Differential Expression of Tissular miRNA-155 in Pediatric Gastritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Histopathological Analysis and Division of the Study Groups
2.3. Assessment of miR-155 Expression
2.4. Statistical Analysis
2.5. Ethics
3. Results
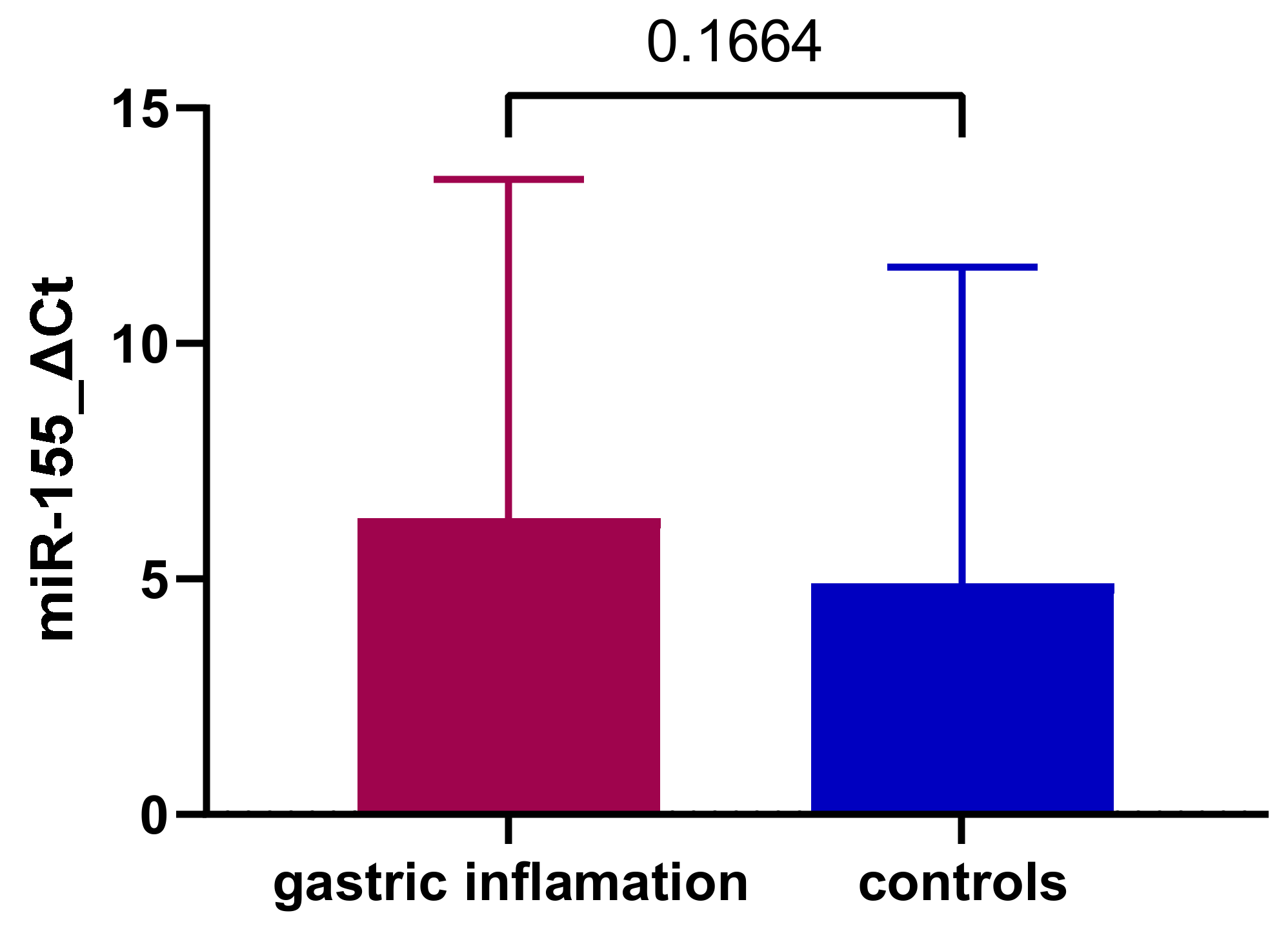
3.1. Expression of miR-155 in Gastric Inflammation
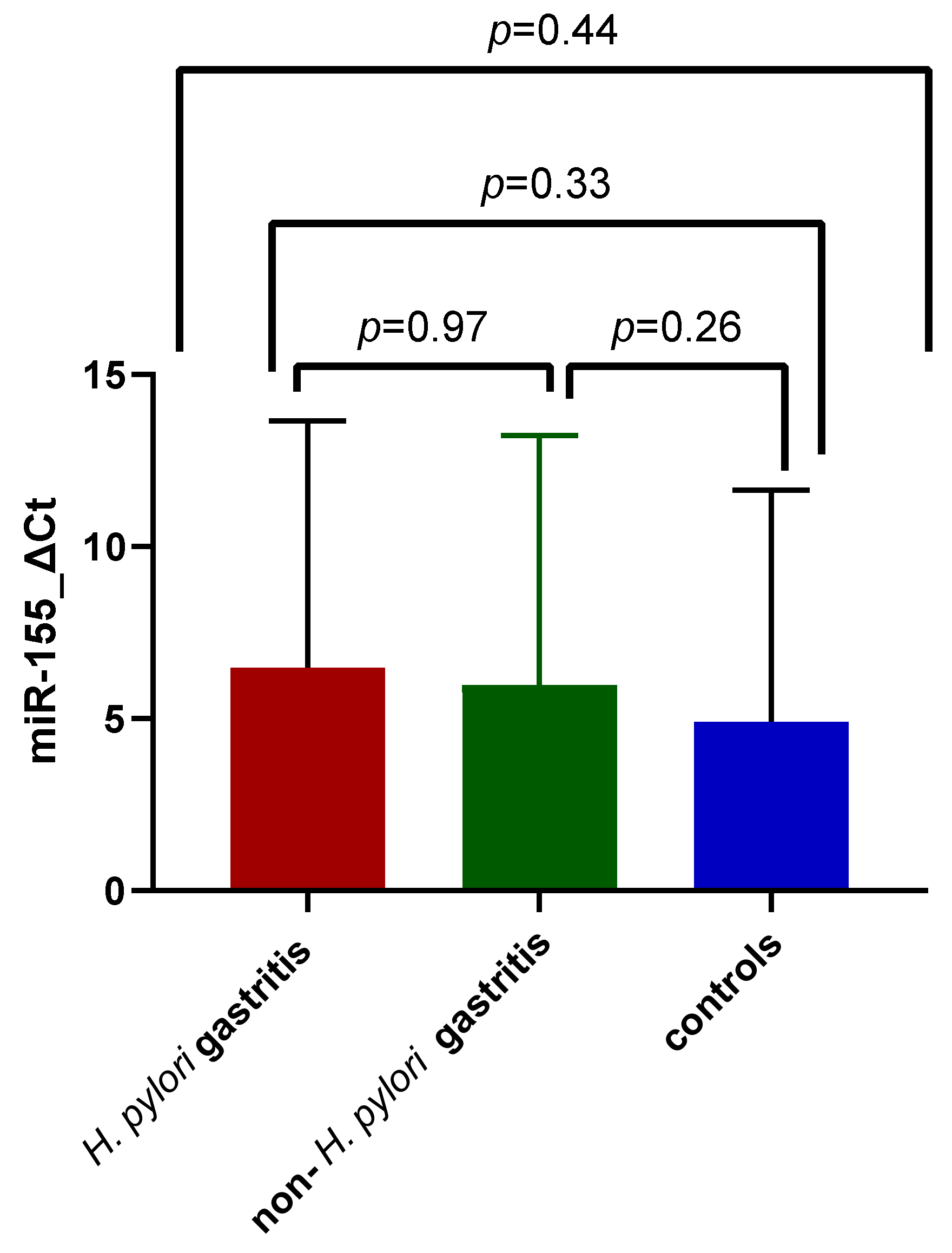
3.2. Expression of miR-155 in Gastric Inflammation Associated with H. pylori Infection
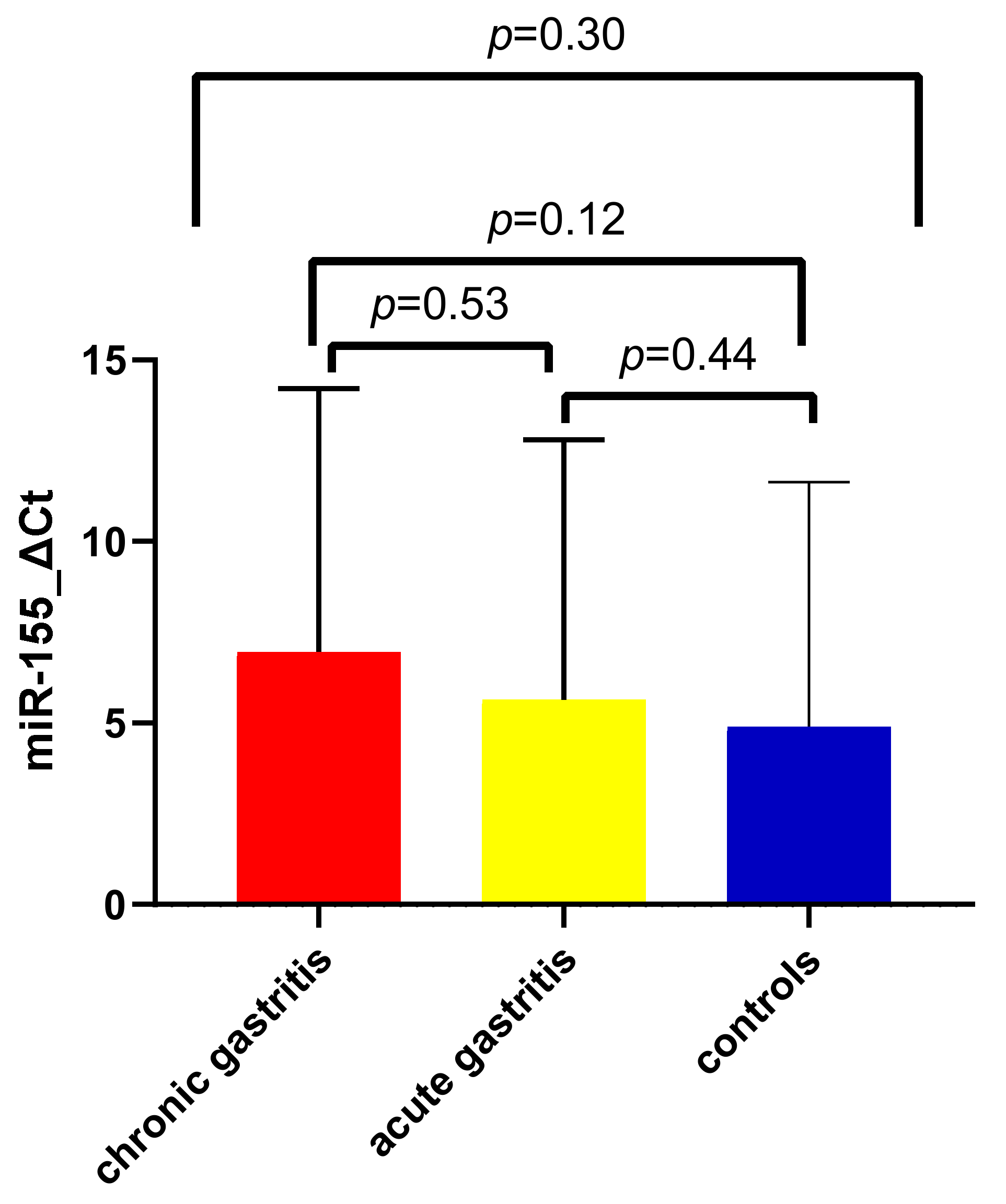
3.3. Expression of miR-155 in Acute and Chronic Gastric Inflammation
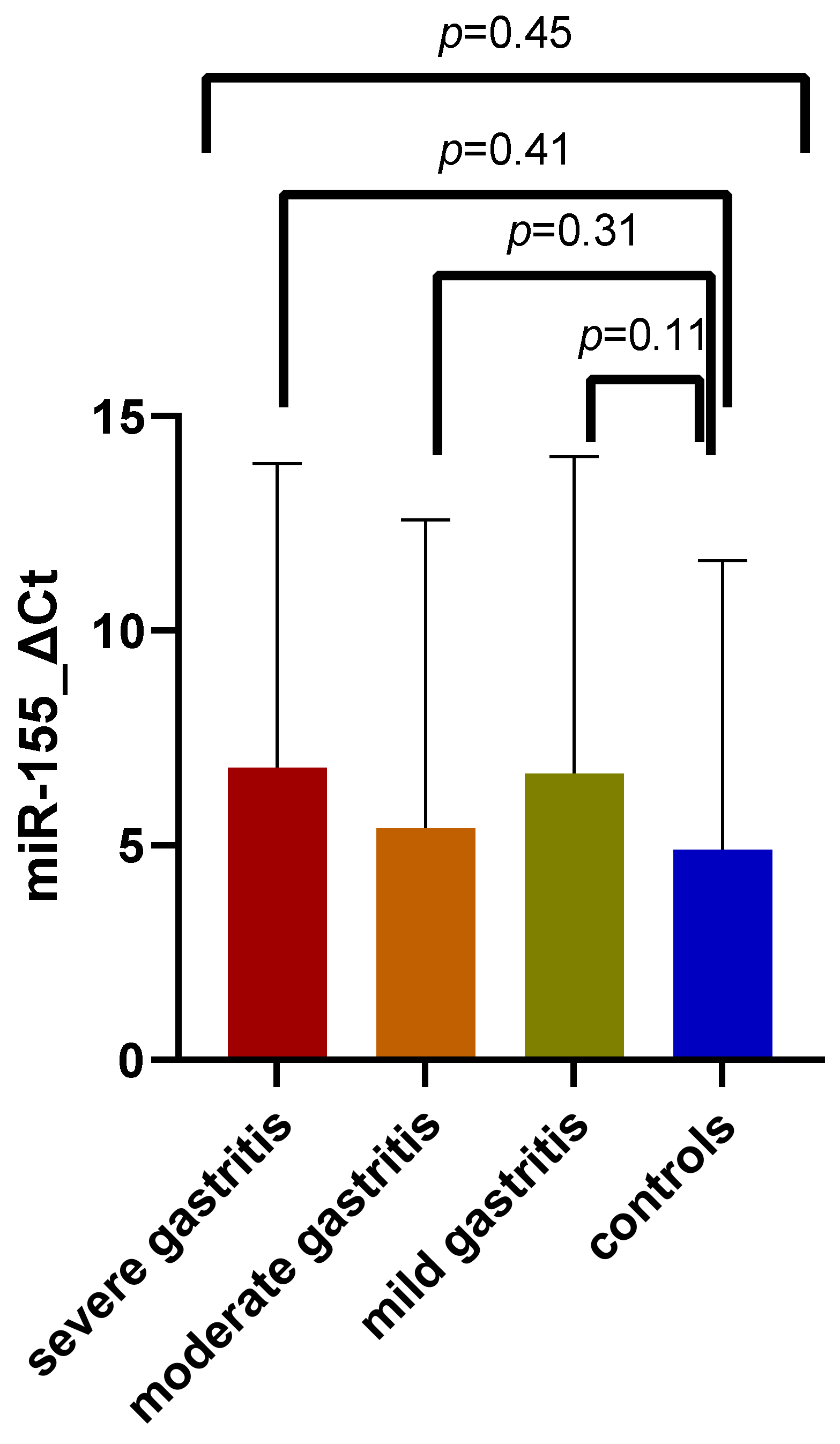
3.4. Expression of miR-155 in Association with Gastritis Severity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sierra, D.; Wood, M.; Kolli, S.; Felipez, L.M. Pediatric Gastritis, Gastropathy, and Peptic Ulcer Disease. Pediatr. Rev. 2018, 39, 542–549. [Google Scholar] [CrossRef] [PubMed]
- De Korwin, J.-D. Helicobacter pylori: When to look for an infection and treat it in adults? Rev. Med. Interne 2021, 42, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Hojo, M.; Nagahara, A.; Kudo, T.; Takeda, T.; Ikuse, T.; Matsumoto, K.; Ueda, K.; Ueyama, H.; Matsumoto, K.; Asaoka, D.; et al. Endoscopic Findings of Helicobacter Pylori Gastritis in Children and Young Adults Based on the Kyoto Classification of Gastritis and Age-Associated Changes. JGH Open 2021, 5, 1197–1202. [Google Scholar] [CrossRef]
- Toh, J.W.T.; Wilson, R.B. Pathways of Gastric Carcinogenesis, Helicobacter Pylori Virulence and Interactions with Antioxidant Systems, Vitamin C and Phytochemicals. Int. J. Mol. Sci. 2020, 21, 6451. [Google Scholar] [CrossRef] [PubMed]
- Herrera, V.; Parsonnet, J. Helicobacter Pylori and Gastric Adenocarcinoma. Clin. Microbiol. Infect. 2009, 15, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef]
- Baek, D.; Villén, J.; Shin, C.; Camargo, F.D.; Gygi, S.P.; Bartel, D.P. The Impact of MicroRNAs on Protein Output. Nature 2008, 455, 64–71. [Google Scholar] [CrossRef]
- Taganov, K.D.; Boldin, M.P.; Baltimore, D. MicroRNAs and Immunity: Tiny Players in a Big Field. Immunity 2007, 26, 133–137. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The Role of MicroRNAs in Human Cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Acunzo, M.; Romano, G.; Wernicke, D.; Croce, C.M. MicroRNA and Cancer—A Brief Overview. Adv. Biol. Regul. 2015, 57, 1–9. [Google Scholar] [CrossRef]
- Banzhaf-Strathmann, J.; Edbauer, D. Good Guy or Bad Guy: The Opposing Roles of MicroRNA 125b in Cancer. Cell Commun. Signal. 2014, 12, 30. [Google Scholar] [CrossRef]
- Săsăran, M.O.; Meliț, L.E.; Dobru, E.D. MicroRNA Modulation of Host Immune Response and Inflammation Triggered by Helicobacter Pylori. Int. J. Mol. Sci. 2021, 22, 1406. [Google Scholar] [CrossRef]
- Zabaleta, J. MicroRNA: A Bridge from H. Pylori Infection to Gastritis and Gastric Cancer Development. Front. Genet. 2012, 3, 294. [Google Scholar] [CrossRef]
- Huang, T.; Wang-Johanning, F.; Zhou, F.; Kallon, H.; Wei, Y. MicroRNAs Serve as a Bridge between Oxidative Stress and Gastric Cancer (Review). Int. J. Oncol. 2016, 49, 1791–1800. [Google Scholar] [CrossRef][Green Version]
- Elton, T.S.; Selemon, H.; Elton, S.M.; Parinandi, N.L. Regulation of the MIR155 Host Gene in Physiological and Pathological Processes. Gene 2013, 532, 1–12. [Google Scholar] [CrossRef]
- Rodriguez, A.; Vigorito, E.; Clare, S.; Warren, M.V.; Couttet, P.; Soond, D.R.; Van Dongen, S.; Grocock, R.J.; Das, P.P.; Miska, E.A.; et al. Requirement of Bic/MicroRNA-155 for Normal Immune Function. Science 2007, 316, 608–611. [Google Scholar] [CrossRef]
- Thai, T.-H.; Calado, D.P.; Casola, S.; Ansel, K.M.; Xiao, C.; Xue, Y.; Murphy, A.; Frendewey, D.; Valenzuela, D.; Kutok, J.L.; et al. Regulation of the Germinal Center Response by MicroRNA-155. Science 2007, 316, 604–608. [Google Scholar] [CrossRef]
- Prinz, C.; Weber, D. MicroRNA (MiR) Dysregulation during Helicobacter Pylori-Induced Gastric Inflammation and Cancer Development: Critical Importance of MiR-155. Oncotarget 2020, 11, 894–904. [Google Scholar] [CrossRef]
- Xiao, B.; Liu, Z.; Li, B.-S.; Tang, B.; Li, W.; Guo, G.; Shi, Y.; Wang, F.; Wu, Y.; Tong, W.-D.; et al. Induction of MicroRNA-155 during Helicobacter Pylori Infection and Its Negative Regulatory Role in the Inflammatory Response. J. Infect. Dis. 2009, 200, 916–925. [Google Scholar] [CrossRef]
- Matsushima, K.; Isomoto, H.; Inoue, N.; Nakayama, T.; Hayashi, T.; Nakayama, M.; Nakao, K.; Hirayama, T.; Kohno, S. MicroRNA Signatures in Helicobacter Pylori-Infected Gastric Mucosa. Int. J. Cancer 2011, 128, 361–370. [Google Scholar] [CrossRef]
- Chen, D.; Ping, S.; Xu, Y.; Wang, M.; Jiang, X.; Xiong, L.; Zhang, L.; Yu, H.; Xiong, Z. Non-Coding RNAs in Gastric Cancer: From Malignant Hallmarks to Clinical Applications. Front. Cell Dev. Biol. 2021, 9, 732036. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, P.-W.; Yang, W.-Q.; Mi, H.; Pan, J.-L.; Huang, Y.-C.; Hou, Z.-K.; Hou, Q.-K.; Luo, Q.; Liu, F.-B. Identification of Non-Invasive Biomarkers for Chronic Atrophic Gastritis from Serum Exosomal MicroRNAs. BMC Cancer 2019, 19, 129. [Google Scholar] [CrossRef]
- Cortés-Márquez, A.C.; Mendoza-Elizalde, S.; Arenas-Huertero, F.; Trillo-Tinoco, J.; Valencia-Mayoral, P.; Consuelo-Sánchez, A.; Zarate-Franco, J.; Dionicio-Avendaño, A.R.; Herrera-Esquivel, J.D.J.; Recinos-Carrera, E.G.; et al. Differential Expression of MiRNA-146a and MiRNA-155 in Gastritis Induced by Helicobacter Pylori Infection in Paediatric Patients, Adults, and an Animal Model. BMC Infect. Dis. 2018, 18, 463. [Google Scholar] [CrossRef]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Histological Classification of Gastritis and Helicobacter Pylori Infection: An Agreement at Last? The International Workshop on the Histopathology of Gastritis. Helicobacter 1997, 2 (Suppl. S1), S17–S24. [Google Scholar] [CrossRef] [PubMed]
- Hyams, J.S.; Di Lorenzo, C.; Saps, M.; Shulman, R.J.; Staiano, A.; Van Tilburg, M. Childhood functional gastrointestinal disorders: Child/adolescent. Gastroenterology 2016, 150, 1456–1468. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.L.; Koletzko, S.; Goodman, K.; Bontems, P.; Cadranel, S.; Casswall, T.; Czinn, S.; Gold, B.D.; Guarner, J.; Elitsur, Y.; et al. Joint ESPGHAN/NASPGHAN Guidelines for the Management of Helicobacter Pylori in Children and Adolescents (Update 2016). J. Pediatr. Gastroenterol. Nutr. 2017, 64, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Lomba-Viana, R.; Dinis-Ribeiro, M.; Fonseca, F.; Vieira, A.S.; Bento, M.J.B.; Lomba-Viana, H. Serum Pepsinogen Test for Early Detection of Gastric Cancer in a European Country. Eur. J. Gastroenterol. Hepatol. 2012, 24, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Bornschein, J.; Selgrad, M.; Wex, T.; Kuester, D.; Malfertheiner, P. Serological Assessment of Gastric Mucosal Atrophy in Gastric Cancer. BMC Gastroenterol. 2012, 12, 10. [Google Scholar] [CrossRef]
- Syrjänen, K. Accuracy of Serum Biomarker Panel (GastroPanel®) in the Diagnosis of Atrophic Gastritis of the Corpus. Systematic Review and Meta-Analysis. Anticancer Res. 2022, 42, 1679–1696. [Google Scholar] [CrossRef]
- Li, X.; Zhu, M.; Zhao, G.; Zhou, A.; Min, L.; Liu, S.; Zhang, N.; Zhu, S.; Guo, Q.; Zhang, S.; et al. MiR-1298-5p Level Downregulation Induced by Helicobacter Pylori Infection Inhibits Autophagy and Promotes Gastric Cancer Development by Targeting MAP2K6. Cell. Signal. 2022, 93, 110286. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Li, J.; Xu, Y. Upregulation of MicroRNA-650 by PBX1 Is Correlated with the Development of Helicobacter Pylori-Associated Gastric Carcinoma. Neoplasma 2021, 68, 262–272. [Google Scholar] [CrossRef]
- Oertli, M.; Engler, D.B.; Kohler, E.; Koch, M.; Meyer, T.F.; Müller, A. MicroRNA-155 Is Essential for the T Cell-Mediated Control of Helicobacter Pylori Infection and for the Induction of Chronic Gastritis and Colitis. J. Immunol. 2011, 187, 3578–3586. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, J. Downregulation of MicroRNA-204 Increases the Expression of Matrix Metallopeptidase 9 in Pediatric Patients with Pulpitis and Helicobacter Pylori Infection in the Stomach. Exp. Ther. Med. 2019, 18, 253–259. [Google Scholar] [CrossRef]
- Feng, J.; Guo, J.; Wang, J.-P.; Chai, B.-F. MiR-32-5p Aggravates Intestinal Epithelial Cell Injury in Pediatric Enteritis Induced by Helicobacter Pylori. World J. Gastroenterol. 2019, 25, 6222–6237. [Google Scholar] [CrossRef]
- Vasapolli, R.; Venerito, M.; Schirrmeister, W.; Thon, C.; Weigt, J.; Wex, T.; Malfertheiner, P.; Link, A. Inflammatory MicroRNAs in Gastric Mucosa Are Modulated by Helicobacter Pylori Infection and Proton-Pump Inhibitors but Not by Aspirin or NSAIDs. PLoS ONE 2021, 16, e0249282. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, S.G.; Choi, J.M.; Yang, H.-J.; Kim, J.S.; Jung, H.C. Helicobacter Pylori Is Associated with MiR-133a Expression through Promoter Methylation in Gastric Carcinogenesis. Gut Liver 2018, 12, 58–66. [Google Scholar] [CrossRef]
- Isomoto, H.; Matsushima, K.; Inoue, N.; Hayashi, T.; Nakayama, T.; Kunizaki, M.; Hidaka, S.; Nakayama, M.; Hisatsune, J.; Nakashima, M.; et al. Interweaving MicroRNAs and Proinflammatory Cytokines in Gastric Mucosa with Reference to H. Pylori Infection. J. Clin. Immunol. 2012, 32, 290–299. [Google Scholar] [CrossRef]
- Petrocca, F.; Visone, R.; Onelli, M.R.; Shah, M.H.; Nicoloso, M.S.; De Martino, I.; Iliopoulos, D.; Pilozzi, E.; Liu, C.-G.; Negrini, M.; et al. E2F1-Regulated MicroRNAs Impair TGFbeta-Dependent Cell-Cycle Arrest and Apoptosis in Gastric Cancer. Cancer Cell 2008, 13, 272–286. [Google Scholar] [CrossRef]
- Chaves, J.R.; De Souza, C.R.T.; Modesto, A.A.C.; Moreira, F.C.; Teixeira, E.B.; Sarraf, J.S.; Allen, T.S.R.; Araújo, T.M.T.; Khayat, A.S. Effects of Alkaline Water Intake on Gastritis and MiRNA Expression (MiR-7, MiR-155, MiR-135b and MiR-29c). Am. J. Transl. Res. 2020, 12, 4043–4050. [Google Scholar] [CrossRef]
- Link, A.; Schirrmeister, W.; Langner, C.; Varbanova, M.; Bornschein, J.; Wex, T.; Malfertheiner, P. Differential Expression of MicroRNAs in Preneoplastic Gastric Mucosa. Sci. Rep. 2015, 5, 8270. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.; Bellosillo, B.; Ferraro, M.; Seoane, A.; Sanchez-Gonzalez, B.; Pairet, S.; Pons, A.; Barranco, L.; Vela, M.C.; Gimeno, E.; et al. MicroRNAs 142-3p, MiR-155 and MiR-203 Are Deregulated in Gastric MALT Lymphomas Compared to Chronic Gastritis. Cancer Genom. Proteom. 2016, 14, 75–82. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, W.-T.; Kuo, S.-H.; Kuo, Y.-C.; Lin, C.-W. MiR-155-Regulated MTOR and Toll-like Receptor 5 in Gastric Diffuse Large B-Cell Lymphoma. Cancer Med. 2022, 11, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Jarry, J.; Schadendorf, D.; Greenwood, C.; Spatz, A.; Van Kempen, L.C. The Validity of Circulating MicroRNAs in Oncology: Five Years of Challenges and Contradictions. Mol. Oncol. 2014, 8, 819–829. [Google Scholar] [CrossRef]



| Parameter | Control Group (n = 93) | Gastric Inflammation (n = 99) | p Value | |
|---|---|---|---|---|
| Age-years (mean ± SD) a | 12.32 ± 3.24 | 12.19 ± 3.85 | p = 0.09 | |
| Sex (%) b | Female | 26.56 | 30.20 | p = 0.6, OR = 1.16 (CI: 0.67–2.03) |
| Male | 21.87 | 21.35 | ||
| Background b | Urban | 29.16 | 17.70 | p < 0.01, OR = 2.89 (CI: 1.57–5.29) |
| Rural | 19.27 | 33.85 | ||
| Parameter | Control Group (n = 93) | H. pylori Gastritis (n = 39) | Non-H. pylori Gastritis (n = 60) | p Value | |
|---|---|---|---|---|---|
| Age-years (mean ± SD) a | 12.32 ± 3.24 | 12.85 ± 3.39 | 11.77 ± 4.10 | p = 0.48 | |
| Sex (%) b | Female | 26.56 | 11.45 | 18.75 | p = 0.81 |
| Male | 21.87 | 8.85 | 12.50 | ||
| Background b | Urban | 29.16 | 3.64 | 14.06 | p < 0.01 |
| Rural | 19.27 | 16.66 | 17.18 | ||
| Parameter | Control Group (n = 93) | Acute Gastritis (n = 50) | Chronic Gastritis (n = 43) | p Value | |
|---|---|---|---|---|---|
| Age-years (mean ± SD) a | 12.32 ± 3.24 | 11.38 ± 4.18 | 13.02 ± 3.32 | p = 0.13 | |
| Sex (%) b | Female | 26.56 | 15.62 | 14.58 | p = 0.83 |
| Male | 21.87 | 10.41 | 10.93 | ||
| Background b | Urban | 29.16 | 12.5 | 5.20 | p = 0.01 |
| Rural | 19.27 | 13.54 | 20.31 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oana, S.M.; Claudia, B.; Lelia, R.A.; Simona, M.; Claudia, C.; Daniela, D.E. Differential Expression of Tissular miRNA-155 in Pediatric Gastritis. J. Clin. Med. 2022, 11, 3351. https://doi.org/10.3390/jcm11123351
Oana SM, Claudia B, Lelia RA, Simona M, Claudia C, Daniela DE. Differential Expression of Tissular miRNA-155 in Pediatric Gastritis. Journal of Clinical Medicine. 2022; 11(12):3351. https://doi.org/10.3390/jcm11123351
Chicago/Turabian StyleOana, Săsăran Maria, Bănescu Claudia, Riza Anca Lelia, Mocan Simona, Cârstea Claudia, and Dobru Ecaterina Daniela. 2022. "Differential Expression of Tissular miRNA-155 in Pediatric Gastritis" Journal of Clinical Medicine 11, no. 12: 3351. https://doi.org/10.3390/jcm11123351
APA StyleOana, S. M., Claudia, B., Lelia, R. A., Simona, M., Claudia, C., & Daniela, D. E. (2022). Differential Expression of Tissular miRNA-155 in Pediatric Gastritis. Journal of Clinical Medicine, 11(12), 3351. https://doi.org/10.3390/jcm11123351






