Model Informed Precision Dosing Tool Forecasts Trough Infliximab and Associates with Disease Status and Tumor Necrosis Factor-Alpha Levels of Inflammatory Bowel Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Laboratory Measurements
2.2. Model Informed Precision Dosing Tool
2.3. Statistical Analysis
3. Results
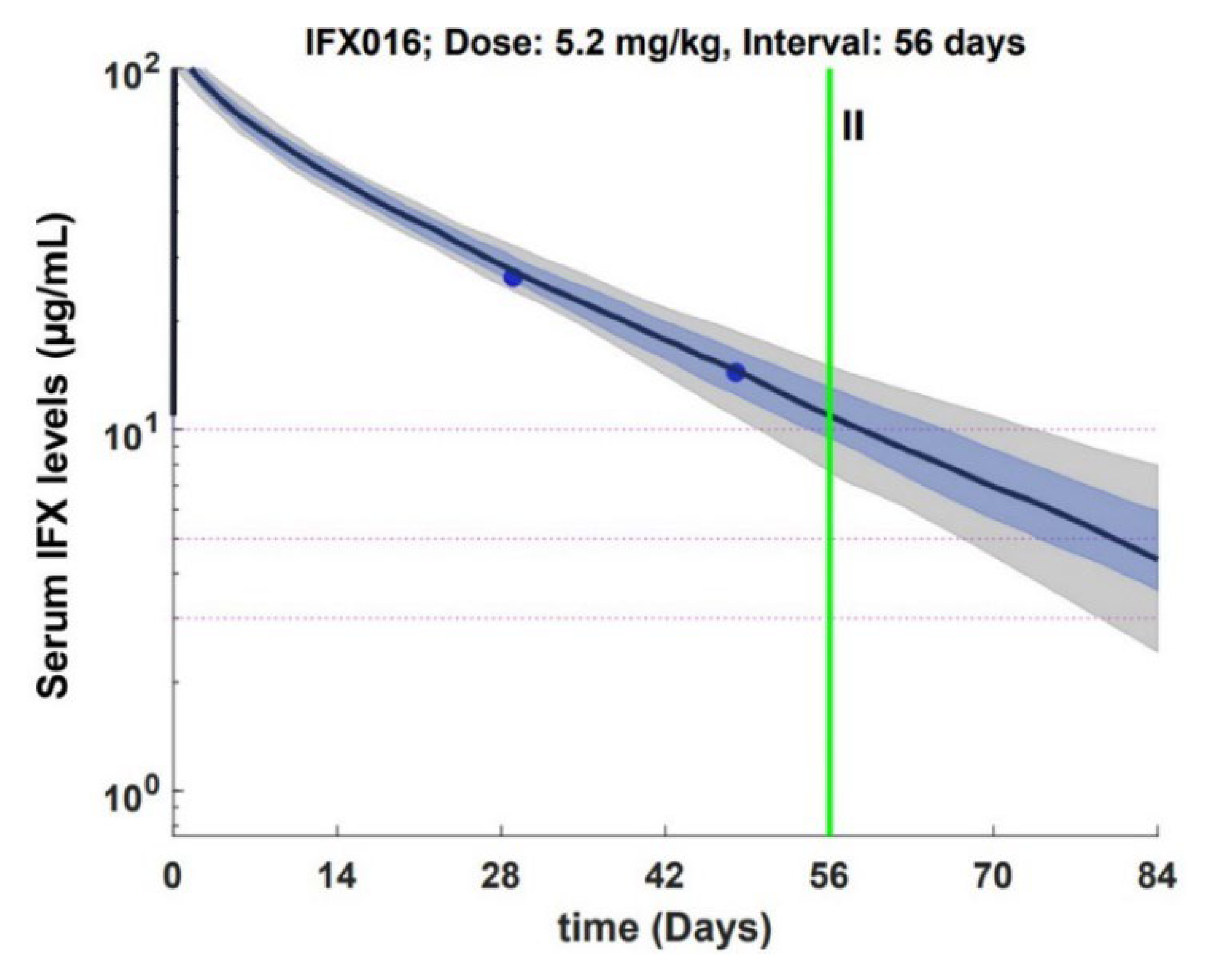
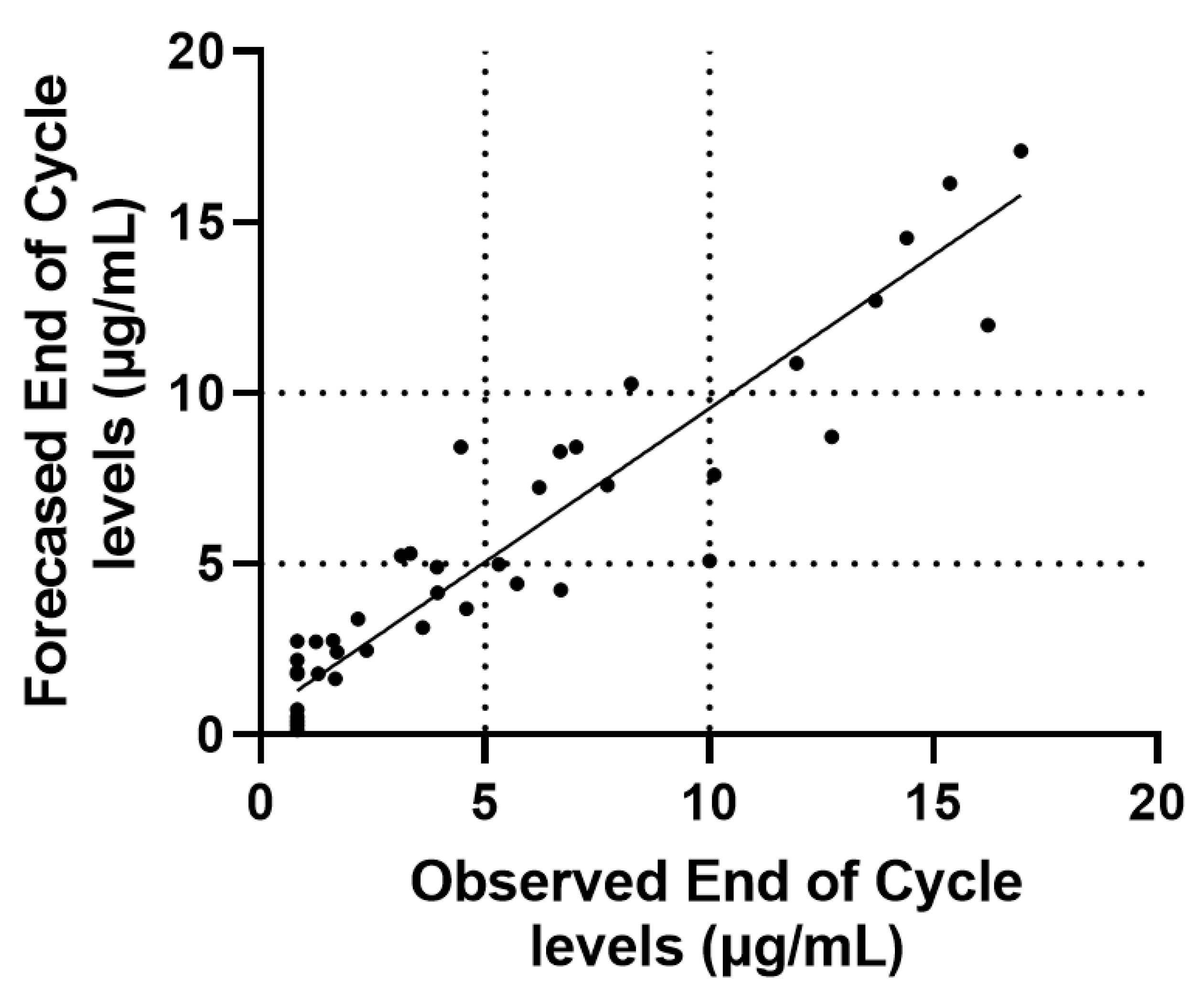
3.1. End of Cycle IFX Levels Can Be Forecasted Using Mid Cycle Determinations
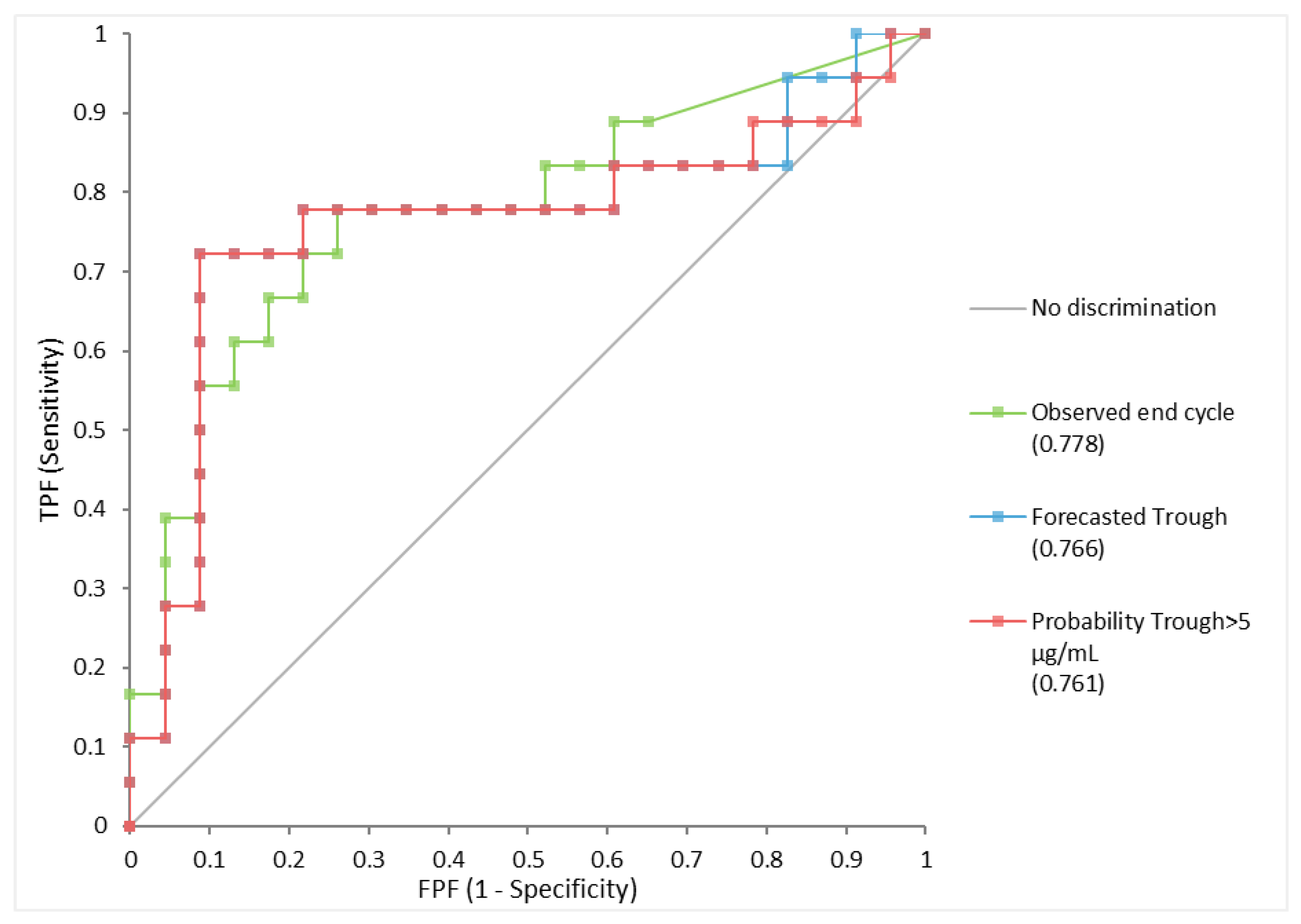
3.2. Forecasted IFX Levels Associate with Clinical and Biochemical Remission
3.3. Circulating TNF-α and CRP Levels Independently and Additively Impact IFX Clearance in IBD
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vande Casteele, N.; Herfarth, H.; Katz, J.; Flack-Ytter, Y.; Singh, S. American Gastroenterological Association Institute Technical Review on the Role of Therapeutic Drug Monitoring in the Management of Inflammatory Bowel Diseases. Gastroenterology 2017, 153, 835–857.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feuerstein, J.D.; Nguyen, G.C.; Kupfer, S.S.; Falck-Ytter, Y.; Singh, S.; Gerson, L.; Hirano, I.; Rubenstein, J.H.; Smalley, W.E.; Stollman, N.; et al. American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology 2017, 153, 827–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negoescu, D.M.; Enns, A.E.; Swanhorst, B.; Baumgartner, B.; Campbell, J.P.; Osterman, M.T.; Papamichael, K.; Cheifetz, A.S.; Vaughn, B.P. Proactive Vs Reactive Therapeutic Drug Monitoring of Infliximab in Crohn’s Disease: A Cost-Effectiveness Analysis in a Simulated Cohort. Inflamm. Bowel Dis. 2019, 26, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Vajravelu, R.K.; Vaughn, B.P.; Osterman, M.T.; Cheifetz, A.S. Proactive Infliximab Monitoring Following Reactive Testing is Associated with Better Clinical Outcomes Than Reactive Testing Alone in Patients With Inflammatory Bowel Disease. J. Crohns Colitis 2018, 12, 804–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubinsky, M.C.; Phan, B.L.; Singh, N.; Rabizadeh, S.; Mould, D.R. Pharmacokinetic Dashboard-Recommended Dosing is Different than Standard of Care Dosing in Infliximab-Treated Pediatric IBD Patients. AAPS J. 2017, 19, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Strik, A.S.; Löwenberg, M.; Mould, D.R.; Berends, S.E.; Ponsioen, C.I.; Brande, J.M.H.V.D.; Jansen, J.M.; Hoekman, D.R.; Brandse, J.F.; Duijvestein, M.; et al. Efficacy of dashboard driven dosing of Infliximab in inflammatory bowel disease patients; a randomized controlled trial. Scand. J. Gastroenterol. 2021, 56, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Mould, D.; Hu, C.; Ford, J.; Keen, M.; Davis, H.; Zhou, H. Population pharmacokinetic analysis of infliximab in pediatrics using integrated data from six clinical trials. Clin. Pharmacol. Drug Dev. 2012, 1, 203. [Google Scholar]
- Yarur, A.J.; Jain, A.; Sussman, D.A.; Barkin, J.S.; Quintero, M.A.; Princen, F.; Kirkland, R.; Deshpande, A.R.; Singh, S.; Abreu, M.T. The association of tissue anti-TNF drug levels with serological and endoscopic disease activity in inflammatory bowel disease: The ATLAS study. Gut 2016, 65, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Miyasaka, N.; Tatsuki, Y.; Yano, T.; Yoshinari, T.; Abe, T.; Koike, T. Baseline tumour necrosis factor alpha levels predict the necessity for dose escalation of infliximab therapy in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2011, 70, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-L.; Ohrmund, L.; Hauenstein, S.; Salbato, J.; Reddy, R.; Monk, P.; Lockton, S.; Ling, N.; Singh, S. Development and validation of a homogeneous mobility shift assay for the measurement of infliximab and antibodies-to-infliximab levels in patient serum. J. Immunol. Methods 2012, 382, 177–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todd, J.; Simpson, P.; Estis, J.; Torres, V.; Wub, A.H.B. Reference range and short- and long-term biological variation of interleukin (IL)-6, IL-17A and tissue necrosis factor-alpha using high sensitivity assays. Cytokine 2013, 64, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Mizuno, T.; Colman, R.; Hyams, J.; Noe, J.D.; Boyle, B.; Tsai, Y.-T.; Dong, M.; Jackson, K.; Punt, N.; et al. Real-World Infliximab Pharmacokinetic Study Informs an Electronic Health Record-Embedded Dashboard to Guide Precision Dosing in Children with Crohn’s Disease. Clin. Pharmacol. Ther. 2021, 109, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Goswami, S.; Keizer, R.J.; Winger, B.A.; Kharbanda, S.; Dvorak, C.; Long-Boyle, J. Assessment of a Model-Informed Precision Dosing Platform Use in Routine Clinical Care for Personalized Busulfan Therapy in the Pediatric Hematopoietic Cell Transplantation (HCT) Population. Front. Pharmacol. 2020, 11, 888. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.H.; Tong, D.M.H.; Lucas, S.S.; Faldasz, J.D.; Goswami, S.; Keizer, R.J. Continuous Learning in Model-Informed Precision Dosing: A Case Study in Pediatric Dosing of Vancomycin. Clin. Pharmacol. Ther. 2021, 109, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Maier, C.; Hartung, N.; Kloft, C.; Huisinga, W.; de Wiljes, J. Reinforcement learning and Bayesian data assimilation for model-informed precision dosing in oncology. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Maier, C.; Hartung, N.; de Wiljes, J.; Klots, C.; Huisinga, W. Bayesian Data Assimilation to Support Informed Decision Making in Individualized Chemotherapy. CPT Pharmacomet. Syst. Pharmacol. 2020, 9, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Berkhout, L.C.; L’Ami, M.J.; Ruwaard, J.; Hart, M.H.; Heer, P.O.-D.; Bloem, K.; Nurmohamed, M.T.; van Vollenhoven, R.F.; Boers, M.; Alvarez, D.F.; et al. Dynamics of circulating TNF during adalimumab treatment using a drug-tolerant TNF assay. Sci. Transl. Med. 2019, 11, eaat3356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komatsu, M.; Kobayashi, D.; Saito, K.; Furuya, D.; Yagihashi, A.; Araake, H.; Tsuji, N.; Sakamaki, S.; Niitsu, Y.; Watanabe, N. Tumor necrosis factor-alpha in serum of patients with inflammatory bowel disease as measured by a highly sensitive immuno-PCR. Clin. Chem. 2001, 47, 1297–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, V.; Andus, T.; Caesar, I.; Schölmerich, J. Evidence for continuous stimulation of interleukin-6 production in Crohn’s disease. Gastroenterology 1992, 102, 514–519. [Google Scholar] [CrossRef]
| Estimate | |
|---|---|
| Gender (female) | 46.3% (19/41) |
| Age (years) | 43 (34–49) |
| CD Diagnosis (vs UC) | 76% (31/41) |
| Concomitant Immunomodulators | 20% (8/41) |
| Albumin (g/L) | 42.6 (40.4–44.3) |
| IFX (µg/mL) | |
| Mid cycle | 13.2 (6.2–20.5) |
| End cycle | 3.9 (1.2–7.7) |
| ATI status | |
| Mid cycle | 15% (6/41) |
| End cycle | 19% (8/41) |
| CRP (mg/L) | |
| Mid cycle | 2.4 (0.6–5.4) |
| End cycle | 2.7 (0.1–8.8) |
| TNF-α (pg/mL) | |
| Mid cycle | 4.6 (3.1–8.8) |
| End cycle | 8.0 (4.8–12.0) |
| Disease activity | |
| HBI | 2 (1–5) |
| Partial Mayo | 0 (0–3) |
| Clinical & Biochemical Remission | 44% (18/41) |
| n/N | |
|---|---|
| Crohn’s Disease | |
| Age: | |
| ≤16 (A1) | 2/31 |
| 17–40 (A2) | 25/31 |
| >40 (A3) | 4/31 |
| Location | |
| Terminal Ileum (L1) | 2/31 |
| Colon (L2) | 6/31 |
| Ileocolon (L3) | 23/31 |
| Behavior | |
| Non structuring, non-penetrating (B1) | 15/31 |
| Stricturing (B2) | 12/31 |
| Penetrating (B3) | 3/31 |
| Perianal | 1/31 |
| Ulcerative Colitis | |
| Proctitis (E1) | 0/10 |
| Left sided (E2) | 3/10 |
| Pancolitis (E3) | 7/10 |
| Observed End of Cycle > 5 µg/mL | Forecasted Trough > 5 µg/mL | |||||
|---|---|---|---|---|---|---|
| Estimate | −95CI | +95CI | Estimate | −95CI | +95CI | |
| Sensitivity | 0.67 | 0.44 | 0.84 | 0.67 | 0.44 | 0.84 |
| Specificity | 0.78 | 0.58 | 0.90 | 0.91 | 0.73 | 0.98 |
| False positive | 0.22 | 0.10 | 0.42 | 0.09 | 0.02 | 0.27 |
| False Negative | 0.33 | 0.16 | 0.56 | 0.33 | 0.16 | 0.56 |
| Positive LR | 3.1 | 1.4 | 7.2 | 7.7 | 2.3 | 28.4 |
| Negative LR | 0.4 | 0.2 | 0.8 | 0.36 | 0.2 | 0.6 |
| Odds Ratio | 7.2 | 1.8 | 30.2 | 21.0 | 3.9 | 109.0 |
| Intercept | Slope ± SEM | p Value | Marginal R2 | |
|---|---|---|---|---|
| CRP | 10.0 ±1.2 | −0.13 ± 0.07 | 0.047 | 0.058 |
| TNF-α | 12.4 ± 1.2 | −0.33 ± 0.08 | <0.001 | 0.213 |
| CRP + TNF-α | 13.4 ± 1.3 | −0.14 ± 0.06 −0.33 ± 0.07 | 0.022 <0.001 | 0.271 |
| End of Cycle | CRP ≤ 3 mg/L and TNF-α ≤ 8 pg/mL | CRP > 3 mg/L or TNF-α > 8 pg/mL | CRP > 3 mg/L and TNF-α > 8 pg/mL | p Value |
|---|---|---|---|---|
| Observed end cycle µg/mL | 7.0 (4.6–14.4) | 3.3 (0.8–7.7) | 1.7 (0.8–3.9) | <0.01 |
| Forecasted Trough µg/mL | 6.7 (5.3–9.0) | 3.7 (0.7–7.7) | 2.0 (1.1–3.2) | <0.01 |
| Probability Trough > 5 µg/mL | 0.68 (0.47–0.90) | 0.25 (0.05–0.70) | 0.10 (0.04–0.19) | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Primas, C.; Reinisch, W.; Panetta, J.C.; Eser, A.; Mould, D.R.; Dervieux, T. Model Informed Precision Dosing Tool Forecasts Trough Infliximab and Associates with Disease Status and Tumor Necrosis Factor-Alpha Levels of Inflammatory Bowel Diseases. J. Clin. Med. 2022, 11, 3316. https://doi.org/10.3390/jcm11123316
Primas C, Reinisch W, Panetta JC, Eser A, Mould DR, Dervieux T. Model Informed Precision Dosing Tool Forecasts Trough Infliximab and Associates with Disease Status and Tumor Necrosis Factor-Alpha Levels of Inflammatory Bowel Diseases. Journal of Clinical Medicine. 2022; 11(12):3316. https://doi.org/10.3390/jcm11123316
Chicago/Turabian StylePrimas, Christian, Walter Reinisch, John C. Panetta, Alexander Eser, Diane R. Mould, and Thierry Dervieux. 2022. "Model Informed Precision Dosing Tool Forecasts Trough Infliximab and Associates with Disease Status and Tumor Necrosis Factor-Alpha Levels of Inflammatory Bowel Diseases" Journal of Clinical Medicine 11, no. 12: 3316. https://doi.org/10.3390/jcm11123316
APA StylePrimas, C., Reinisch, W., Panetta, J. C., Eser, A., Mould, D. R., & Dervieux, T. (2022). Model Informed Precision Dosing Tool Forecasts Trough Infliximab and Associates with Disease Status and Tumor Necrosis Factor-Alpha Levels of Inflammatory Bowel Diseases. Journal of Clinical Medicine, 11(12), 3316. https://doi.org/10.3390/jcm11123316






