H. Prognostic value of systemic immune-inflammation index (SII) in cancers: A systematic review and meta-analysis. J. Lab. Precis. Med. 2018, 9, 3–29. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 29, 106. [Google Scholar] [CrossRef] [Green Version]
- Ying, H.-Q.; Deng, Q.-W.; He, B.-S.; Pan, Y.-Q.; Wang, F.; Sun, H.-L.; Chen, J.; Liu, X.; Wang, S.-K. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med. Oncol. 2014, 31, 305. [Google Scholar] [CrossRef]
- Gan, Y.; Zhou, X.; Niu, X.; Li, J.; Wang, T.; Zhang, H.; Yang, Y.; Liu, Y.; Mao, Q. Neutrophil/lymphocyte ratio is an independent prognostic factor in elderly patients with high-grade gliomas. World Neurosurg. 2019, 127, e261–e267. [Google Scholar] [CrossRef]
- Nolte, I.; Przibylla, H.; Bostel, T.; Groden, C.; Brockamnn, M.A. Tumor-platelet interactions: Glioblastoma growth is accompanied by increasing platelet counts. Clin. Neurol. Neurosurg. 2008, 110, 339–342. [Google Scholar] [CrossRef]
- Deshmukh, R.; Allega, M.F.; Tardito, S. A map of the altered glioma metabolism. Trends Mol. Med. 2021, 27, 1045–1059. [Google Scholar] [CrossRef]
- Best, M.G.; Sol, N.; Kooi, I.; Tannous, J.; Westerman, B.A.; Rustenburg, F.; Schellen, P.; Verschueren, H.; Post, E.; Koster, J.; et al. RNA-Seq of Tumor-Educated Platelets Enables Blood-Based Pan-Cancer, Multiclass, and Molecular Pathway Cancer Diagnostics. Cancer Cell 2015, 28, 666–676. [Google Scholar] [CrossRef] [Green Version]
- Pudakalakatti, S.; Audia, A.; Mukhopadhyay, A.; Enriquez, J.S.; Bourgeois, D.; Tayob, N.; Zacharias, N.M.; Millward, S.W.; Carson, D.; Farach-Carson, M.C.; et al. NMR spectroscopy-based metabolomics of platelets to analyze brain tumors. Reports 2012, 4, 32. [Google Scholar] [CrossRef]
- Lei, Y.-Y.; Li, Y.-T.; Hu, Q.-L.; Wang, J.; Sui, A.-X. Prognostic impact of neutrophil-to-lymphocyte ratio in gliomas: A systematic review and meta-analysis. World J. Surg. Oncol. 2019, 31, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.P.; Kang, K.; Lin, Q.; Hai, J. Prognostic significance of preoperative systemic cellular inflammatory markers in gliomas: A systematic review and meta-analysis. Clin. Transl. Sci. 2020, 13, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Cupp, M.A.; Cariolou, M.; Tzoulaki, I.; Aune, D.; Evangelou, E.; Berlanga-Taylor, A.J. Neutrophil to lymphocyte ratio and cancer prognosis: An umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020, 20, 360. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-J.; He, D.-S.; Tang, R.-X.; Ren, F.-H.; Chen, G. Ki-67 is a valuable prognostic factor in gliomas: Evidence from a systematic review and meta-analysis. Asian Pac. J. Cancer Prev. 2015, 16, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; He, L.; Zhang, G.; Yu, J.; Chen, Y.; Yin, H.; Goyal, H.; Zhang, G.-M.; Xiao, Y.; Gu, C.; et al. Normal reference intervals of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and systemic immune inflammation index in healthy adults: A large multi-center study from Western China. Clin. Lab. 2019, 1, 65. [Google Scholar] [CrossRef]
- Qi, Q.; Zhuang, L.; Shen, Y.; Geng, Y.; Yu, S.; Chen, H.; Liu, L.; Meng, Z.; Wang, P.; Chen, Z.; et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer 2016, 122, 2158–2167. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 15 February 2022).
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [Green Version]
- Tylutka, A.; Morawin, B.; Gramacki, A.; Zembron-Lacny, A. Pre-Existing hypertension is related with disproportions in t-lymphocytes in older age. J. Clin. Med. 2022, 6, 291. [Google Scholar] [CrossRef]
- Hong, H.; Wang, Q.; Li, J.; Liu, H.; Meng, X.; Zhang, H. Aging, cancer and immunity. J. Cancer 2019, 10, 3021–3027. [Google Scholar] [CrossRef] [Green Version]
- Chao, B.; Ju, X.; Zhang, L.; Xu, X.; Zhao, Y. A novel prognostic marker systemic inflammation response index (SIRI) for operable cervical cancer patients. Front Oncol. 2020, 13, 766. [Google Scholar]
- Granot, Z.; Jablonska, J. Distinct Functions of Neutrophil in cancer and its regulation. Mediat. Inflamm. 2015, 2015, 701067. [Google Scholar] [CrossRef] [Green Version]
- De Larco, J.E.; Wuertz, B.R.K.; Furcht, L.T. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin. Cancer Res. 2004, 1, 4895–4900. [Google Scholar] [CrossRef] [Green Version]
- Ardi, V.C.; Kupriyanova, T.A.; Deryugina, E.I.; Quigley, J.P. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA 2007, 18, 20262–20267. [Google Scholar] [CrossRef] [Green Version]
- Jablonska, E.; Puzewska, W.; Grabowska, Z.; Jablonski, J.; Talarek, Ł. VEGF, IL-18 and NO production by neutrophils and their serum levels in patients with oral cavity cancer. Cytokine 2005, 7, 93–99. [Google Scholar] [CrossRef]
- Weng, W.; Chen, X.; Gong, S.; Guo, L.; Zhang, X. Preoperative neutrophil-lymphocyte ratio correlated with glioma grading and glioblastoma survival. Neurol. Res. 2018, 40, 917–922. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Cropet, C.; Van Glabbeke, M.; Sebban, C.; Le Cesne, A.; Judson, I.; Tredan, O.; Verweij, J.; Biron, P.; Labidi, I.; et al. European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group: Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009, 69, 5383–5391. [Google Scholar] [CrossRef] [Green Version]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 29, 1960–1964. [Google Scholar] [CrossRef] [Green Version]
- Ding, P.-R.; An, X.; Zhang, R.-X.; Fang, Y.-J.; Li, L.-R.; Chen, G.; Wu, X.-J.; Lu, Z.-H.; Lin, J.-Z.; Kong, L.-H.; et al. Elevated preoperative neutrophil to lymphocyte ratio predicts risk of recurrence following curative resection for stage IIA colon cancer. Int. J. Colorectal. Dis. 2010, 25, 1427–1433. [Google Scholar] [CrossRef]
- Liao, R.; Jiang, N.; Tang, Z.-W.; Li, D.W.; Huang, P.; Luo, S.-Q.; Gong, J.-P.; Du, C.-Y. Systemic and intratumoral balances between monocytes/macrophages and lymphocytes predict prognosis in hepatocellular carcinoma patients after surgery. Oncotarget 2016, 24, 30951–30961. [Google Scholar] [CrossRef]
- Mantovani, A.; Schioppa, T.; Porta, C.; Allavena, P.; Sica, A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006, 25, 315–322. [Google Scholar] [CrossRef]
- Mezouar, S.; Frère, C.; Darbousset, R.; Mege, D.; Crescence, L.; Dignat-George, F.; Panicot-Dubois, L.; Dubois, C. Role of platelets in cancer and cancer-associated thrombosis: Experimental and clinical evidences. Thromb. Res. 2016, 139, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Stone, R.L.; Nick, A.M.; McNeish, I.A.; Balkwill, F.; Han, H.D.; Bottsford-Miller, J.; Rupairmoole, R.; Armaiz-Pena, R.N.; Pecot, C.V.; Coward, J.; et al. Paraneoplastic thrombocytosis in ovarian cancer. N. Engl. J. Med. 2012, 366, 610–618. [Google Scholar] [CrossRef] [Green Version]
- Woo, J.K.; Yun-Sik, D.; Chan-Young, O.; Jin, W.K.; Seung, H.C.; Soon-Tae, L.; Han, K.; Tae, M.K.; Chul-Kee, P. Clinical observation of lymphopenia in patients with newly diagnosed glioblastoma. J. Neurooncol. 2019, 143, 321–328. [Google Scholar]
- Keeratikarn, B.; Kenneth, R.H.; Jie, Y.; Lihong, L.; Qianghu, W.; Ravesanker, E.; Auia, A.; Alfaro-Munoz, K.D.; de Groot, J.F.; Bhat, K.P.; et al. A relative increase in circulating platelets following chemoradiation predicts for poor survival of patients with glioblastoma. Oncotarget 2017, 27, 90488–90495. [Google Scholar]
- Schneider, M.; Schäfer, N.; Apallas, S.; Potthoff, A.-L.; Bode, C.; Güresir, E.; Heimann, M.; Lehmann, F.; Scharnböck, E.; Schaub, C.; et al. Red blood cell distribution width to platelet ratio substantiates preoperative survival prediction in patients with newly-di.agnosed glioblastoma. J. Neurooncol. 2021, 154, 229–235. [Google Scholar] [CrossRef]
- Ya-Jui, L.; Kuo-Chen, W.; Pin-Yuan, C.; Michael, L.; Tsong-Long, H. Roles of neutrophils in glioma and brain metastases. Front Immunol. 2021, 12, 701383. [Google Scholar]
- Kaya, V.; Yıldırım, M.; Yazıcı, G.; Yalçın, A.Y.; Orhan, N.; Güzel, A. Prognostic Significance of Indicators of Systemic Inflammatory Responses in Glioblastoma Patients. Asian Pac. J. Cancer Prev. 2017, 18, 3287–3291. [Google Scholar]
- Shaul, M.E.; Fridlender, Z.G. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 2019, 16, 601–620. [Google Scholar] [CrossRef]
- Fossati, G.; Ricevuti, G.; Edwards, S.W.; Walker, C.; Dalton, A.; Rossi, M.L. Neutrophil infiltration into human gliomas. Acta Neuropathol. 1999, 98, 349–354. [Google Scholar] [CrossRef]
- Mostofa, A.G.M.; Punganuru, S.R.; Rao Madala, H.; Al-Obaide, M.; Srivenugopal, K.S. The Process and regulatory components of inflammation in brain oncogenesis. Biomolecules 2017, 27, 34. [Google Scholar] [CrossRef] [Green Version]
- Topkan, E.; Kucuk, E.; Ozdemir, Y.; Mertsoylu, H.; Besen, A.A.; Sezen, D.; Bolukbasi, Y.; Pehlivan, B.; Selek, U. Systemic inflammation response index predicts survival outcomes in glioblastoma multiforme patients treated with standard stupp protocol. J. Immunol. Res. 2020, 14, 8628540. [Google Scholar] [CrossRef] [PubMed]
- Roesler, R.; Dini, S.A.; Isolan, G.R. Neuroinflammation and immunoregulation in glioblastoma and brain metastases: Recent developments in imaging approaches. Clin. Exp. Immunol. 2021, 206, 314–324. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).