Levels of lncRNA GAS5 in Plasma of Patients with Severe Traumatic Brain Injury: Correlation with Systemic Inflammation and Early Outcome
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Sample Collection
2.3. RNA Extraction and Plasma GAS5 Assessment
2.4. Statistical Analysis
3. Results
3.1. Clinical Basic Data
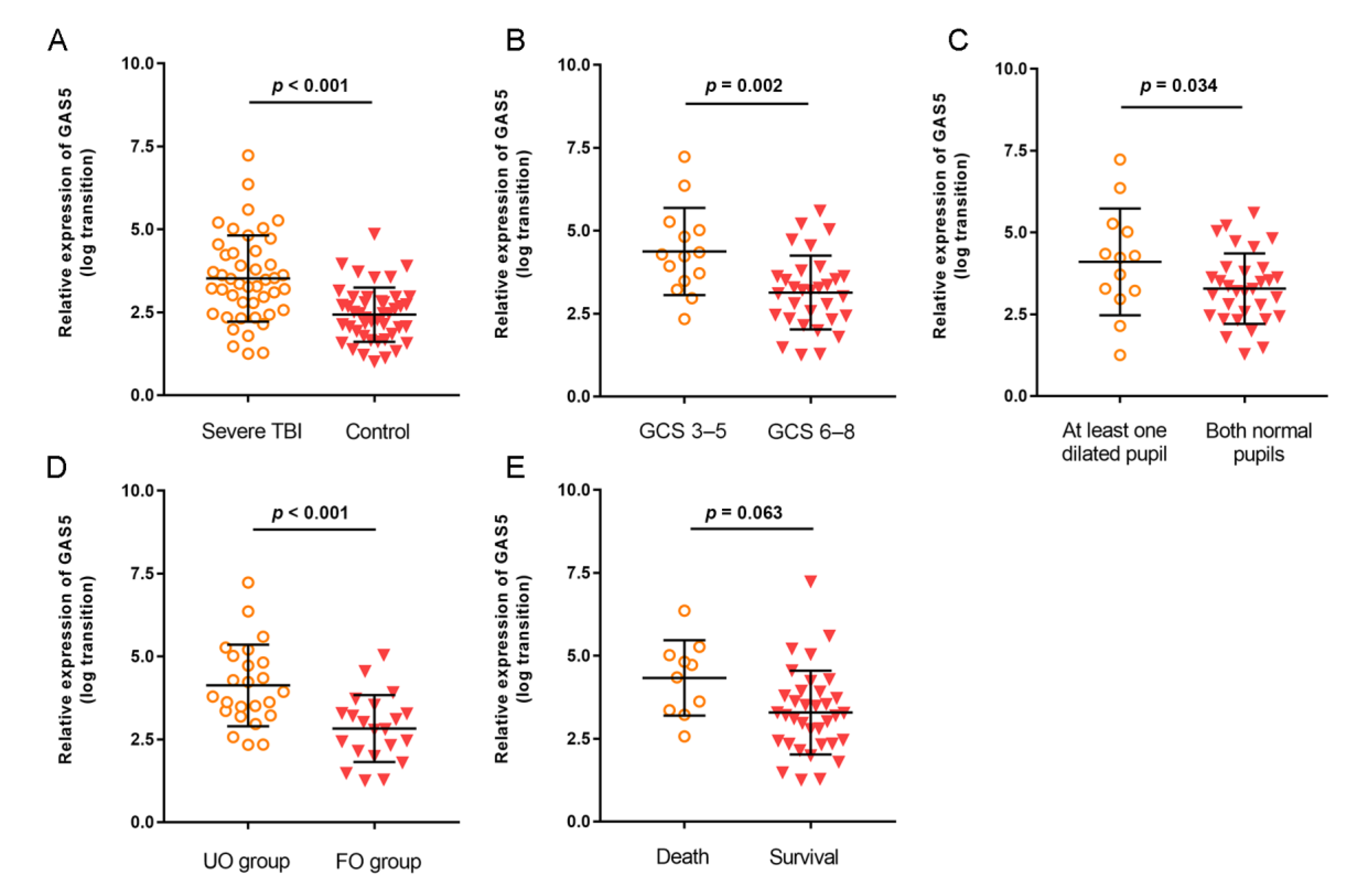
3.2. Expression of GAS5 in Severe TBI Patients
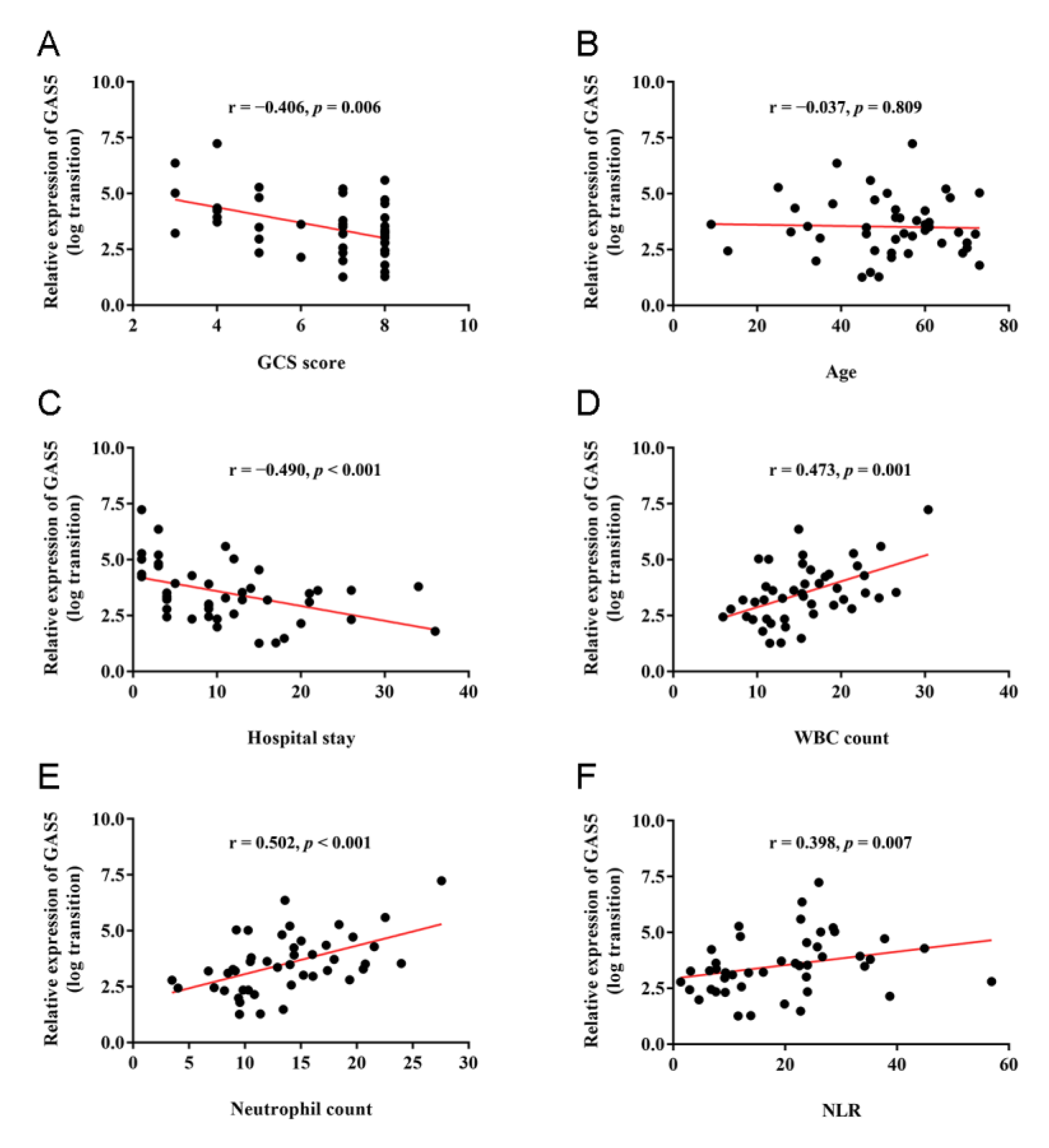
3.3. Correlations of GAS5 Expression with Clinical and Inflammatory Parameters
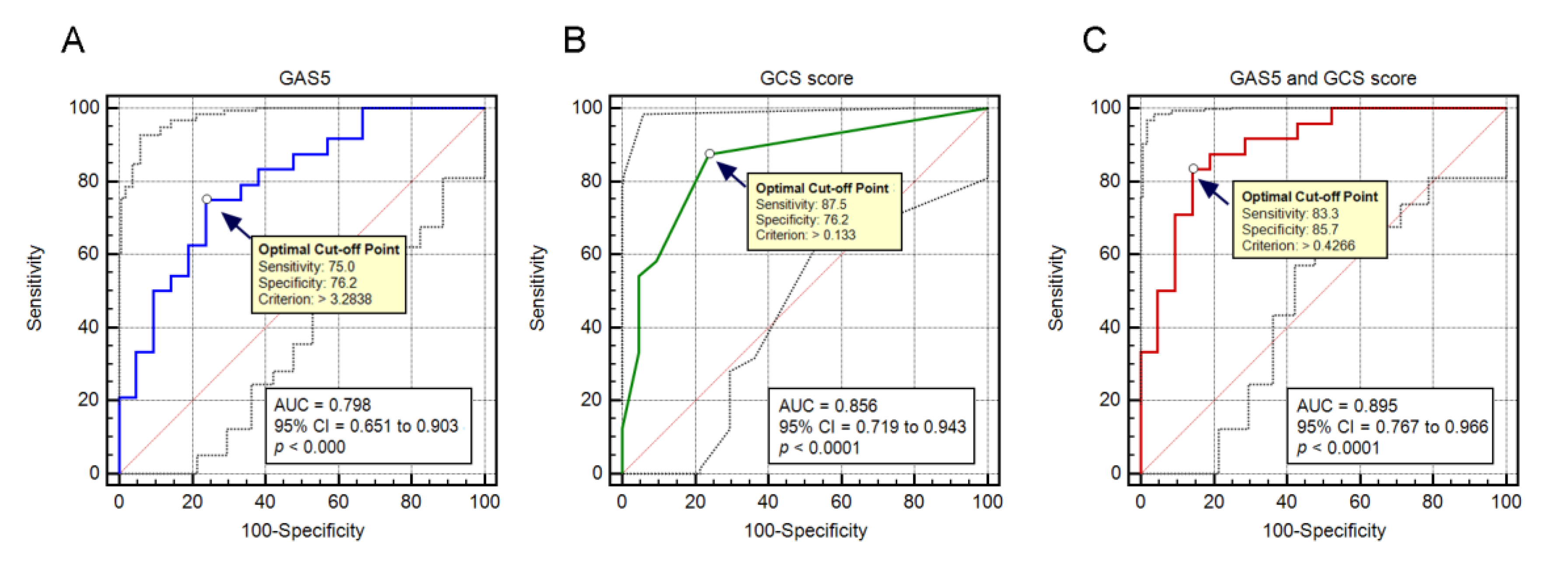
3.4. Prognostic Value of GAS5 Expression in Predicting Patient Outcome of Severe TBI
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maas, A.I.; Stocchetti, N.; Bullock, R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008, 7, 728–741. [Google Scholar] [CrossRef]
- Gao, G.; Wu, X.; Feng, J.; Hui, J.; Mao, Q.; Lecky, F.; Lingsma, H.; Maas, A.I.R.; Jiang, J.; China CENTER-TBI Registry Participants. Clinical characteristics and outcomes in patients with traumatic brain injury in China: A prospective, multicentre, longitudinal, observational study. Lancet Neurol. 2020, 19, 670–677. [Google Scholar] [CrossRef]
- Lei, J.; Gao, G.; Jiang, J. Acute traumatic brain injury: Is current management evidence based? An empirical analysis of systematic reviews. J. Neurotrauma 2013, 30, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Nagalakshmi, B.; Sagarkar, S.; Sakharkar, A.J. Epigenetic Mechanisms of Traumatic Brain Injuries. Prog. Mol. Biol. Transl. Sci. 2018, 157, 263–298. [Google Scholar]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadakkuzha, B.M.; Liu, X.A.; McCrate, J.; Shankar, G.; Rizzo, V.; Afinogenova, A.; Young, B.; Fallahi, M.; Carvalloza, A.C.; Raveendra, B.; et al. Transcriptome analyses of adult mouse brain reveal enrichment of lncRNAs in specific brain regions and neuronal populations. Front. Cell Neurosci. 2015, 9, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Wang, H. Long non-coding RNA in CNS injuries: A new target for therapeutic intervention. Mol. Ther. Nucleic Acids 2019, 17, 754–766. [Google Scholar] [CrossRef] [Green Version]
- Kino, T.; Hurt, D.E.; Ichijo, T.; Nader, N.; Chrousos, G.P. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci. Signal. 2010, 3, ra8. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Shi, X.; Zhu, Q.; Li, Q.; Liu, Y.; Yao, Y.; Song, Y. The growth arrest-specific transcript 5 (GAS5): A pivotal tumor suppressor long noncoding RNA in human cancers. Tumour Biol. 2016, 37, 1437–1444. [Google Scholar] [CrossRef]
- Zhao, R.B.; Zhu, L.H.; Shu, J.P.; Qiao, L.X.; Xia, Z.K. GAS5 silencing protects against hypoxia/ischemia-induced neonatal brain injury. Biochem. Biophys. Res. Commun. 2018, 497, 285–291. [Google Scholar] [CrossRef]
- Li, J.; Lv, H.; Che, Y.Q. Long non-coding RNA Gas5 potentiates the effects of microRNA-21 downregulation in response to ischaemic brain injury. Neuroscience 2020, 437, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, X.; Pan, J.; Zhao, S.; Li, Y.; Wang, Z.; Yang, J.; Zhang, X.; Wang, Y.; Liu, M. GAS5 knockdown alleviates spinal cord injury by reducing VAV1 expression via RNA binding protein CELF2. Sci. Rep. 2021, 11, 3628. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Zhao, C.C.; Weng, W.J.; Lei, J.; Lin, Y.; Mao, Q.; Gao, G.Y.; Feng, J.F.; Jiang, J.Y. Alteration in long non-coding RNA expression after traumatic brain injury in rats. J. Neurotrauma 2017, 34, 2100–2108. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.Y.; Gao, G.Y.; Feng, J.F.; Mao, Q.; Chen, L.G.; Yang, X.F.; Liu, J.F.; Wang, Y.H.; Qiu, B.H.; Huang, X.J. Traumatic brain injury in China. Lancet Neurol. 2019, 18, 286–295. [Google Scholar] [CrossRef]
- Stein, S.C.; Georgoff, P.; Meghan, S.; Mizra, K.; Sonnad, S.S. 150 years of treating severe traumatic brain injury: A systematic review of progress in mortality. J. Neurotrauma 2010, 27, 1343–1353. [Google Scholar] [CrossRef]
- Kumar, A.; Loane, D.J. Neuroinflammation after traumatic brain injury: Opportunities for therapeutic intervention. Brain Behav. Immun. 2012, 26, 1191–1201. [Google Scholar] [CrossRef]
- Sun, D.; Yu, Z.; Fang, X.; Liu, M.; Pu, Y.; Shao, Q.; Wang, D.; Zhao, X.; Huang, A.; Xiang, Z.; et al. LncRNA GAS5 inhibits microglial M2 polarization and exacerbates demyelination. EMBO Rep. 2017, 18, 1801–1816. [Google Scholar] [CrossRef]
- Dai, X.; Yi, M.; Wang, D.; Chen, Y.; Xu, X. Changqin NO. 1 inhibits neuronal apoptosis via suppressing GAS5 expression in a traumatic brain injury mice model. Biol. Chem. 2019, 400, 753–763. [Google Scholar] [CrossRef]
- Mayama, T.; Marr, A.K.; Kino, T. Differential expression of glucocorticoid receptor noncoding RNA repressor Gas5 in autoimmune and inflammatory Diseases. Horm. Metab. Res. 2016, 48, 550–557. [Google Scholar] [CrossRef]
- Moharamoghli, M.; Hassan-Zadeh, V.; Dolatshahi, E.; Alizadeh, Z.; Farazmand, A. The expression of GAS5, THRIL, and RMRP lncRNAs is increased in T cells of patients with rheumatoid arthritis. Clin. Rheumatol. 2019, 38, 3073–3080. [Google Scholar] [CrossRef]
- Suo, Q.F.; Sheng, J.; Qiang, F.Y.; Tang, Z.S.; Yang, Y.Y. Association of long non-coding RNA GAS5 and miR-21 levels in CD4(+) T cells with clinical features of systemic lupus erythematosus. Exp. Ther. Med. 2018, 15, 345–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Zhang, X.; Liu, X. Long noncoding RNA GAS5 protects against Mycoplasma pneumoniae pneumonia by regulating the microRNA2223p/TIMP3 axis. Mol. Med. Rep. 2021, 23, 380. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Ma, H.; Gao, M.; Chen, A.; Zha, S.; Yan, J. Long non-coding RNA GAS5 aggravates myocardial depression in mice with sepsis via the microRNA-449b/HMGB1 axis and the NF-kappaB signaling pathway. Biosci. Rep. 2021, 41, BSR20201738. [Google Scholar] [CrossRef] [PubMed]
- Needham, E.J.; Helmy, A.; Zanier, E.R.; Jones, J.L.; Coles, A.J.; Menon, D.K. The immunological response to traumatic brain injury. J. Neuroimmunol. 2019, 332, 112–125. [Google Scholar] [CrossRef]
- Xu, L.B.; Yue, J.K.; Korley, F.; Puccio, A.M.; Yuh, E.L.; Sun, X.; Rabinowitz, M.; Vassar, M.J.; Taylor, S.R.; Winkler, E.A.; et al. High-Sensitivity C-Reactive Protein is a Prognostic Biomarker of Six-Month Disability after Traumatic Brain Injury: Results from the TRACK-TBI Study. J. Neurotrauma 2021, 38, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, G.; Maas, A.; Lecky, F.; Manley, G.; Stocchetti, N.; Murray, G. The Glasgow Coma Scale at 40 years: Standing the test of time. Lancet Neurol. 2014, 13, 844–854. [Google Scholar] [CrossRef]
- Laureys, S.; Bodart, O.; Gosseries, O. The Glasgow Coma Scale: Time for critical reappraisal? Lancet Neurol. 2014, 13, 755–757. [Google Scholar] [CrossRef] [Green Version]
- Tong, Y.S.; Wang, X.W.; Zhou, X.L.; Liu, Z.H.; Yang, T.X.; Shi, W.H.; Xie, H.W.; Lv, J.; Wu, Q.Q.; Cao, X.F. Identification of the long non-coding RNA POU3F3 in plasma as a novel biomarker for diagnosis of esophageal squamous cell carcinoma. Mol. Cancer 2015, 14, 3. [Google Scholar] [CrossRef] [Green Version]
- Hartl, R.; Medary, M.B.; Ruge, M.; Arfors, K.E.; Ghajar, J. Early white blood cell dynamics after traumatic brain injury: Effects on the cerebral microcirculation. J. Cereb. Blood Flow. Metab. 1997, 17, 1210–1220. [Google Scholar] [CrossRef] [Green Version]
- Sabouri, E.; Majdi, A.; Jangjui, P.; Rahigh Aghsan, S.; Naseri Alavi, S.A. Neutrophil-to-Lymphocyte Ratio and Traumatic Brain Injury: A Review Study. World Neurosurg. 2020, 140, 142–147. [Google Scholar] [CrossRef]
| UO Group (n = 24) | FO Group (n = 21) | Total (n = 45) | p Value | |
|---|---|---|---|---|
| Age (years) | 52.4 ± 14.9 | 49.7 ± 15.9 | 51.1 ± 15.2 | 0.552 |
| Gender (Male/Female) | 20/4 | 13/8 | 33/12 | 0.105 |
| Cause of injury, n (%) | 0.904 | |||
| Traffic accident | 11 (45.8%) | 12 (57.1%) | 23 (51.1%) | |
| Fall | 10 (41.7%) | 6 (28.6%) | 16 (35.6%) | |
| Assault | 2 (8.3%) | 2 (9.5%) | 4 (8.9%) | |
| Other | 1 (4.2%) | 1 (4.8%) | 2 (4.4%) | |
| GCS score (point) | 5.5 ± 1.7 | 7.6 ± 0.9 | 6.5 ± 1.7 | <0.001 |
| Pupil reaction, n (%) | ||||
| Both normal | 15 (62.5%) | 17 (81.0%) | 32 (71.1%) | |
| One pupil dilated | 5 (20.8%) | 4 (19.0%) | 9 (20%) | 0.122 |
| Both pupils dilated | 4 (16.7%) | 0 (0%) | 4 (8.9%) | |
| Neurosurgery | 19 (79.1%) | 13 (61.9%) | 32 (71.1%) | 0.203 |
| Length of hospital stay (days) | 8.7 ± 8.9 | 13.8 ± 7.6 | 11.1 ± 8.7 | 0.048 |
| WBC (103/μL) | 17.2 ± 5.1 | 13.8 ± 5.5 | 15.6 ± 5.5 | 0.040 |
| Neutrophil count (103/μL) | 15.2 ± 4.8 | 11.9 ± 5.3 | 13.6 ± 5.3 | 0.037 |
| NLR | 21.3 ± 10.9 | 17.4 ± 13.6 | 19.4 ± 12.2 | 0.296 |
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| GCS score | 0.336 | 0.169–0.666 | 0.002 | 0.318 | 0.132–0.767 | 0.011 |
| GAS5 expression | 3.119 | 1.462–6.652 | 0.003 | 2.771 | 1.025–7.494 | 0.045 |
| Age | 1.012 | 0.973–1.053 | 0.543 | |||
| Sex | 0.325 | 0.081–1.303 | 0.113 | |||
| Cause of injury | ||||||
| Pupil reaction | 0.392 | 0.100–1.539 | 0.180 | |||
| Neurosurgery | 2.338 | 0.624–8.766 | 0.208 | |||
| Lohs | 0.927 | 0.857–1.004 | 0.161 | |||
| WBC count | 1.134 | 1.001–1.285 | 0.049 | 1.547 | 0.624–3.836 | 0.347 |
| Neutrophil count | 1.144 | 1.003–1.306 | 0.045 | 1.146 | 0.246–1.697 | 0.376 |
| NLR | 1.028 | 0.977–1.082 | 0.285 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, J.; Zhang, X.; Tan, R.; Li, Y.; Zhao, K.; Niu, H. Levels of lncRNA GAS5 in Plasma of Patients with Severe Traumatic Brain Injury: Correlation with Systemic Inflammation and Early Outcome. J. Clin. Med. 2022, 11, 3319. https://doi.org/10.3390/jcm11123319
Lei J, Zhang X, Tan R, Li Y, Zhao K, Niu H. Levels of lncRNA GAS5 in Plasma of Patients with Severe Traumatic Brain Injury: Correlation with Systemic Inflammation and Early Outcome. Journal of Clinical Medicine. 2022; 11(12):3319. https://doi.org/10.3390/jcm11123319
Chicago/Turabian StyleLei, Jin, Xiao Zhang, Rui Tan, Yu Li, Kai Zhao, and Hongquan Niu. 2022. "Levels of lncRNA GAS5 in Plasma of Patients with Severe Traumatic Brain Injury: Correlation with Systemic Inflammation and Early Outcome" Journal of Clinical Medicine 11, no. 12: 3319. https://doi.org/10.3390/jcm11123319
APA StyleLei, J., Zhang, X., Tan, R., Li, Y., Zhao, K., & Niu, H. (2022). Levels of lncRNA GAS5 in Plasma of Patients with Severe Traumatic Brain Injury: Correlation with Systemic Inflammation and Early Outcome. Journal of Clinical Medicine, 11(12), 3319. https://doi.org/10.3390/jcm11123319






