Dupilumab Improves Skin Barrier Function in Adults with Atopic Dermatitis: A Prospective Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Outcomes and Measures
2.2.1. Clinical Assessment
2.2.2. Skin Barrier Function Parameters
2.2.3. Other Variables
2.3. Statistical Analysis
2.4. Ethics
3. Results
3.1. Baseline Demographic and Clinical Characteristics
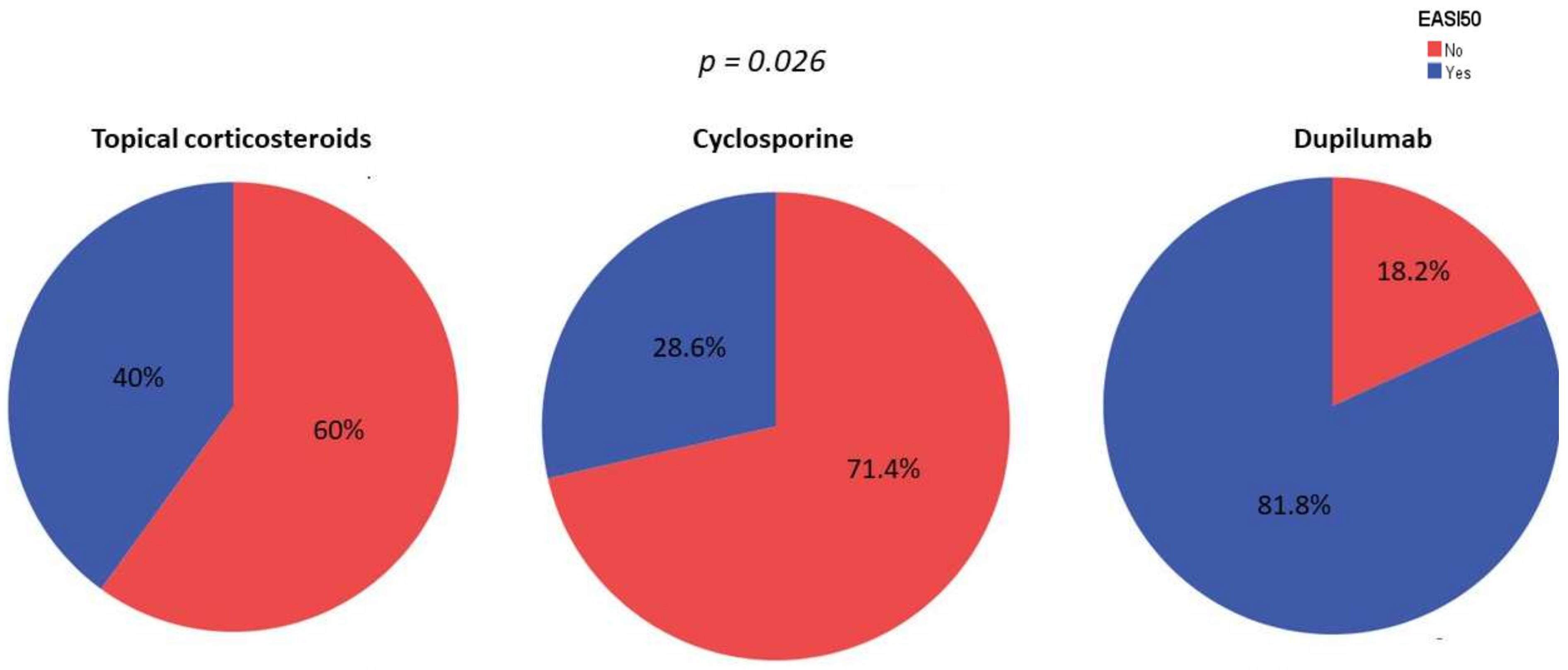
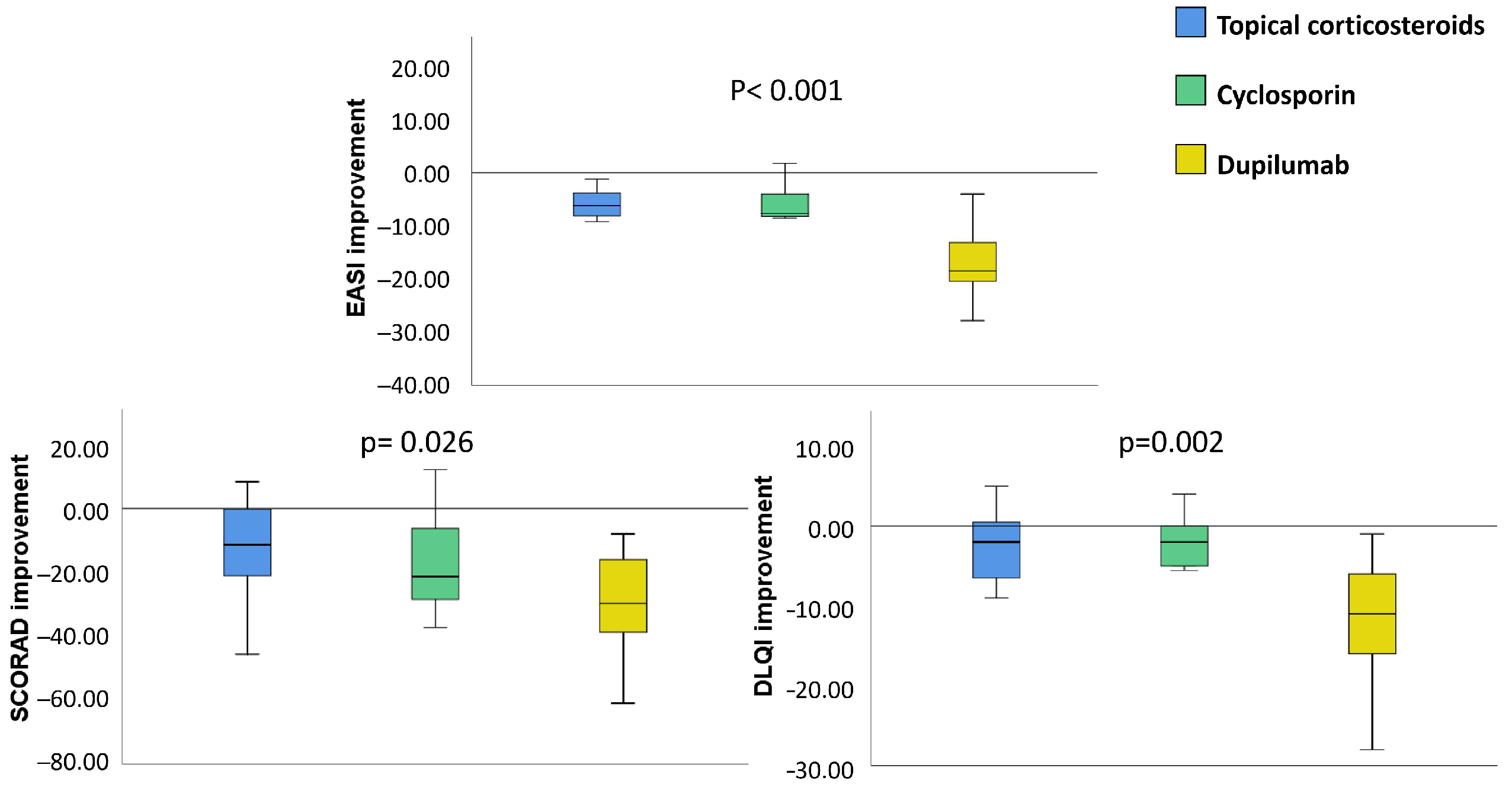
3.2. Clinical Improvement
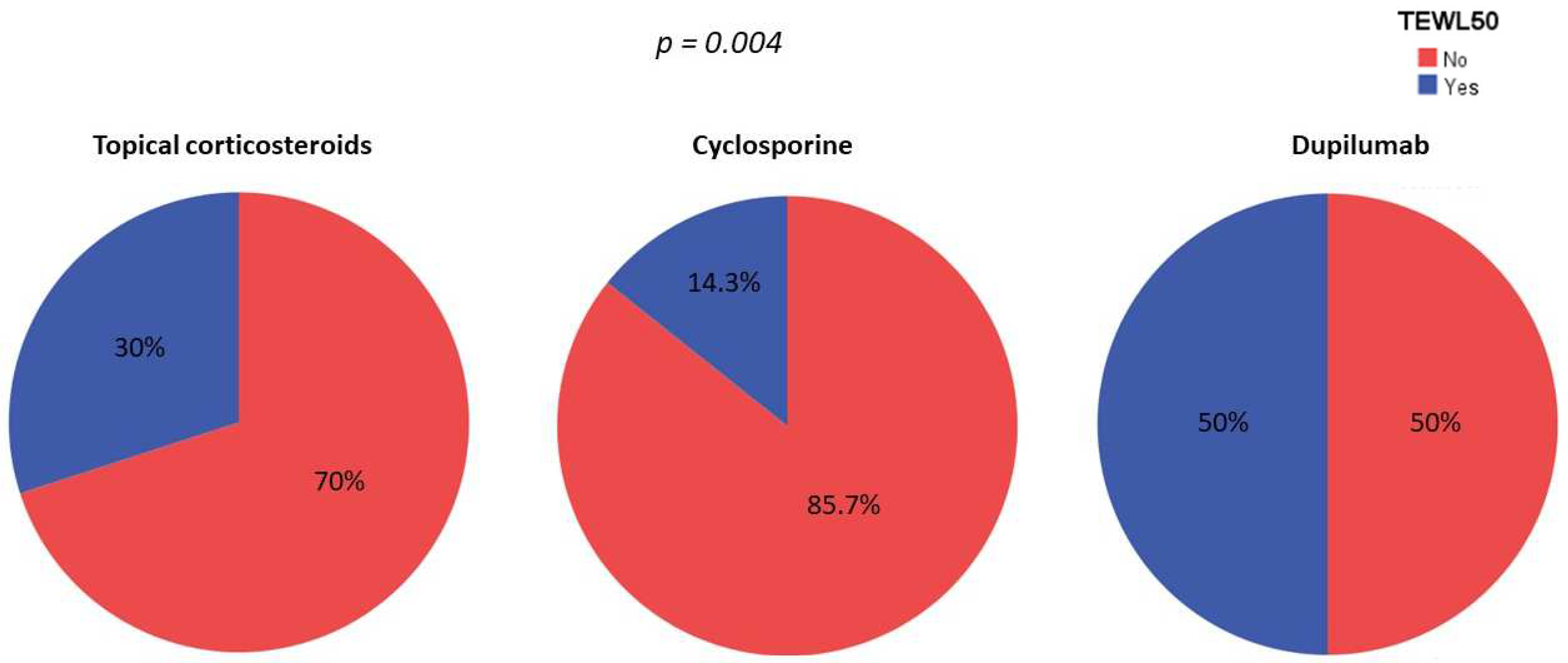
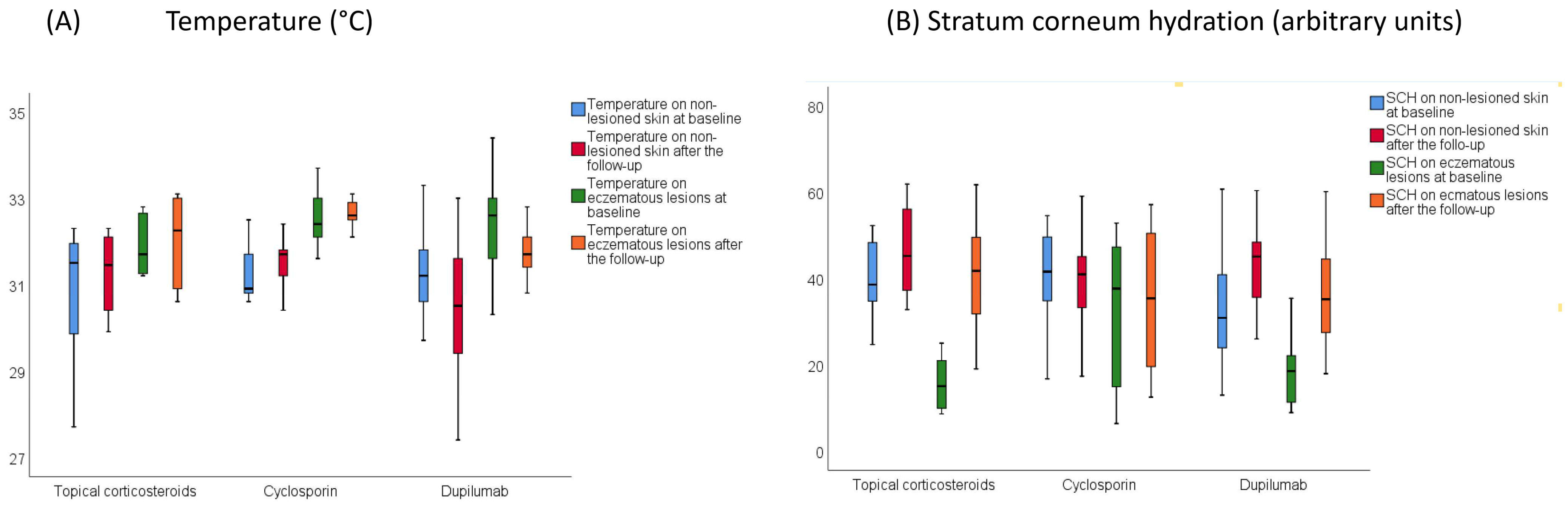
3.3. Skin Barrier Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wollenberg, A.; Christen-Zach, S.; Taieb, A.; Paul, C.; Thyssen, J.P.; De Bruin-Weller, M.; Vestergaard, C.; Seneschal, J.; Werfel, T.; Cork, M.J.; et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2717–2744. [Google Scholar] [CrossRef]
- Stander, S. Atopic Dermatitis. N. Engl. J. Med. 2021, 384, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Bylund, S.; Kobyletzki, L.B.; Svalstedt, M.; Svensson, A. Prevalence and Incidence of Atopic Dermatitis: A Systematic Review. Acta Derm. Venereol. 2020, 100, adv00160. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Lavery, A.; Solman, L.; Grindlay, D.J.C.; Rogers, N.K.; Thomas, K.S.; Harman, K.E. What’s new in atopic eczema? An analysis of systematic reviews published in 2016. Part 2: Epidemiology, aetiology and risk factors. Clin. Exp. Dermatol. 2019, 44, 370–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esparza-Gordillo, J.; Dekio, I. Atopic Dermatitis—Disease Etiology and Clinical Management [Internet]; IntechOpen: London, UK, 2012; 416p, Available online: https://www.intechopen.com/books/941 (accessed on 7 June 2022). [CrossRef]
- Ali, F.; Vyas, J.; Finlay, A.Y. Counting the Burden: Atopic Dermatitis and Health-related Quality of Life. Acta Derm. Venereol. 2020, 100, adv00161. [Google Scholar] [CrossRef]
- Luger, T.; Amagai, M.; Dreno, B.; Dagnelie, M.A.; Liao, W.; Kabashima, K.; Schikowski, T.; Proksch, E.; Elias, P.M.; Simon, M.; et al. Atopic dermatitis: Role of the skin barrier, environment, microbiome, and therapeutic agents. J. Dermatol. Sci. 2021, 102, 142–157. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.E.; Leung, D.Y.M. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc. 2019, 40, 84–92. [Google Scholar] [CrossRef]
- Kelleher, M.; Dunn-Galvin, A.; Hourihane, J.O.; Murray, D.; Campbell, L.E.; McLean, W.H.I.; Irvine, A.D. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J. Allergy Clin. Immunol. 2015, 135, 930–935.e1. [Google Scholar] [CrossRef] [Green Version]
- David Boothe, W.; Tarbox, J.A.; Tarbox, M.B. Atopic Dermatitis: Pathophysiology. Adv. Exp. Med. Biol. 2017, 1027, 21–37. [Google Scholar] [CrossRef]
- Tsakok, T.; Woolf, R.; Smith, C.H.; Weidinger, S.; Flohr, C. Atopic dermatitis: The skin barrier and beyond. Br. J. Dermatol. 2019, 180, 464–474. [Google Scholar] [CrossRef]
- Flohr, C.; Perkin, M.; Logan, K.; Marrs, T.; Radulovic, S.; Campbell, L.E.; MacCallum, S.F.; McLean, W.H.I.; Lack, G. Atopic dermatitis and disease severity are the main risk factors for food sensitization in exclusively breastfed infants. J. Investig. Dermatol. 2014, 134, 345–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montero-Vilchez, T.; Segura-Fernandez-Nogueras, M.V.; Perez-Rodriguez, I.; Soler-Gongora, M.; Martinez-Lopez, A.; Fernandez-Gonzalez, A.; Molina-Leyva, A.; Arias-Santiago, S. Skin Barrier Function in Psoriasis and Atopic Dermatitis: Transepidermal Water Loss and Temperature as Useful Tools to Assess Disease Severity. J. Clin. Med. 2021, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Chopra, R.; Silverberg, J.I. Assessing the severity of atopic dermatitis in clinical trials and practice. Clin. Dermatol. 2018, 36, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Severity scoring of atopic dermatitis: The SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 1993, 186, 23–31. [CrossRef] [PubMed]
- Zhao, C.Y.; Wijayanti, A.; Doria, M.C.; Harris, A.G.; Jain, S.V.; Legaspi, K.N.; Dlova, N.C.; Law, M.G.; Murrell, D.F. The reliability and validity of outcome measures for atopic dermatitis in patients with pigmented skin: A grey area. Int. J. Womens Dermatol. 2015, 1, 150–154. [Google Scholar] [CrossRef] [Green Version]
- Hon, K.L.; Kung, J.S.C.; Ng, W.G.; Tsang, K.Y.C.; Cheng, N.; Leung, T.F. Are skin equipment for assessing childhood eczema any good? J. Dermatol. Treat. 2021, 32, 45–48. [Google Scholar] [CrossRef]
- Chopra, R.; Vakharia, P.P.; Sacotte, R.; Patel, N.; Immaneni, S.; White, T.; Kantor, R.; Hsu, D.Y.; Silverberg, J.I. Relationship between EASI and SCORAD severity assessments for atopic dermatitis. J. Allergy Clin. Immunol. 2017, 140, 1708–1710.e1. [Google Scholar] [CrossRef] [Green Version]
- Halling, A.S.; Loft, N.; Silverberg, J.I.; Guttman-Yassky, E.; Thyssen, J.P. Real-world evidence of dupilumab efficacy and risk of adverse events: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2021, 84, 139–147. [Google Scholar] [CrossRef]
- Pereyra-Rodriguez, J.J.; Alcantara-Luna, S.; Dominguez-Cruz, J.; Galan-Gutierrez, M.; Ruiz-Villaverde, R.; Vilar-Palomo, S.; Armario-Hita, J.C. Short-Term Effectiveness and Safety of Biologics and Small Molecule Drugs for Moderate to Severe Atopic Dermatitis: A Systematic Review and Network Meta-Analysis. Life 2021, 11, 927. [Google Scholar] [CrossRef]
- Blauvelt, A.; Teixeira, H.D.; Simpson, E.L.; Costanzo, A.; De Bruin-Weller, M.; Barbarot, S.; Prajapati, V.H.; Lio, P.; Hu, X.; Wu, T.; et al. Efficacy and Safety of Upadacitinib vs. Dupilumab in Adults With Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2021, 157, 1047–1055. [Google Scholar] [CrossRef]
- Nakahara, T.; Izuhara, K.; Onozuka, D.; Nunomura, S.; Tamagawa-Mineoka, R.; Masuda, K.; Ichiyama, S.; Saeki, H.; Kabata, Y.; Abe, R.; et al. Exploration of biomarkers to predict clinical improvement of atopic dermatitis in patients treated with dupilumab: A study protocol. Medicine 2020, 99, e22043. [Google Scholar] [CrossRef] [PubMed]
- Breiteneder, H.; Peng, Y.Q.; Agache, I.; Diamant, Z.; Eiwegger, T.; Fokkens, W.J.; Traidl-Hoffmann, C.; Nadeau, K.; O’Hehir, R.E.; O’Mahony, L.; et al. Biomarkers for diagnosis and prediction of therapy responses in allergic diseases and asthma. Allergy 2020, 75, 3039–3068. [Google Scholar] [CrossRef] [PubMed]
- Alexander, H.; Brown, S.; Danby, S.; Flohr, C. Research Techniques Made Simple: Transepidermal Water Loss Measurement as a Research Tool. J. Investig. Dermatol. 2018, 138, 2295–2300.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinosa-Rueda, M.I.; Montero-Vilchez, T.; Martinez-Lopez, A.; Molina-Leyva, A.; Sierra-Sanchez, A.; Arias-Santiago, S.; Buendia-Eisman, A. Cutaneous homeostasis and epidermal barrier function in a young healthy Caucasian population. Eur. J. Dermatol. 2021, 31, 176–182. [Google Scholar] [CrossRef]
- Rudzki, E.; Samochocki, Z.; Rebandel, P.; Saciuk, E.; Galecki, W.; Raczka, A.; Szmurlo, A. Frequency and significance of the major and minor features of Hanifin and Rajka among patients with atopic dermatitis. Dermatology 1994, 189, 41–46. [Google Scholar] [CrossRef]
- Oranje, A.P.; Glazenburg, E.J.; Wolkerstorfer, A.; De Waard-van der Spek, F.B. Practical issues on interpretation of scoring atopic dermatitis: The SCORAD index, objective SCORAD and the three-item severity score. Br. J. Dermatol. 2007, 157, 645–648. [Google Scholar] [CrossRef]
- Schmitt, J.; Spuls, P.I.; Thomas, K.S.; Simpson, E.; Furue, M.; Deckert, S.; Dohil, M.; Apfelbacher, C.; Singh, J.A.; Chalmers, J.; et al. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J. Allergy Clin. Immunol. 2014, 134, 800–807. [Google Scholar] [CrossRef]
- Hanifin, J.M.; Thurston, M.; Omoto, M.; Cherill, R.; Tofte, S.J.; Graeber, M. The eczema area and severity index (EASI): Assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp. Dermatol. 2001, 10, 11–18. [Google Scholar] [CrossRef]
- Finlay, A.Y.; Khan, G.K. Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J.; Chen, R.; Wang, Y.; Ding, Y.; Xu, S.; Zou, Y.; Yi, X.; Shi, Y. The efficacy and safety of dupilumab for the treatment of atopic dermatitis among Chinese patients in clinical practice: A single-center retrospective study. Dermatol. Ther. 2022, 35, e15385. [Google Scholar] [CrossRef]
- Jahn, S.; Fohr, J.; Diamanti, E.; Herbst, M. Treatment of atopic dermatitis with dupilumab: A retrospective cohort analysis from dermatological practice. Hautarzt 2021, 72, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, M.E.; Nahm, D.H. Real Clinical Practice Data of Monthly Dupilumab Therapy in Adult Patients With Moderate-to-Severe Atopic Dermatitis: Clinical Efficacy and Predictive Markers for a Favorable Clinical Response. Allergy Asthma Immunol. Res. 2021, 13, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Thyssen, J.P.; Fahrbach, K.; Mickle, K.; Cappelleri, J.C.; Romero, W.; Cameron, M.C.; Myers, D.E.; Clibborn, C.; DiBonaventura, M. Comparative efficacy and safety of systemic therapies used in moderate-to-severe atopic dermatitis: A systematic literature review and network meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1797–1810. [Google Scholar] [CrossRef]
- Nettis, E.; Ferrucci, S.M.; Ortoncelli, M.; Pellacani, G.; Foti, C.; Di Leo, E.; Patruno, C.; Rongioletti, F.; Argenziano, G.; Macchia, L.; et al. Use of Dupilumab for 543 Adult Patients with Moderate-To-Severe Atopic Dermatitis: A Multicenter, Retrospective Study. J. Investig. Allergol. Clin. Immunol. 2022, 32, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Siegels, D.; Heratizadeh, A.; Abraham, S.; Binnmyr, J.; Brockow, K.; Irvine, A.D.; Halken, S.; Mortz, C.G.; Flohr, C.; Schmid-Grendelmeier, P.; et al. Systemic treatments in the management of atopic dermatitis: A systematic review and meta-analysis. Allergy 2021, 76, 1053–1076. [Google Scholar] [CrossRef] [PubMed]
- Drucker, A.M.; Ellis, A.G.; Bohdanowicz, M.; Mashayekhi, S.; Yiu, Z.Z.N.; Rochwerg, B.; Di Giorgio, S.; Arents, B.W.M.; Burton, T.; Spuls, P.I.; et al. Systemic Immunomodulatory Treatments for Patients With Atopic Dermatitis: A Systematic Review and Network Meta-analysis. JAMA Dermatol. 2020, 156, 659–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariens, L.F.M.; Gadkari, A.; Van Os-Medendorp, H.; Ayyagari, R.; Terasawa, E.; Kuznik, A.; Chen, Z.; Bego-Le Bagousse, G.; Lu, Y.; Rizova, E.; et al. Dupilumab Versus Cyclosporine for the Treatment of Moderate-to-Severe Atopic Dermatitis in Adults: Indirect Comparison Using the Eczema Area and Severity Index. Acta Derm. Venereol. 2019, 99, 851–857. [Google Scholar] [CrossRef] [Green Version]
- Montero-Vilchez, T.A.-O.; Cuenca-Barrales, C.A.-O.; Rodriguez-Pozo, J.A.; Diaz-Calvillo, P.; Tercedor-Sanchez, J.A.-O.X.; Martinez-Lopez, A.; Molina-Leyva, A.A.-O.; Arias-Santiago, S.A.-O. Epidermal Barrier Function and Skin Homeostasis in Atopic Dermatitis: The Impact of Age. Life 2022, 12, 132. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Kezic, S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 792–799. [Google Scholar] [CrossRef]
- Danby, S.G.; Cork, M.J. pH in Atopic Dermatitis. Curr. Probl. Dermatol. 2018, 54, 95–107. [Google Scholar] [CrossRef]
- Brunner, P.M.; Guttman-Yassky, E.; Leung, D.Y. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J. Allergy Clin. Immunol. 2017, 139, S65–S76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guttman-Yassky, E.; Ungar, B.; Malik, K.; Dickstein, D.; Suprun, M.; Estrada, Y.D.; Xu, H.; Peng, X.; Oliva, M.; Todd, D.; et al. Molecular signatures order the potency of topically applied anti-inflammatory drugs in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2017, 140, 1032–1042.e13. [Google Scholar] [CrossRef] [Green Version]
- Guttman-Yassky, E.; Bissonnette, R.; Ungar, B.; Suarez-Farinas, M.; Ardeleanu, M.; Esaki, H.; Suprun, M.; Estrada, Y.; Xu, H.; Peng, X.; et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin. Immunol. 2019, 143, 155–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manfredini, M.; Liberati, S.; Ciardo, S.; Bonzano, L.; Guanti, M.; Chester, J.; Kaleci, S.; Pellacani, G. Microscopic and functional changes observed with dynamic optical coherence tomography for severe refractory atopic dermatitis treated with dupilumab. Skin Res. Technol. 2020, 26, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Cristaudo, A.; Pigliacelli, F.; Sperati, F.; Orsini, D.; Cameli, N.; Morrone, A.; Mariano, M. Instrumental evaluation of skin barrier function and clinical outcomes during dupilumab treatment for atopic dermatitis: An observational study. Skin Res. Technol. 2021, 27, 810–813. [Google Scholar] [CrossRef]
- Furuhashi, T.; Oda, T.; Torii, K.; Nishida, E.; Morita, A. Dupilumab probably reduces transepidermal water loss but does not increase stratum corneum hydration in atopic dermatitis. J. Dermatol. 2021, 48, e74–e75. [Google Scholar] [CrossRef]
- Berdyshev, E.; Goleva, E.; Bissonnette, R.; Bronova, I.; Bronoff, A.S.; Richers, B.; Garcia, S.; Gama, M.R.; Taylor, P.; Bologna, G.; et al. Dupilumab treatment significantly improves skin barrier function in adult and adolescent patients with moderate to severe atopic dermatitis. J. Allergy Clin. Immunol. 2022, 149, AB10. [Google Scholar] [CrossRef]
- Ferrucci, S.; Romagnuolo, M.; Maronese, C.A.; Germiniasi, F.; Tavecchio, S.; Angileri, L.; Casazza, G.; Marzano, A.V.; Genovese, G. Skin barrier status during dupilumab treatment in patients with severe atopic dermatitis. Ther. Adv. Chronic Dis. 2021, 12, 20406223211058332. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, S.E.; Shin, K.O.; Park, K.; Lee, S.E. Dupilumab Therapy Improves Stratum Corneum Hydration and Skin Dysbiosis in Patients With Atopic Dermatitis. Allergy Asthma Immunol. Res. 2021, 13, 762–775. [Google Scholar] [CrossRef]
- McAleer, M.A.; Jakasa, I.; Stefanovic, N.; McLean, W.H.I.; Kezic, S.; Irvine, A.D. Topical corticosteroids normalize both skin and systemic inflammatory markers in infant atopic dermatitis. Br. J. Dermatol. 2021, 185, 153–163. [Google Scholar] [CrossRef]
| Topical Corticosteroids (n = 10) | Cyclosporin (n = 14) | Dupilumab (n = 22) | p * | |
|---|---|---|---|---|
| Age (years) | 19.90 (3.76) | 35.64 (22.42) | 28.95 (10.83) | 0.042 * |
| Female sex, % (n) | 80% (8) | 71.4% (10) | 68.2% (15) | 0.789 |
| Marital status | ||||
| - Single | 100% (10) | 64.3% (9) | 81.8% (18) | 0.036 * |
| - Married | 0 | 35.7% (5) | 4.5% (1) | |
| - Divorced | 0 | 0 | 13.6% (3) | |
| Mandatory educational level (yes) | 80% (8) | 71.4% (10) | 88.9% (16) | 0.457 |
| Smoking habit (yes) | 30% (3) | 28.57% (4) | 9.1% (2) | 0.202 |
| Alcohol consumption (yes) | 20% (2) | 42.86% (6) | 27.3% (6) | 0.350 |
| BMI (kg/m2) | 20.55 (0.97) | 22.66 (1.71) | 22.02 (3.78) | 0.566 |
| Frecuecy of emollient use (times per week) | 5.12 (1.72) | 5.25 (3.50) | 6.33 (1.19) | 0.378 |
| Skin hydration > 4 times/week (yes) | 70% (7) | 92.9% (13) | 72.72% (16) | 0.253 |
| AD family history (yes) | 40% (4) | 71.43% (10) | 68.18% (15) | 0.111 |
| Signs of atopic march (yes) | 60% (6) | 64.29% (9) | 68.18% (15) | 0.835 |
| Disease evolution (years) | 12.20 (7.68) | 8.38 (7.45) | 20.67 (12.39) | 0.004 * |
| EASI | 11.72 (3.91) | 15.04 (4.90) | 24.60 (5.36) | <0.001 * |
| SCORAD | 37.28 (12.11) | 47.41 (9.43) | 57.30 (13.83) | <0.001 * |
| DLQI | 12.20 (6.20) | 9.29 (4.05) | 17.59 (7.28) | 0.001 |
| BSA | 14.23 (5.31) | 22.63 (7.96) | 39.54 (18.47) | <0.001 |
| IGA | 2.5 (0.53) | 3.21 (0.58) | 3.73 (0.46) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montero-Vilchez, T.; Rodriguez-Pozo, J.-A.; Diaz-Calvillo, P.; Salazar-Nievas, M.; Tercedor-Sanchez, J.; Molina-Leyva, A.; Arias-Santiago, S. Dupilumab Improves Skin Barrier Function in Adults with Atopic Dermatitis: A Prospective Observational Study. J. Clin. Med. 2022, 11, 3341. https://doi.org/10.3390/jcm11123341
Montero-Vilchez T, Rodriguez-Pozo J-A, Diaz-Calvillo P, Salazar-Nievas M, Tercedor-Sanchez J, Molina-Leyva A, Arias-Santiago S. Dupilumab Improves Skin Barrier Function in Adults with Atopic Dermatitis: A Prospective Observational Study. Journal of Clinical Medicine. 2022; 11(12):3341. https://doi.org/10.3390/jcm11123341
Chicago/Turabian StyleMontero-Vilchez, Trinidad, Juan-Angel Rodriguez-Pozo, Pablo Diaz-Calvillo, Maria Salazar-Nievas, Jesús Tercedor-Sanchez, Alejandro Molina-Leyva, and Salvador Arias-Santiago. 2022. "Dupilumab Improves Skin Barrier Function in Adults with Atopic Dermatitis: A Prospective Observational Study" Journal of Clinical Medicine 11, no. 12: 3341. https://doi.org/10.3390/jcm11123341
APA StyleMontero-Vilchez, T., Rodriguez-Pozo, J.-A., Diaz-Calvillo, P., Salazar-Nievas, M., Tercedor-Sanchez, J., Molina-Leyva, A., & Arias-Santiago, S. (2022). Dupilumab Improves Skin Barrier Function in Adults with Atopic Dermatitis: A Prospective Observational Study. Journal of Clinical Medicine, 11(12), 3341. https://doi.org/10.3390/jcm11123341









