Calcification in Salivary Gland Cancer Mimicking Sialolithiasis—A Diagnostic Pitfall on Imaging: Report of Two Cases and Brief Review of the Literature
Abstract
:1. Introduction
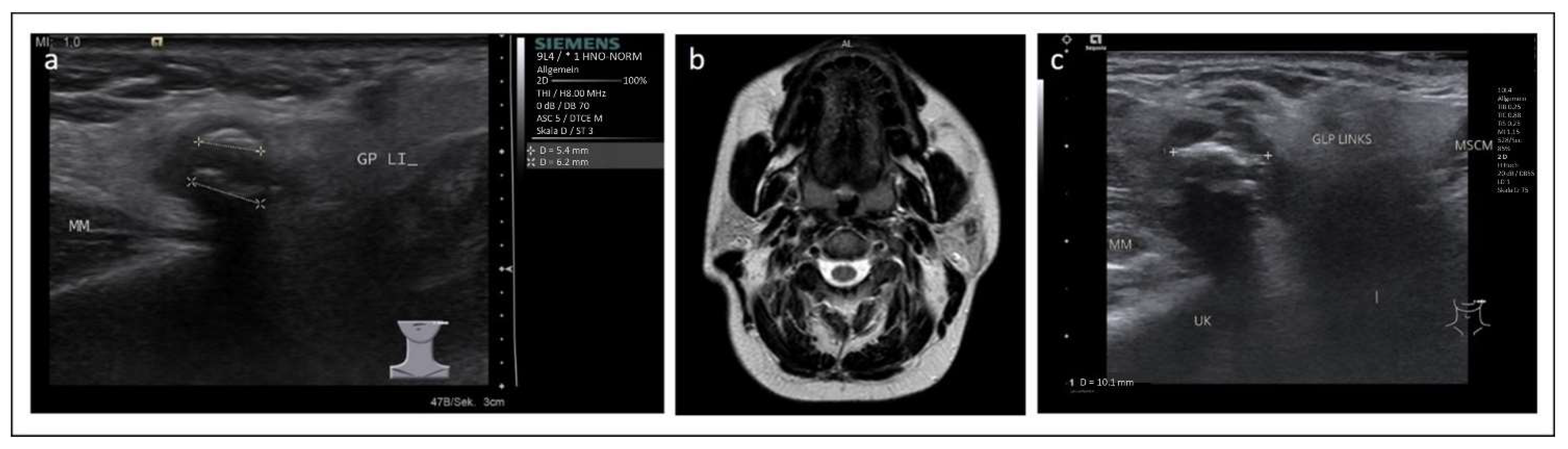
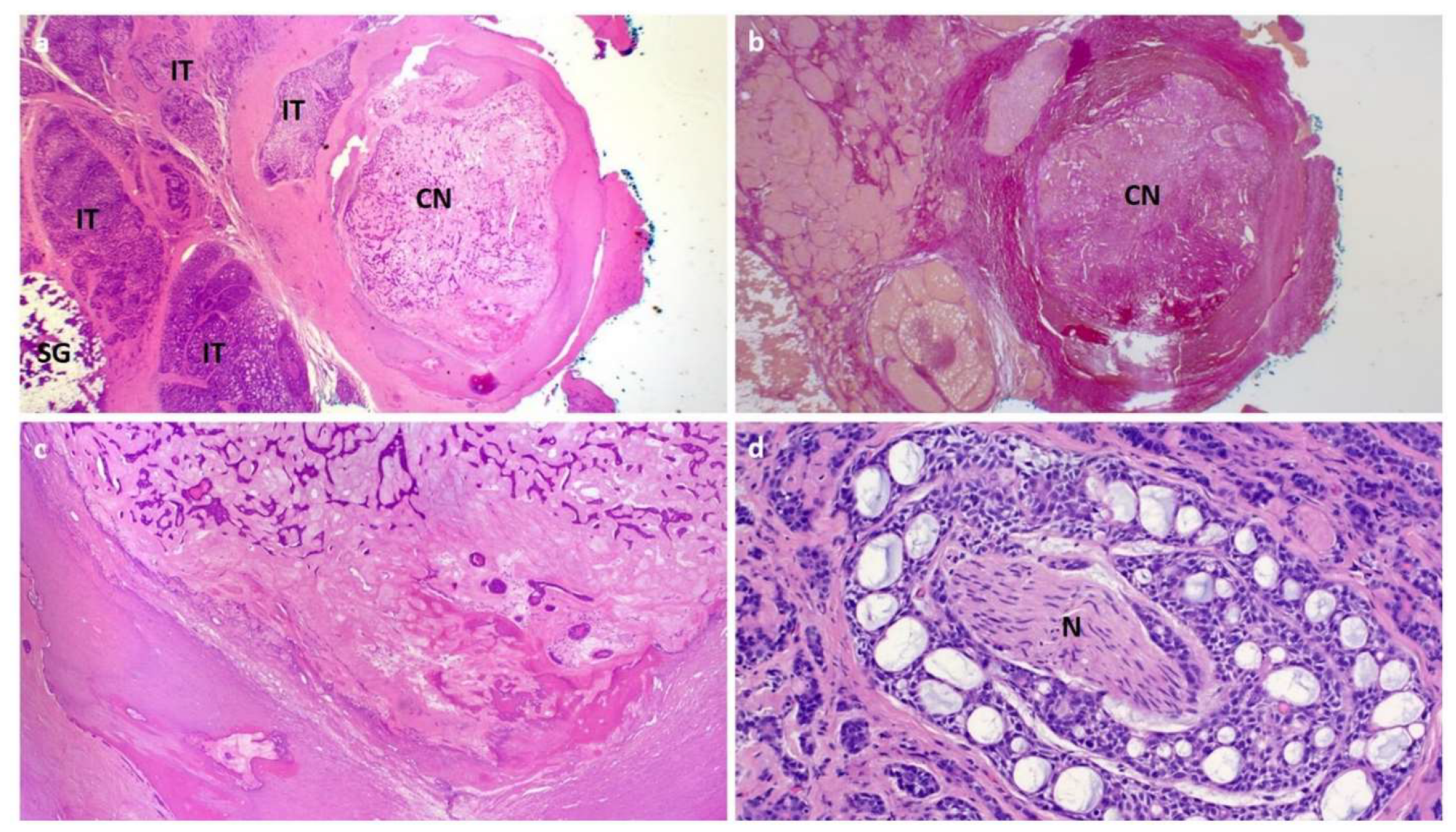
2. Case 1
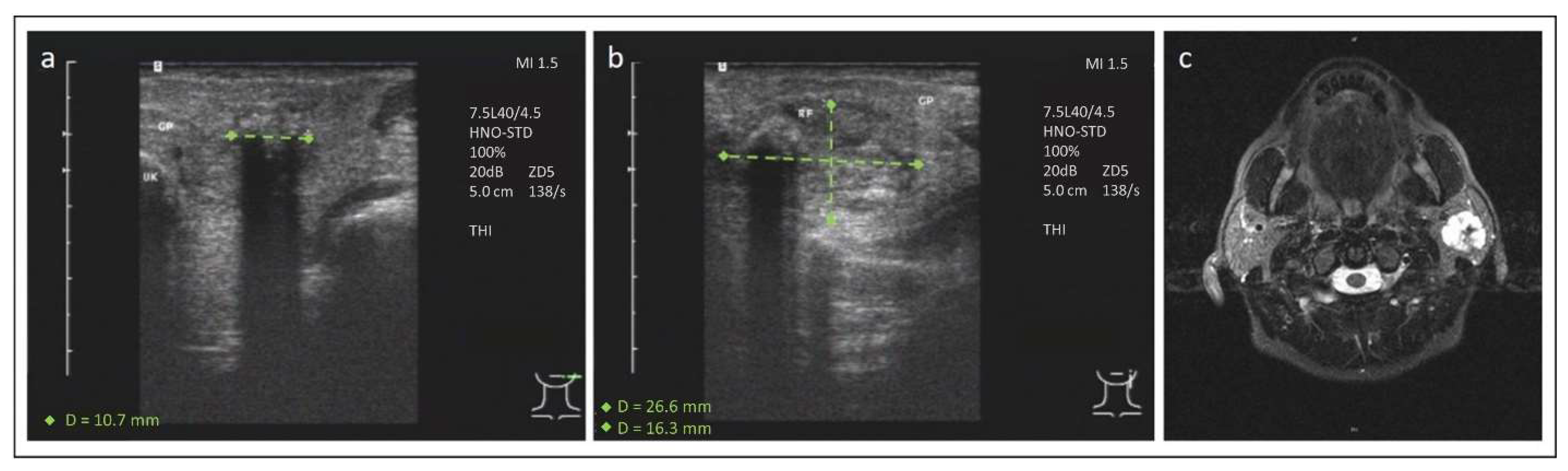
3. Case 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Escudier, M.P.; McGurk, M. Symptomatic sialoadenitis and sialolithiasis in the English population, an estimate of the cost of hospital treatment. Br. Dent. J. 1999, 186, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Sigismund, P.E.; Zenk, J.; Koch, M.; Schapher, M.; Rudes, M.; Iro, H. Nearly 3000 salivary stones: Some clinical and epidemiologic aspects. Laryngoscope 2015, 125, 1879–1882. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, M.; Mantsopoulos, K.; Schapher, M.; Iro, H.; Koch, M. Ultrasound Supplemented by Sialendoscopy: Diagnostic Value in Sialolithiasis. Otolaryngol. Head Neck Surg. 2018, 159, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Marchal, F.; Becker, C.D.; Dulguerov, P.; Georgakopoulos, G.; Lehmann, W.; Terrier, F. Sialolithiasis and salivary ductal stenosis: Diagnostic accuracy of MR sialography with a three-dimensional extended-phase conjugate-symmetry rapid spin-echo sequence. Radiology 2000, 217, 347–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, C.; Judson, B.L.; Prasad, M.L. Vascular malformation with phleboliths involving the parotid gland: A case report with a review of the literature. Ear Nose Throat J. 2015, 94, E1–E5. [Google Scholar] [PubMed]
- Song, H.U.; Kwon, K.J.; Koh, K.J. Adenocarcinoma of the parotid gland with calcification. Dentomaxillofacial Radiol. 2003, 32, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Shocket, E.; Manheimer, L.H.; Benson, J.; Lubin, J. Calcified parotid carcinoma masquerading as benign calculus with acute parotitis. South Med. J. 1982, 75, 1273–1274. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.C.; Chang, C.M.; Wang, C.T.; Cheng, P.W.; Liao, L.J. A Novel Sonographic Scoring Model in the Prediction of Major Salivary Gland Tumors. Laryngoscope 2021, 131, E157–E162. [Google Scholar] [CrossRef] [PubMed]
- House, J.W.; Brackmann, D.E. Facial nerve grading system. Otolaryngol. Head Neck Surg. 1985, 93, 146–147. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Zenk, J.; Iro, H. Algorithms for treatment of salivary gland obstructions. Otolaryngol. Clin. N. Am. 2009, 42, 1173–1192. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Lee, J.C.; Kim, J.H.; Lee, Y.M.; Lee, H.J. Cavernous hemangioma with large phlebolith of the parotid gland. J. Craniofacial Surg. 2013, 24, e621–e623. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, M.; De Luca, P.; Scarpa, A.; Savignano, L.; Cassandro, C.; Iemma, M.; Cassandro, E. Acinic cell carcinoma of the parotid gland: From pathogenesis to management: A literature review. Eur. Arch. Otorhinolaryngol. 2020, 277, 2673–2679. [Google Scholar] [CrossRef] [PubMed]
- Spiro, R.H.; Huvos, A.G.; Strong, E.W. Adenoid cystic carcinoma of salivary origin. A clinicopathologic study of 242 cases. Am. J. Surg. 1974, 128, 512–520. [Google Scholar] [CrossRef]
- Shenoy, V.S.; Kamath, M.P.; Sreedharan, S.; Suhas, S.S. Adenoid cystic carcinoma of the parotid gland associated with salivary calculi: An unusual presentation. J. Cancer Res. Ther. 2015, 11, 652. [Google Scholar] [CrossRef] [PubMed]
- Spiro, R.H.; Huvos, A.G. Stage means more than grade in adenoid cystic carcinoma. Am. J. Surg. 1992, 164, 623–628. [Google Scholar] [CrossRef]
- Zbaren, P.; Triantafyllou, A.; Devaney, K.O.; Poorten, V.V.; Hellquist, H.; Rinaldo, A.; Ferlito, A. Preoperative diagnostic of parotid gland neoplasms: Fine-needle aspiration cytology or core needle biopsy? Eur. Arch. Otorhinolaryngol. 2018, 275, 2609–2613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanege, F.M.; Tuysuz, O.; Sakallioglu, O.; Arslan Solmaz, O. Diagnostic value of preoperative fine needle aspiration cytology in parotid gland tumors. Diagn. Cytopathol. 2020, 48, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thimsen, V.; Fauck, V.; Wiesmüller, M.; Agaimy, A.; Schapher, M.; Iro, H.; Koch, M.; Mantsopoulos, K. Calcification in Salivary Gland Cancer Mimicking Sialolithiasis—A Diagnostic Pitfall on Imaging: Report of Two Cases and Brief Review of the Literature. J. Clin. Med. 2022, 11, 3329. https://doi.org/10.3390/jcm11123329
Thimsen V, Fauck V, Wiesmüller M, Agaimy A, Schapher M, Iro H, Koch M, Mantsopoulos K. Calcification in Salivary Gland Cancer Mimicking Sialolithiasis—A Diagnostic Pitfall on Imaging: Report of Two Cases and Brief Review of the Literature. Journal of Clinical Medicine. 2022; 11(12):3329. https://doi.org/10.3390/jcm11123329
Chicago/Turabian StyleThimsen, Vivian, Vanessa Fauck, Marco Wiesmüller, Abbas Agaimy, Mirco Schapher, Heinrich Iro, Michael Koch, and Konstantinos Mantsopoulos. 2022. "Calcification in Salivary Gland Cancer Mimicking Sialolithiasis—A Diagnostic Pitfall on Imaging: Report of Two Cases and Brief Review of the Literature" Journal of Clinical Medicine 11, no. 12: 3329. https://doi.org/10.3390/jcm11123329
APA StyleThimsen, V., Fauck, V., Wiesmüller, M., Agaimy, A., Schapher, M., Iro, H., Koch, M., & Mantsopoulos, K. (2022). Calcification in Salivary Gland Cancer Mimicking Sialolithiasis—A Diagnostic Pitfall on Imaging: Report of Two Cases and Brief Review of the Literature. Journal of Clinical Medicine, 11(12), 3329. https://doi.org/10.3390/jcm11123329







