First 24-Hour Potassium Concentration and Variability and Association with Mortality in Patients Requiring Continuous Renal Replacement Therapy in Intensive Care Units: A Hospital-Based Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CRRT Protocol
2.3. K+ Measurements and Other Confounders
2.4. Endpoint
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Cohort
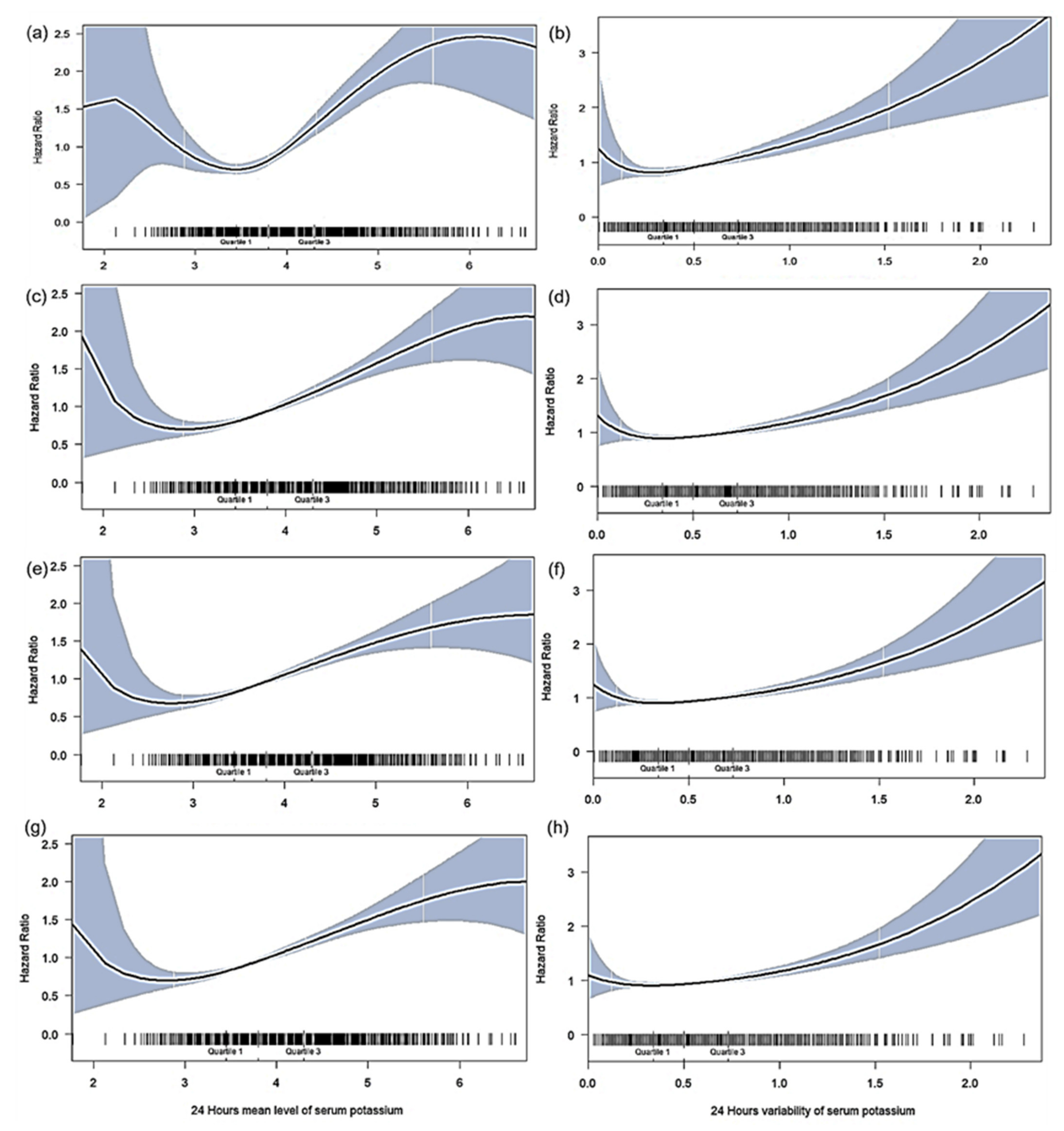
3.2. Association of Mean K+ Levels after CRRT Initiation with Mortality
3.3. Association of K+ Variability after CRRT Initiation with Mortality
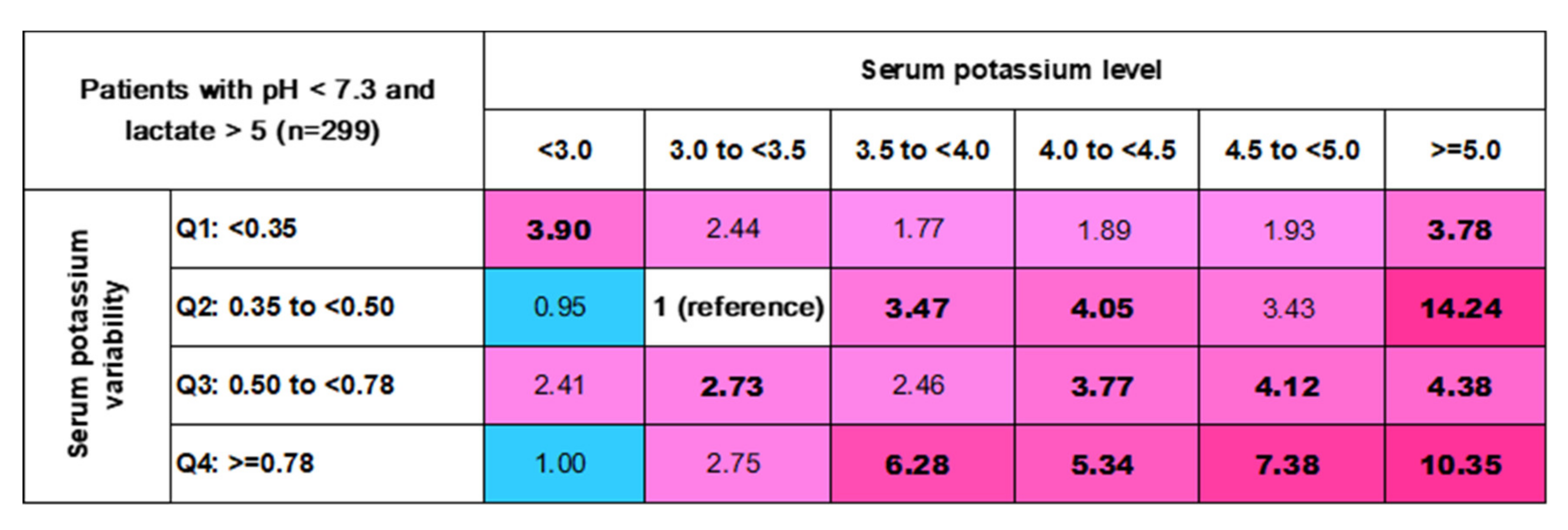
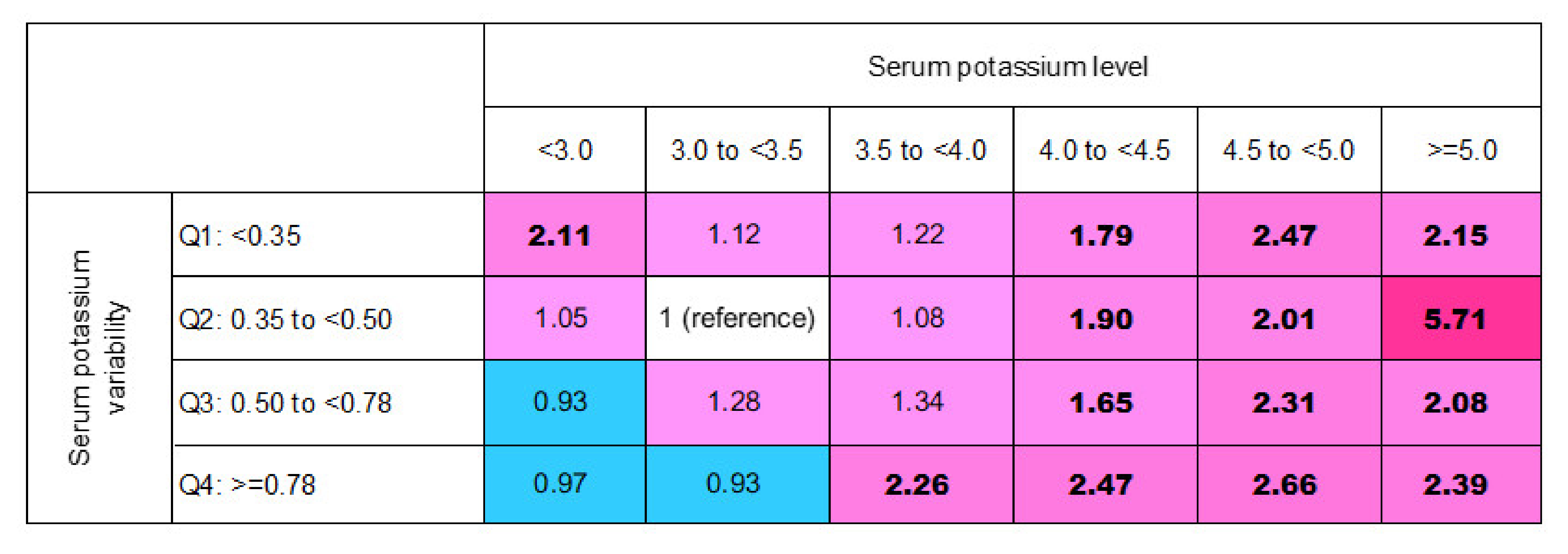
3.4. In-Hospital Mortality Risk Matrix of Mean (K+) Levels and Their Variability
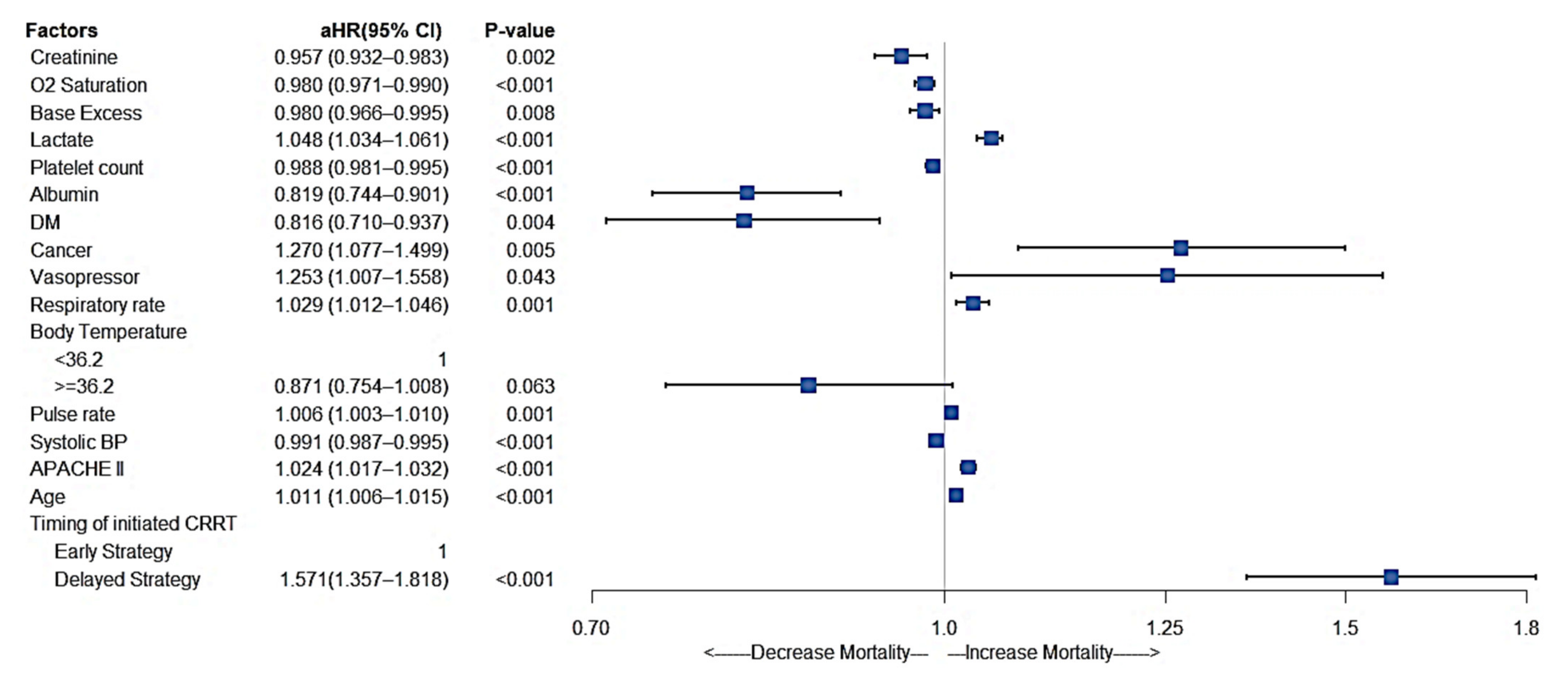
3.5. Other Significant Factors Affecting In-Hospital Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| (K+) (mmol/L) | |
|---|---|
| >3.5 | No KCl mixed in the 5 L Prismasol B0 |
| 3.1~3.5 | 40 mmol KCL mixed in the 5 L Prismasol B0 |
| 2.6~3.0 | 80 mmol KCL mixed in the 5 L Prismasol B0 |
| 2.1~2.5 | 120 mmol KCL mixed in the 5 L Prismasol B0 |
Appendix B
| 72-Hour Mortality | 28-Day Mortality | 90-Day Mortality | In-Hospital Mortality | |||||
|---|---|---|---|---|---|---|---|---|
| cHR (95% CI) | p Value | cHR (95% CI) | p Value | cHR (95% CI) | p Value | cHR (95% CI) | p Value | |
| Serum (K+) level | ||||||||
| <3.0 | 1.56 (1.02–2.39) | 0.040 | 1.28 (0.95–1.73) | 0.104 | 1.23 (0.93–1.63) | 0.142 | 1.28 (0.97–1.70) | 0.082 |
| 3.0 to <3.5 | Reference | Reference | Reference | Reference | ||||
| 3.5 to <4.0 | 1.05 (0.81–1.35) | 0.718 | 1.06 (0.90–1.25) | 0.477 | 1.08 (0.93–1.26) | 0.289 | 1.10 (0.95–1.27) | 0.200 |
| 4.0 to <4.5 | 1.48 (1.13–1.93) | 0.004 | 1.29 (1.08–1.54) | 0.006 | 1.30 (1.10–1.53) | 0.002 | 1.32 (1.12–1.56) | 0.001 |
| 4.5 to <5.0 | 1.95 (1.46–2.61) | <0.001 | 1.47 (1.20–1.82) | <0.001 | 1.43 (1.18–1.73) | <0.001 | 1.46 (1.20–1.76) | <0.001 |
| ≥5.0 | 2.85 (2.12–3.84) | <0.001 | 1.90 (1.51–2.38) | <0.001 | 1.66 (1.33–2.06) | <0.001 | 1.77 (1.42–2.19) | <0.001 |
| Serum (K+) variability | ||||||||
| Q1: <0.35 | 1.09 (0.84–1.41) | 0.521 | 1.09 (0.92–1.30) | 0.299 | 1.05 (0.90–1.23) | 0.539 | 1.09 (0.93–1.27) | 0.273 |
| Q2: 0.35 to<0.50 | Reference | Reference | Reference | Reference | ||||
| Q3: 0.50 to<0.78 | 1.29 (1.02–1.63) | 0.033 | 1.14 (0.97–1.33) | 0.101 | 1.11 (0.96–1.28) | 0.149 | 1.14 (0.99–1.32) | 0.067 |
| Q4: ≥0.78 | 2.49 (1.9–3.27) | <0.001 | 1.71 (1.40–2.10) | <0.001 | 1.61 (1.33–1.95) | <0.001 | 1.62 (1.34–1.96) | <0.001 |
Appendix C
References
- Gemmell, L.; Docking, R.; Black, E. Renal replacement therapy in critical care. BJA Educ. 2017, 17, 88–93. [Google Scholar] [CrossRef]
- Hoste, E.A.J.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Chuasuwan, A.; Gojaseni, P.; Chittinandana, A. Continuous veno-venous hemofiltration in Bhumibol Adulyadej Hospital. J. Med. Assoc. Thai. 2006, 89 (Suppl. S2), S86–S97. [Google Scholar] [PubMed]
- Wong, D.T.; Knaus, W.A. Predicting outcome in critical care: The current status of the APACHE prognostic scoring system. Can. J. Anaesth. 1991, 38, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Douma, C.E.; Redekop, W.K.; van der Meulen, J.H.; van Olden, R.W.; Haeck, J.; Struijk, D.G.; Krediet, R.T. Predicting mortality in intensive care patients with acute renal failure treated with dialysis. J. Am. Soc. Nephrol. 1997, 8, 111–117. [Google Scholar] [CrossRef]
- Chertow, G.M.; Levy, E.M.; Hammermeister, K.E.; Grover, F.; Daley, J. Independent association between acute renal failure and mortality following cardiac surgery. Am. J. Med. 1998, 104, 343–348. [Google Scholar] [CrossRef]
- Metnitz, P.G.; Krenn, C.G.; Steltzer, H.; Lang, T.; Ploder, J.; Lenz, K.; Le Gall, J.R.; Druml, W. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit. Care Med. 2002, 30, 2051–2058. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Appel, L.J.; Grams, M.E.; Gutekunst, L.; McCullough, P.A.; Palmer, B.F.; Pitt, B.; Sica, D.A.; Townsend, R.R. Potassium Homeostasis in Health and Disease: A Scientific Workshop Cosponsored by the National Kidney Foundation and the American Society of Hypertension. Am. J. Kidney Dis. 2017, 70, 844–858. [Google Scholar] [CrossRef]
- Udensi, U.K.; Tchounwou, P.B. Potassium Homeostasis, Oxidative Stress, and Human Disease. Int. J. Clin. Exp. Physiol. 2017, 4, 111–122. [Google Scholar] [CrossRef]
- Buckley, M.S.; Leblanc, J.M.; Cawley, M.J. Electrolyte disturbances associated with commonly prescribed medications in the intensive care unit. Crit. Care Med. 2010, 38, S253–S264. [Google Scholar] [CrossRef]
- Dashputre, A.A.; Potukuchi, P.K.; Sumida, K.; Kar, S.; Obi, Y.; Thomas, F.; Molnar, M.Z.; Streja, E.; Kalantar-Zadeh, K.; Kovesdy, C.P. Predialysis Potassium Variability and Postdialysis Mortality in Patients With Advanced CKD. Kidney Int. Rep. 2021, 6, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Xu, F.; Fan, L.; Xiong, L.; Li, H.; Cao, S.; Lin, X.; Zheng, Z.; Yu, X.; Mao, H. Serum potassium levels and its variability in incident peritoneal dialysis patients: Associations with mortality. PLoS ONE 2014, 9, e86750. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, L.J.; Balzer, F.; Müller, M.C.; Grunow, J.J.; Spies, C.D.; Christopher, K.B.; Weber-Carstens, S.; Wollersheim, T. Association between potassium concentrations, variability and supplementation, and in-hospital mortality in ICU patients: A retrospective analysis. Ann. Intensive Care 2019, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Mena, L.; Pintos, S.; Queipo, N.V.; Aizpúrua, J.A.; Maestre, G.; Sulbarán, T. A reliable index for the prognostic significance of blood pressure variability. J. Hypertens 2005, 23, 505–511. [Google Scholar] [CrossRef]
- Hansen, T.W.; Thijs, L.; Li, Y.; Boggia, J.; Kikuya, M.; Björklund-Bodegård, K.; Richart, T.; Ohkubo, T.; Jeppesen, J.; Torp-Pedersen, C.; et al. Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension 2010, 55, 1049–1057. [Google Scholar] [CrossRef]
- Parati, G.; Rizzoni, D. Assessing the prognostic relevance of blood pressure variability: Discrepant information from different indices. J. Hypertens 2005, 23, 483–486. [Google Scholar] [CrossRef]
- Hessels, L.; Hoekstra, M.; Mijzen, L.J.; Vogelzang, M.; Dieperink, W.; Lansink, A.O.; Nijsten, M.W. The relationship between serum potassium, potassium variability and in-hospital mortality in critically ill patients and a before-after analysis on the impact of computer-assisted potassium control. Crit. Care 2015, 19, 4. [Google Scholar] [CrossRef]
- Goyal, A.; Spertus, J.A.; Gosch, K.; Venkitachalam, L.; Jones, P.G.; Van den Berghe, G.; Kosiborod, M. Serum potassium levels and mortality in acute myocardial infarction. JAMA 2012, 307, 157–164. [Google Scholar] [CrossRef]
- Macdonald, J.E.; Struthers, A.D. What is the optimal serum potassium level in cardiovascular patients? J. Am. Coll. Cardiol. 2004, 43, 155–161. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, L. The association between fluid balance and mortality in patients with ARDS was modified by serum potassium levels: A retrospective study. PeerJ 2015, 3, e752. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tisdale, J.E.; Patel, R.; Webb, C.R.; Borzak, S.; Zarowitz, B.J. Electrophysiologic and proarrhythmic effects of intravenous inotropic agents. Prog. Cardiovasc. Dis. 1995, 38, 167–180. [Google Scholar] [CrossRef]
- Weiss, J.N.; Qu, Z.; Shivkumar, K. Electrophysiology of Hypokalemia and Hyperkalemia. Circ. Arrhythm. Electrophysiol. 2017, 10, e004667. [Google Scholar] [CrossRef] [PubMed]
- Campese, V.M.; Adenuga, G. Electrophysiological and clinical consequences of hyperkalemia. Kidney Int. Suppl. 2016, 6, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Gambaro, G.; Ferraro, P.M. Serum potassium variability is associated with increased mortality in a large cohort of hospitalized patients. Nephrol. Dial. Transpl. 2021, gfab211. [Google Scholar] [CrossRef]
- Vogelzang, M.; Zijlstra, F.; Nijsten, M.W.N. Design and implementation of GRIP: A computerized glucose control system at a surgical intensive care unit. BMC Med. Inform. Decis. Mak. 2005, 5, 38. [Google Scholar] [CrossRef]
- Shiyovich, A.; Gilutz, H.; Plakht, Y. Potassium Fluctuations Are Associated With Inhospital Mortality From Acute Myocardial Infarction. Soroka Acute Myocardial Infarction II (SAMI-II) Project. Angiology 2018, 69, 709–717. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Cheungpasitporn, W.; Radhakrishnan, Y.; Zabala Genovez, J.L.; Petnak, T.; Shawwa, K.; Qureshi, F.; Mao, M.A.; Kashani, K.B. Association of serum potassium derangements with mortality among patients requiring continuous renal replacement therapy. Apher Dial. 2022. [Google Scholar] [CrossRef]
- Atrash, A.K.; De Vasconcellos, K. Low albumin levels are associated with mortality in the critically ill: A retrospective observational study in a multidisciplinary intensive care unit. S. Afr. J. Crit. Care 2020, 36, 74–79. [Google Scholar] [CrossRef]
- Hu, C.; Hu, B.; Wang, J.; Peng, Z.; Kashani, K.; Li, J. Diabetes Mellitus is Associated with Lower Mortality in Intensive Care Unit: A Multicenter Historical Cohort Study. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Zarbock, A.; Kellum, J.A.; Schmidt, C.; Van Aken, H.; Wempe, C.; Pavenstädt, H.; Boanta, A.; Gerß, J.; Meersch, M. Effect of Early vs Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients With Acute Kidney Injury: The ELAIN Randomized Clinical Trial. JAMA 2016, 315, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhang, Y. Inotrope Use and Intensive Care Unit Mortality in Patients With Cardiogenic Shock: An Analysis of a Large Electronic Intensive Care Unit Database. Front. Cardiovasc. Med. 2021, 8, 696138. [Google Scholar] [CrossRef] [PubMed]
- Assi, H.I.; Halim, N.A.; Alameh, I.; Khoury, J.; Nahra, V.; Sukhon, F.; Charafeddine, M.; El Nakib, C.; Moukalled, N.; Bou Zerdan, M.; et al. Outcomes of Patients with Malignancy Admitted to the Intensive Care Units: A Prospective Study. Crit. Care Res. Pr. 2021, 2021, 4792309. [Google Scholar] [CrossRef]
- Kaur, G.; Banoth, P.; Yerram, P.; Misra, M. A case of hypothermia on CRRT. Hemodial. Int. 2017, 21 (Suppl. S2), S57–S61. [Google Scholar] [CrossRef] [PubMed]
- Jones, S. Heat loss and continuous renal replacement therapy. AACN Clin. Issues 2004, 15, 223–230. [Google Scholar] [CrossRef]


| The Levels of Serum (K+) within 24 h after Initial CRRT | p Value | ||||||
|---|---|---|---|---|---|---|---|
| <3.0 | 3.0 to <3.5 | 3.5 to <4.0 | 4.0 to <4.5 | 4.5 to <5.0 | ≥5.0 | ||
| Sample size | 86 | 477 | 663 | 394 | 217 | 154 | |
| Gender, Male | 50 (58.1%) | 286 (60%) | 427 (64.4%) | 251 (63.7%) | 147 (67.7%) | 106 (68.8%) | 0.189 |
| Age | 66 (56–78) | 69 (56–80) | 70 (59–80) | 73 (62–81) | 71 (60–80) | 70 (58–82) | 0.007 |
| BMI | 23.1 (20.3–26.6) | 24.4 (21.5–28) | 24.6 (21.4–27.5) | 24.2 (20.9–28.2) | 25 (21.8–27.8) | 24 (22.1–28.9) | 0.261 |
| APACHE II at admission | 29 (23–37) | 28 (22–35) | 29 (22–35) | 29 (22–35) | 30 (24–36) | 30 (23–38) | 0.071 |
| ICU type | |||||||
| Medicine ICU | 54 (62.8%) | 355 (74.4%) | 531 (80.1%) | 319 (81%) | 175 (80.6%) | 127 (82.5%) | 0.001 |
| Surgery ICU | 32 (37.2%) | 122 (25.6%) | 132 (19.9%) | 75 (19%) | 42 (19.4%) | 27 (17.5%) | |
| The average level of serum (K+) within 24 h after initial CRRT | 2.9 (2.7–2.9) | 3.3 (3.2–3.4) | 3.7 (3.6–3.9) | 4.2 (4.1–4.4) | 4.7 (4.6–4.9) | 5.4 (5.2–5.7) | <0.001 |
| The variability of serum (K+) within 24 h after initial CRRT | 0.51 (0.36–0.71) | 0.40 (0.26–0.58) | 0.46 (0.33–0.66) | 0.55 (0.39–0.78) | 0.64 (0.46–0.91) | 0.78 (0.58–1.11) | <0.001 |
| Q1: <0.35 | 20 (23.3%) | 190 (39.8%) | 193 (29.1%) | 74 (18.8%) | 30 (13.8%) | 13 (8.4%) | <0.001 |
| Q2: 0.35 to <0.50 | 22 (25.6%) | 116 (24.3%) | 178 (26.8%) | 100 (25.4%) | 35 (16.1%) | 15 (9.7%) | |
| Q3: 0.50 to <0.78 | 35 (40.7%) | 144 (30.2%) | 249 (37.6%) | 175 (44.4%) | 107 (49.3%) | 72 (46.8%) | |
| Q4: ≥0.78 | 9 (10.5%) | 27 (5.7%) | 43 (6.5%) | 45 (11.4%) | 45 (20.7%) | 54 (35.1%) | |
| Diagnostic criteria for AKI | |||||||
| Oliguria | 19 (22.1%) | 146 (30.6%) | 201 (30.3%) | 114 (28.9%) | 65 (30%) | 30 (19.5%) | <0.001 |
| Anuria | 11 (12.8%) | 93 (19.5%) | 155 (23.4%) | 104 (26.4%) | 49 (22.6%) | 40 (26%) | |
| AKI achieves KDIGO-defined serum Creatinine elevation | 22 (25.6%) | 107 (22.4%) | 152 (22.9%) | 109 (27.7%) | 59 (27.2%) | 52 (33.8%) | |
| Others | 34 (39.5%) | 131 (27.5%) | 155 (23.4%) | 67 (17%) | 44 (20.3%) | 32 (20.8%) | |
| Timing of initiated CRRT * | |||||||
| Early strategy | 56 (65.1%) | 279 (58.5%) | 412 (62.1%) | 225 (57.1%) | 124 (57.1%) | 85 (55.2%) | 0.323 |
| Delayed strategy | 30 (34.9%) | 198 (41.5%) | 251 (37.9%) | 169 (42.9%) | 93 (42.9%) | 69 (44.8%) | |
| Vital Sign | |||||||
| Systolic BP (mmHg) | 110 (104–123) | 109 (102–122) | 110 (101–120) | 111 (102–120) | 110 (101–125) | 110 (99–121) | 0.851 |
| Diastolic BP (mmHg) | 58 (52–66) | 59 (52–66) | 59 (52–68) | 57 (51–65) | 58 (52–67) | 59 (51–67) | 0.564 |
| Pulse rate (bpm) | 107 (88–122) | 105 (88–117) | 103 (88–117) | 100 (85–115) | 105 (90–117) | 101 (87–114) | 0.158 |
| Body temperature (degree Celsius) | 36.2 (35.5–37.2) | 36.2 (35.4–36.9) | 36.2 (35.4–36.8) | 36.1 (35.4–36.8) | 36.2 (35.6–36.9) | 36.2 (35.7–36.9) | 0.556 |
| Respiratory rate (/min) | 20 (17–24) | 20 (17–23) | 20 (17–23) | 20 (17–22) | 21 (18–23) | 21 (18–25) | 0.009 |
| SPO2 | 98 (95–99) | 97 (95–99) | 97 (95–99) | 97 (95–99) | 97 (95–99) | 96 (94–98) | 0.118 |
| Multiple organ support before CRRT—no. (%) | |||||||
| Invasive mechanical ventilation | 77 (89.5%) | 423 (88.7%) | 578 (87.2%) | 333 (84.5%) | 187 (86.2%) | 127 (82.5%) | 0.270 |
| Extracorporeal Membrane Oxygenation (ECMO) | 10 (11.6%) | 73 (15.3%) | 92 (13.9%) | 30 (7.6%) | 12 (5.5%) | 4 (2.6%) | <0.001 |
| Vasopressors support with norepinephrine or epinephrine | 70 (81.4%) | 400 (83.9%) | 547 (82.5%) | 332 (84.3%) | 184 (84.8%) | 129 (83.8%) | 0.942 |
| Medication use before CRRT—no. (%) | |||||||
| Sedative | 54 (62.8%) | 358 (75.1%) | 482 (72.7%) | 284 (72.1%) | 146 (67.3%) | 111 (72.1%) | 0.136 |
| Corticosteroids | 49 (57%) | 264 (55.3%) | 372 (56.1%) | 225 (57.1%) | 129 (59.4%) | 94 (61%) | 0.807 |
| Loop diuretic | 86(100%) | 477(100%) | 663(100%) | 394(100%) | 217(100%) | 154(100%) | -- |
| Parental nutrition | 79 (91.9%) | 401 (84.1%) | 537 (81.0%) | 330 (83.8%) | 178 (82.0%) | 144 (93.5%) | 0.002 |
| Furosemide | 47 (54.7%) | 237 (49.7%) | 307 (46.3%) | 206 (52.3%) | 105 (48.4%) | 83 (53.9%) | 0.292 |
| Antibiotics | 84 (97.7%) | 459 (96.2%) | 619 (93.4%) | 369 (93.7%) | 210 (96.8%) | 146 (94.8%) | 0.113 |
| Insulin use within 24 h after initial CRRT | 36 (41.9%) | 254 (53.2%) | 355 (53.5%) | 230 (58.4%) | 130 (59.9%) | 108 (70.1%) | <0.001 |
| Urine Output before CRRT—ml/24 h | 25 (6–52) | 16 (4–35) | 10 (2–29) | 10 (3–29) | 6 (0–22) | 14 (2–42) | <0.001 |
| Coexisting conditions—no. (%) | |||||||
| Hypertension | 20 (23.3%) | 170 (35.6%) | 260 (39.2%) | 152 (38.6%) | 81 (37.3%) | 59 (38.3%) | 0.101 |
| Diabetes Mellitus | 24 (27.9%) | 149 (31.2%) | 248 (37.4%) | 162 (41.1%) | 79 (36.4%) | 53 (34.4%) | 0.030 |
| Hyperlipidemia | 10 (11.6%) | 67 (14%) | 122 (18.4%) | 71 (18%) | 37 (17.1%) | 31 (20.1%) | 0.221 |
| Coronary artery disease | 12 (14%) | 100 (21%) | 171 (25.8%) | 107 (27.2%) | 52 (24%) | 33 (21.4%) | 0.050 |
| Congestive heart failure | 4 (4.7%) | 51 (10.7%) | 145 (21.9%) | 95 (24.1%) | 40 (18.4%) | 26 (16.9%) | <0.001 |
| Chronic pulmonary disease | 8 (9.3%) | 80 (16.8%) | 132 (19.9%) | 72 (18.3%) | 52 (24%) | 29 (18.8%) | 0.056 |
| Chronic renal disease | 24 (27.9%) | 145 (30.4%) | 247 (37.3%) | 162 (41.1%) | 91 (41.9%) | 64 (41.6%) | 0.002 |
| Malignancy | 14 (16.3%) | 87 (18.2%) | 91 (13.7%) | 73 (18.5%) | 35 (16.1%) | 32 (20.8%) | 0.163 |
| Cardiac arrhythmia occurrence at the baseline | 8 (9.3%) | 82 (17.19%) | 136 (20.51%) | 88 (22.34%) | 52 (23.96%) | 32 (20.78%) | 0.033 |
| Cardiac arrhythmia occurrence during the 24 h CRRT | 6 (6.98%) | 74 (15.51%) | 124 (18.7%) | 69 (17.51%) | 38 (17.51%) | 20 (12.99%) | 0.073 |
| Laboratory data before CRRT | |||||||
| Albumin, mg/dL | 1.9 (1.5–2.4) | 2.1 (1.7–2.6) | 2.3 (1.9–2.8) | 2.3 (1.8–2.9) | 2.2 (1.7–2.8) | 2.2 (1.7–2.6) | <0.001 |
| Hemoglobin, g/dL | 9 (9–11) | 10 (9–11) | 10 (9–12) | 9 (8–11) | 10 (8–11) | 10 (8–11) | 0.022 |
| WBC count, 1000/uL | 9 (5–13) | 11 (7–18) | 12 (7–17) | 12 (8–18) | 13 (9–21) | 13 (8–20) | <0.001 |
| Platelet count, 1000/uL | 74 (33–121) | 95 (58–170) | 109 (58–181) | 111 (64–183) | 136 (72–208) | 141 (79–228) | <0.001 |
| pH | 7.3 (7.2–7.4) | 7.3 (7.3–7.4) | 7.3 (7.3–7.4) | 7.3 (7.2–7.4) | 7.3 (7.2–7.4) | 7.3 (7.2–7.4) | 0.003 |
| Sodium, mmol/L | 139 (133–145) | 139 (134–143) | 138 (134–142) | 138 (134–142) | 137 (134–141) | 137 (133–142) | 0.103 |
| Lactate, mmol/L | 5.1(1.6–9) | 4(1.9–8.5) | 4.1(1.7–8.7) | 3.6(1.8–8.5) | 3.5(2.1–8.1) | 5.4(1.6–9.7) | 0.737 |
| Calcium, mg/dL | 7 (7–8) | 8 (7–8) | 8 (7–8) | 8 (7–9) | 8 (7–8) | 8 (7–9) | <0.001 |
| Base Excess, mmol/L | −9 (−13–−6) | −8 (−11–−5) | −7 (−11–−4) | −8 (−11–−4) | −8 (−11–−5) | −8 (−12–−4) | 0.235 |
| O2 Saturation, % | 99 (97–100) | 98 (96–100) | 98 (96–100) | 98 (96–100) | 98 (96–100) | 98 (95–100) | 0.220 |
| Creatinine, mg/dL | 2.0 (1.0–3.1) | 1.9 (1.2–3.3) | 2.5 (1.4–4.3) | 2.4 (1.3–5.2) | 2.8 (1.4–5.1) | 2.7 (1.3–4.9) | <0.001 |
| Outcome | |||||||
| 72 h mortality | 27 (31.4%) | 101 (21.2%) | 147 (22.2%) | 117 (29.7%) | 82 (37.8%) | 78 (50.6%) | <0.001 |
| 28-day mortality | 52 (60.5%) | 246 (51.6%) | 353 (53.2%) | 234 (59.4%) | 137 (63.1%) | 107 (69.5%) | <0.001 |
| 90-day mortality | 59 (68.6%) | 295 (61.8%) | 431 (65%) | 276 (70.1%) | 158 (72.8%) | 112 (72.7%) | 0.014 |
| In-hospital mortality | 59 (68.6%) | 302 (63.3%) | 435 (65.6%) | 276 (70.1%) | 160 (73.7%) | 114 (74.0%) | 0.024 |
| 72-h Mortality | 28-Day Mortality | 90-Day Mortality | In-Hospital Mortality | |||||
|---|---|---|---|---|---|---|---|---|
| aHR (95% CI) a | p Value | aHR (95% CI) b | p Value | aHR (95% CI) c | p Value | aHR (95% CI) d | p Value | |
| Serum (K+) level | ||||||||
| <3.0 | 1.10 (0.60–2.02) | 0.756 | 1.10 (0.74–1.63) | 0.630 | 0.99 (0.68–1.44) | 0.968 | 1.24 (0.88–1.75) | 0.228 |
| 3.0 to <3.5 | Reference | Reference | Reference | Reference | ||||
| 3.5 to <4.0 | 1.00 (0.73–1.38) | 0.991 | 1.15 (0.93–1.41) | 0.194 | 1.15 (0.95–1.39) | 0.150 | 1.17 (0.97–1.39) | 0.094 |
| 4.0 to <4.5 | 1.63 (1.17–2.26) | 0.004 | 1.65 (1.32–2.07) | <0.001 | 1.66 (1.35–2.04) | <0.001 | 1.59 (1.31–1.94) | <0.001 |
| 4.5 to <5.0 | 2.12 (1.46–3.09) | <0.001 | 2.00 (1.52–2.64) | <0.001 | 2.06 (1.60–2.66) | <0.001 | 1.85 (1.44–2.36) | <0.001 |
| ≥5.0 | 2.52 (1.69–3.77) | <0.001 | 2.10 (1.54–2.86) | <0.001 | 1.96 (1.45–2.64) | <0.001 | 1.72 (1.30–2.27) | <0.001 |
| Serum (K+) variability | ||||||||
| Q1: <0.35 | 1.03 (0.74–1.42) | 0.870 | 1.10 (0.89–1.36) | 0.381 | 1.07 (0.88–1.30) | 0.490 | 1.12 (0.93–1.35) | 0.242 |
| Q2: 0.35 to <0.50 | Reference | Reference | Reference | Reference | ||||
| Q3: 0.50 to <0.78 | 1.09 (0.81–1.46) | 0.565 | 1.04 (0.86–1.27) | 0.688 | 1.04 (0.87–1.24) | 0.677 | 1.06 (0.89–1.26) | 0.500 |
| Q4: ≥0.78 | 1.61 (1.13–2.29) | 0.009 | 1.39 (1.06–1.82) | 0.018 | 1.43 (1.11–1.83) | 0.005 | 1.31 (1.03–1.65) | 0.027 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, I.-C.; Lin, P.-R.; Wu, S.-H.; Hsu, H.-H.; Hung, P.-S.; Kor, C.-T. First 24-Hour Potassium Concentration and Variability and Association with Mortality in Patients Requiring Continuous Renal Replacement Therapy in Intensive Care Units: A Hospital-Based Retrospective Cohort Study. J. Clin. Med. 2022, 11, 3383. https://doi.org/10.3390/jcm11123383
Mao I-C, Lin P-R, Wu S-H, Hsu H-H, Hung P-S, Kor C-T. First 24-Hour Potassium Concentration and Variability and Association with Mortality in Patients Requiring Continuous Renal Replacement Therapy in Intensive Care Units: A Hospital-Based Retrospective Cohort Study. Journal of Clinical Medicine. 2022; 11(12):3383. https://doi.org/10.3390/jcm11123383
Chicago/Turabian StyleMao, I-Chieh, Pei-Ru Lin, Shin-Hwar Wu, Hsin-Hui Hsu, Pei-Shan Hung, and Chew-Teng Kor. 2022. "First 24-Hour Potassium Concentration and Variability and Association with Mortality in Patients Requiring Continuous Renal Replacement Therapy in Intensive Care Units: A Hospital-Based Retrospective Cohort Study" Journal of Clinical Medicine 11, no. 12: 3383. https://doi.org/10.3390/jcm11123383
APA StyleMao, I.-C., Lin, P.-R., Wu, S.-H., Hsu, H.-H., Hung, P.-S., & Kor, C.-T. (2022). First 24-Hour Potassium Concentration and Variability and Association with Mortality in Patients Requiring Continuous Renal Replacement Therapy in Intensive Care Units: A Hospital-Based Retrospective Cohort Study. Journal of Clinical Medicine, 11(12), 3383. https://doi.org/10.3390/jcm11123383





