Ga-68-PSMA-11 PET/CT in Patients with Biochemical Recurrence of Prostate Cancer after Primary Treatment with Curative Intent—Impact of Delayed Imaging
Abstract: Background
1. Introduction
2. Materials and Methods
2.1. Patients’ Population
2.2. Ga-68-PSMA-11 PET/CT Protocol and Image Interpretation
2.3. Statistical Methods
2.4. Ethics
3. Results
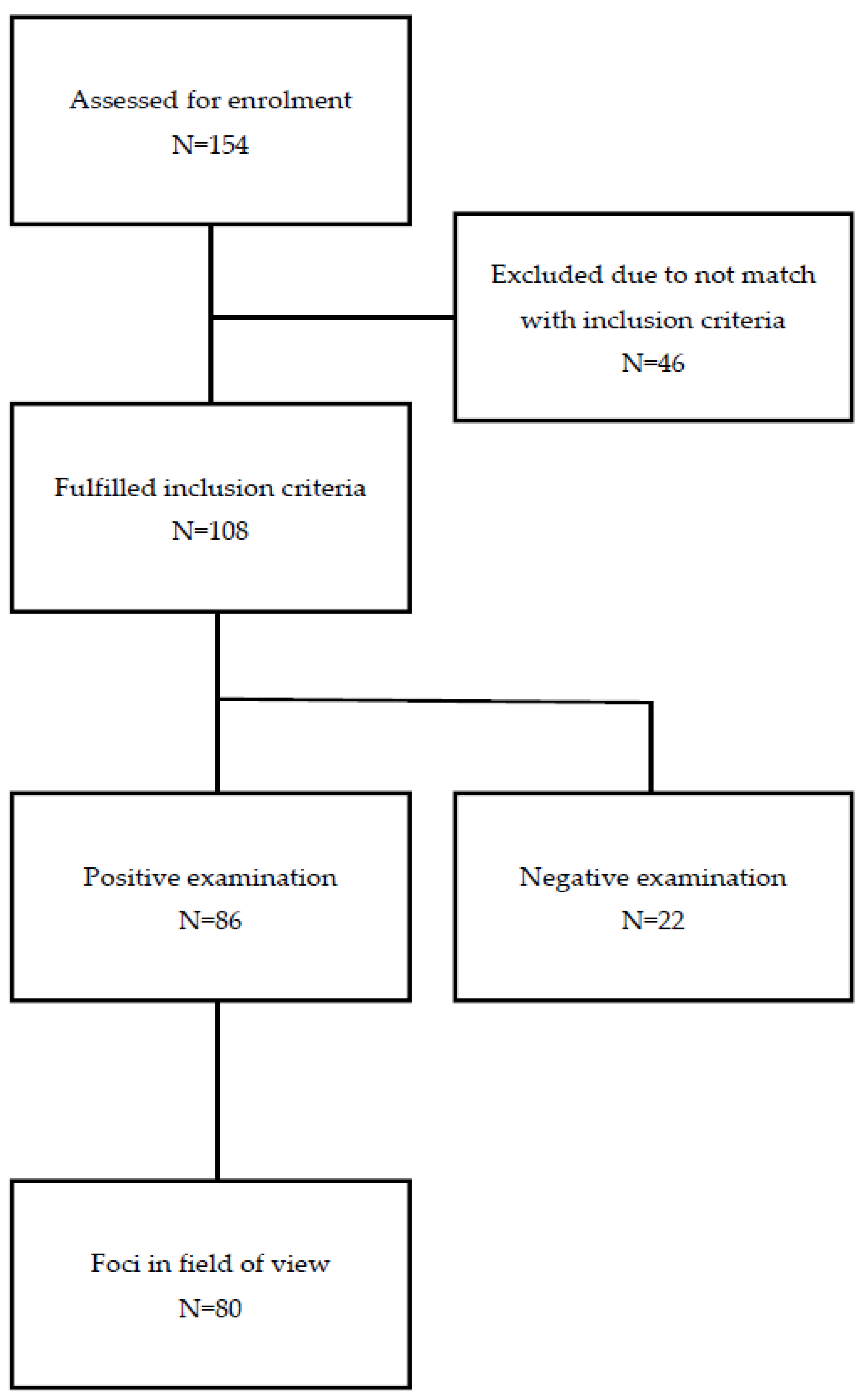
3.1. Patients
3.1.1. Flowchart
3.1.2. Patients’ Characteristics
3.2. PET/CT Ga-68-PSMA-11 Detection Rate
3.3. PET/CT Ga-68-PSMA-11 Lesions-to-Lesion Analysis
3.3.1. Local Recurrence
3.3.2. Local Lymph Node Metastases
3.3.3. Other Local Metastases
3.4. Impact on Clinical Management
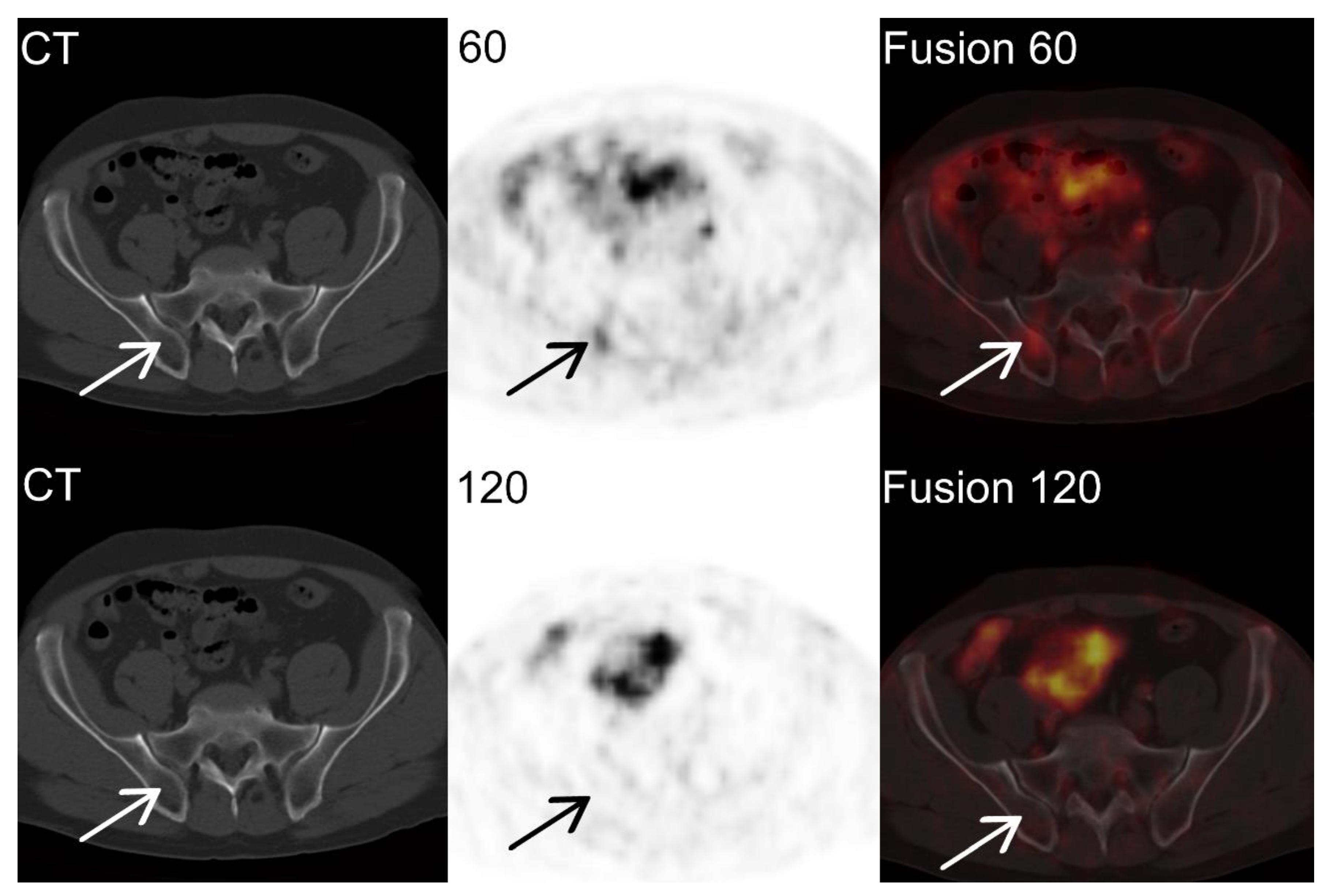
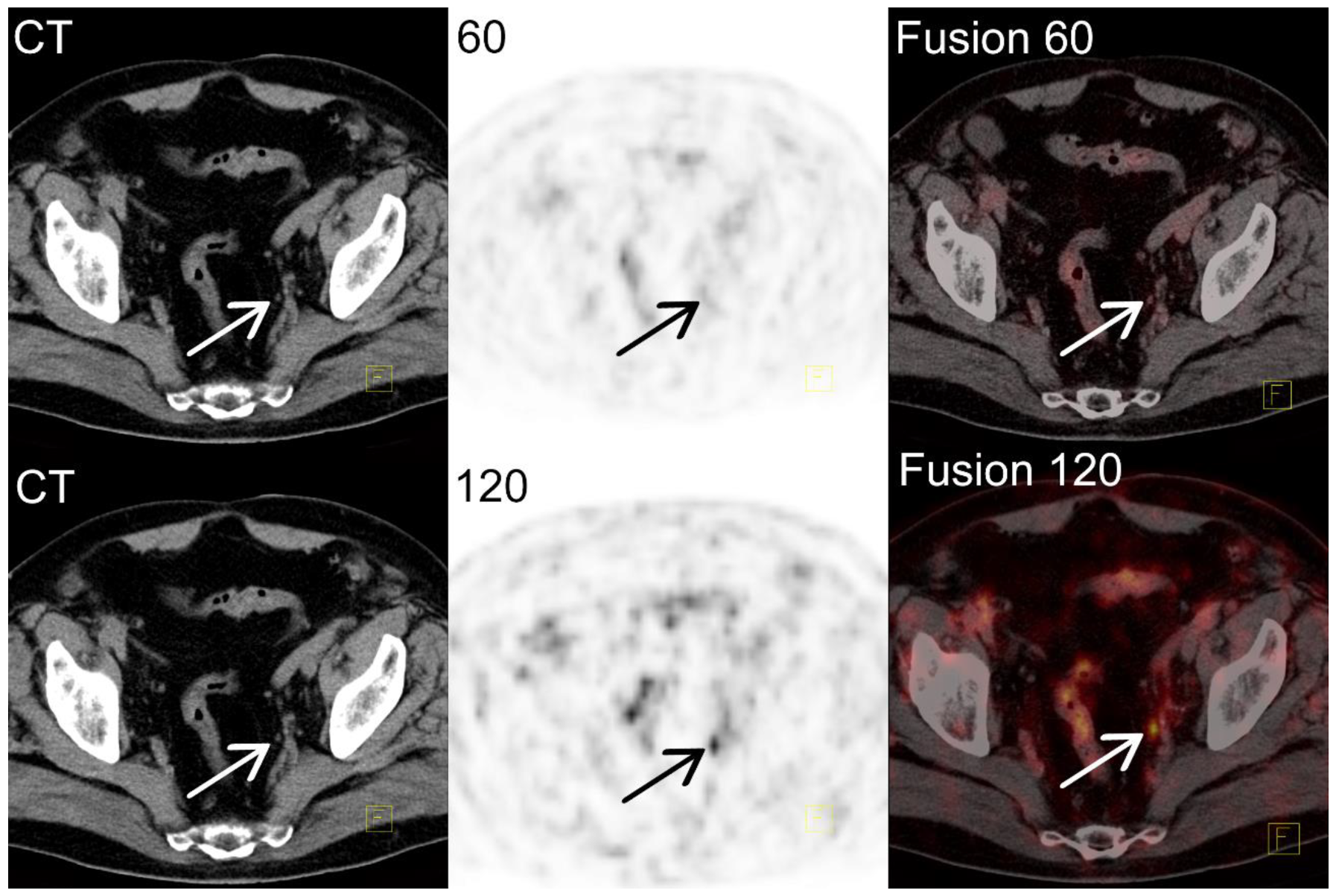
Individual Examples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Stephenson, A.J.; Scardino, P.T.; Eastham, J.A.; Bianco, F.J.; Dotan, Z.A.; Fearn, P.A.; Kattan, M.W. Preoperative Nomogram Predicting the 10-Year Probability of Prostate Cancer Recurrence After Radical Prostatectomy. JNCI J. Natl. Cancer Inst. 2006, 98, 715–717. [Google Scholar] [CrossRef] [Green Version]
- Hull, G.W.; Rabbani, F.; Abbas, F.; Wheeler, T.M.; Kattan, M.W.; Scardino, P.T. Cancer control with radical prostatectomy alone in 1000 consecutive patients. J. Urol. 2002, 167 Pt 1, 528–534. [Google Scholar] [CrossRef]
- Gravas (Chair), S.; Cornu, J.N.; Gacci, M.; Gratzke, C.; Herrmann, T.R.W.; Mamoulakis, C.; Rieken, M.; Speakman, M.J.; Tikkinen, K.A.O. EAU Guidelines. In Proceedings of the EAU Annual Congress Amsterdam 2020, Amsterdam, The Netherlands, 20–24 March 2020; ISBN 978-94-92671-07-3. [Google Scholar]
- American Society for Therapeutic Radiology and Oncology Consensus Panel. Consensus statement: Guidelines for PSA following radiation therapy. American Society for Therapeutic Radiology and Oncology Consensus Panel. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 1035–1041. [Google Scholar]
- Fanti, S.; Minozzi, S.; Antoch, G.; Banks, I.; Briganti, A.; Carrio, I.; Chiti, A.; Clarke, N.; Eiber, M.; De Bono, J.; et al. Consensus on molecular imaging and theranostics in prostate cancer. Lancet Oncol. 2018, 19, e696–e708. [Google Scholar] [CrossRef]
- Ceci, F.; Castellucci, P.; Graziani, T.; Farolfi, A.; Fonti, C.; Lodi, F.; Fanti, S. 68Ga-PSMA-11 PET/CT in recurrent prostate cancer: Efficacy in different clinical stages of PSA failure after radical therapy. Eur. J. Pediatr. 2019, 46, 31–39. [Google Scholar] [CrossRef]
- Perera, M.; Papa, N.; Roberts, M.; Williams, M.; Udovicich, C.; Vela, I.; Christidis, D.; Bolton, D.; Hofman, M.S.; Lawrentschuk, N.; et al. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer-Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-specific Membrane Antigen-avid Lesions: A Systematic Review and Meta-analysis. Eur. Urol. 2020, 77, 403–417. [Google Scholar]
- Rajasekaran, A.K.; Anilkumar, G.; Christiansen, J.J. Is prostate-specific membrane antigen a multifunctional protein? Am. J. Physiol. Physiol. 2005, 288, C975–C981. [Google Scholar] [CrossRef] [Green Version]
- Sokoloff, R.L.; Norton, K.C.; Gasior, C.L.; Marker, K.M.; Grauer, L.S. A dual-monoclonal sandwich assay for prostate-specific membrane antigen: Levels in tissues, seminal fluid and urine. Prostate 2000, 43, 150–157. [Google Scholar] [CrossRef]
- Fendler, W.P.; Eiber, M.; Beheshti, M.; Bomanji, J.; Ceci, F.; Cho, S.; Giesel, F.; Haberkorn, U.; Hope, T.A.; Kopka, K.; et al. 68Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1014–1024. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Hadaschik, B.A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Haufe, S.; et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 486–495. [Google Scholar] [CrossRef]
- Hohberg, M.; Kobe, C.; Täger, P.; Hammes, J.; Schmidt, M.; Dietlein, F.; Wild, M.; Heidenreich, A.; Drzezga, A. Combined Early and Late [68Ga]PSMA-HBED-CC PET Scans Improve Lesion Detectability in Biochemical Recurrence of Prostate Cancer with Low PSA Levels. Mol. Imaging Biol. 2019, 21, 558–566. [Google Scholar] [CrossRef]
- Wondergem, M.; van der Zant, F.M.; Knol, R.J.J.; Lazarenko, S.V.; Pruim, J.; de Jong, I.J. 18F-DCFPyL PET/CT in the Detection of Prostate Cancer at 60 and 120 Minutes: Detection Rate, Image Quality, Activity Kinetics, and Biodistribution. J. Nucl. Med. 2017, 58, 1797–1804. [Google Scholar] [CrossRef] [Green Version]
- Rahbar, K.; Afshar-Oromieh, A.; Seifert, R.; Wagner, S.; Schäfers, M.; Bögemann, M.; Weckesser, M. Diagnostic performance of 18F-PSMA-1007 PET/CT in patients with biochemical recurrent prostate cancer. Eur. J. Pediatr. 2018, 45, 2055–2061. [Google Scholar] [CrossRef] [Green Version]
- Schmuck, S.; Nordlohne, S.; Von Klot, C.-A.; Henkenberens, C.; Sohns, J.S.; Christiansen, H.; Wester, H.-J.; Ross, T.L.; Bengel, F.; Derlin, T. Comparison of standard and delayed imaging to improve the detection rate of [68Ga]PSMA I&T PET/CT in patients with biochemical recurrence or prostate-specific antigen persistence after primary therapy for prostate cancer. Eur. J. Pediatr. 2017, 44, 960–968. [Google Scholar] [CrossRef]
- Kunikowska, J.; Kujda, S.; Królicki, L. 68Ga-PSMA PET/CT in Recurrence Prostate Cancer. Should We Perform Delayed Image in Cases of Negative 60 Minutes Postinjection Examination? Clin. Nucl. Med. 2020, 45, e213–e214. [Google Scholar] [CrossRef]
- Kunikowska, J.; Kuliński, R.; Muylle, K.; Koziara, H.; Królicki, L. 68Ga-Prostate-Specific Membrane Antigen-11 PET/CT: A New Imaging Option for Recurrent Glioblastoma Multiforme. Clin. Nucl. Med. 2020, 45, 11–18. [Google Scholar] [CrossRef]
- Fanti, S.; Minozzi, S.; Morigi, J.J.; Giesel, F.; Ceci, F.; Uprimny, C.; Hofman, M.; Eiber, M.; Schwarzenbock, S.; Castellucci, P.; et al. Development of standardized image interpretation for 68Ga-PSMA PET/CT to detect prostate cancer recurrent lesions. Eur. J. Pediatr. 2017, 44, 1622–1635. [Google Scholar] [CrossRef]
- Beheshti, M.; Paymani, Z.; Brilhante, J.; Geinitz, H.; Gehring, D.; Leopoldseder, T.; Wouters, L.; Pirich, C.; Loidl, W.; Langsteger, W. Optimal time-point for 68Ga-PSMA-11 PET/CT imaging in assessment of prostate cancer: Feasibility of sterile cold-kit tracer preparation? Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1188–1196. [Google Scholar] [CrossRef]
- Emmett, L.; Tang, R.; Nandurkar, R.H.; Hruby, G.; Roach, P.J.; Watts, J.A.; Cusick, T.; Kneebone, A.; Ho, B.; Chan, L.; et al. 3-Year Freedom from Progression After 68Ga-PSMA PET/CT–Triaged Management in Men with Biochemical Recurrence After Radical Prostatectomy: Results of a Prospective Multicenter Trial. J. Nucl. Med. 2020, 61, 866–872. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Steuber, T.; Jilg, C.; Tennstedt, P.; De Bruycker, A.; Tilki, D.; Decaestecker, K.; Zilli, T.; Jereczek-Fossa, B.; Wetterauer, U.; Grosu, A.; et al. Standard of Care Versus Metastases-directed Therapy for PET-detected Nodal Oligorecurrent Prostate Cancer Following Multimodality Treatment: A Multi-institutional Case-control Study. Eur. Urol. Focus 2019, 5, 1007–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bleser, E.; Jereczek-Fossa, B.A.; Pasquier, D.; Zilli, T.; Van As, N.; Siva, S.; Fodor, A.; Dirix, P.; Gomez-Iturriaga, A.; Trippa, F.; et al. Metastasis-directed Therapy in Treating Nodal Oligorecurrent Prostate Cancer: A Multi-institutional Analysis Comparing the Outcome and Toxicity of Stereotactic Body Radiotherapy and Elective Nodal Radiotherapy. Eur. Urol. 2019, 76, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Rischke, H.C.; Schultze-Seemann, W.; Wieser, G.; Krönig, M.; Drendel, V.; Stegmaier, P.; Krauss, T.; Henne, K.; Volegova-Neher, N.; Schlager, D.; et al. Adjuvant radiotherapy after salvage lymph node dissection because of nodal relapse of prostate cancer versus salvage lymph node dissection only. Strahlenther. Onkol. 2015, 191, 310–320. [Google Scholar] [CrossRef]
- Ramey, S.J.; Agrawal, S.; Abramowitz, M.C.; Moghanaki, D.; Pisansky, T.M.; Efstathiou, J.A.; Michalski, J.M.; Spratt, D.E.; Hearn, J.W.; Koontz, B.F.; et al. Multi-institutional Evaluation of Elective Nodal Irradiation and/or Androgen Deprivation Therapy with Postprostatectomy Salvage Radiotherapy for Prostate Cancer. Eur. Urol. 2018, 74, 99–106. [Google Scholar] [CrossRef]
- Pfister, D.; Bolla, M.; Briganti, A.; Carroll, P.; Cozzarini, C.; Joniau, S.; van Poppel, H.; Roach, M.; Stephenson, A.; Wiegel, T.; et al. Early Salvage Radiotherapy Following Radical Prostatectomy. Eur. Urol. 2014, 65, 1034–1043. [Google Scholar] [CrossRef]
- Vaugier, L.; Palpacuer, C.; Rio, E.; Goineau, A.; Pasquier, D.; Buthaud, X.; De Laroche, G.; Beckendorf, V.; Sargos, P.; Créhange, G.; et al. Early Toxicity of a Phase 2 Trial of Combined Salvage Radiation Therapy and Hormone Therapy in Oligometastatic Pelvic Node Relapses of Prostate Cancer (OLIGOPELVIS GETUG P07). Int. J. Radiat. Oncol. 2019, 103, 1061–1067. [Google Scholar] [CrossRef] [Green Version]
- De Bruycker, A.; Spiessens, A.; Dirix, P.; Koutsouvelis, N.; Semac, I.; Liefhooghe, N.; Gomez-Iturriaga, A.; Everaerts, W.; Otte, F.; Papachristofilou, A.; et al. PEACE V—Salvage Treatment of OligoRecurrent nodal prostate cancer Metastases (STORM): A study protocol for a randomized controlled phase II trial. BMC Cancer 2020, 20, 406. [Google Scholar] [CrossRef]
- Ploussard, G.; Gandaglia, G.; Borgmann, H.; de Visschere, P.; Heidegger, I.; Kretschmer, A.; Mathieu, R.; Surcel, C.; Tilki, D.; Tsaur, I.; et al. Salvage Lymph Node Dissection for Nodal Recurrent Prostate Cancer: A Systematic Review. Eur. Urol. 2019, 76, 493–504. [Google Scholar] [CrossRef]
- Maurer, T.; Robu, S.; Schottelius, M.; Schwamborn, K.; Rauscher, I.; van den Berg, N.S.; van Leeuwen, F.W.B.; Haller, B.; Horn, T.; Heck, M.M.; et al. 99mTechnetium-based Prostate-specific Membrane Antigen-radioguided Surgery in Recurrent Prostate Cancer. Eur. Urol. 2019, 75, 659–666. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, F.W.B.; van Oosterom, M.N.; Meershoek, P.; van Leeuwen, P.J.; Berliner, C.; van der Poel, H.G.; Graefen, M.; Maurer, T. Minimal-Invasive Robot-Assisted Image-Guided Resection of Prostate-Specific Membrane Antigen–Positive Lymph Nodes in Recurrent Prostate Cancer. Clin. Nucl. Med. 2019, 44, 580–581. [Google Scholar] [CrossRef] [PubMed]
- Harke, N.N.; Godes, M.; Wagner, C.; Addali, M.; Fangmeyer, B.; Urbanova, K.; Hadaschik, B.; Witt, J.H. Fluorescence-supported lymphography and extended pelvic lymph node dissection in robot-assisted radical prostatectomy: A prospective, randomized trial. World J. Urol. 2018, 36, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Claps, F.; Ramírez-Backhaus, M.; Maresma, M.C.M.; Gómez-Ferrer, Á.; Mascarós, J.M.; Marenco, J.; Serra, A.C.; Ramón-Borja, J.C.; Fons, A.C.; Trombetta, C.; et al. Indocyanine green guidance improves the efficiency of extended pelvic lymph node dissection during laparoscopic radical prostatectomy. Int. J. Urol. 2021, 28, 566–572. [Google Scholar] [CrossRef]
- Opoka, L.; Kunikowska, J.; Podgajny, Z.; Szołkowska, M.; Błasińska-Przerwa, K.; Burakowska, B.; Oniszh, K.; Rudziński, P.; Bestry, I.; Roszkowski-Śliż, K. Accuracy of FDG PET/CT in the evaluation of solitary pulmonary lesions—Own experience. Pneumonologia i Alergologia Polska 2014, 82, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Beheshti, M.; Manafi-Farid, R.; Geinitz, H.; Vali, R.; Loidl, W.; Mottaghy, F.M.; Langsteger, W. Multiphasic 68Ga-PSMA PET/CT in the Detection of Early Recurrence in Prostate Cancer Patients with a PSA Level of Less Than 1 ng/mL: A Prospective Study of 135 Patients. J. Nucl. Med. 2020, 61, 1484–1490. [Google Scholar] [CrossRef]
- Hoffmann, M.A.; Buchholz, H.-G.; Wieler, H.J.; Rosar, F.; Miederer, M.; Fischer, N.; Schreckenberger, M. Dual-Time Point [68Ga]Ga-PSMA-11 PET/CT Hybrid Imaging for Staging and Restaging of Prostate Cancer. Cancers 2020, 12, 2788. [Google Scholar] [CrossRef]
- Derlin, T.; Weiberg, D.; Von Klot, C.; Wester, H.-J.; Henkenberens, C.; Ross, T.; Christiansen, H.; Merseburger, A.S.; Bengel, F. 68Ga-PSMA I&T PET/CT for assessment of prostate cancer: Evaluation of image quality after forced diuresis and delayed imaging. Eur. Radiol. 2016, 26, 4345–4353. [Google Scholar] [CrossRef]
- Schmuck, S.; Mamach, M.; Wilke, F.; von Klot, C.A.; Henkenberens, C.; Thackeray, J.T.; Sohns, J.M.; Geworski, L.; Ross, T.L.; Wester, H.-J.; et al. Multiple Time-Point 68Ga-PSMA I&T PET/CT for Characterization of Primary Prostate Cancer: Value of Early Dynamic and Delayed Imaging. Clin. Nucl. Med. 2017, 42, e286–e293. [Google Scholar]
- Afshar-Oromieh, A.; Sattler, L.P.; Mier, W.; Hadaschik, B.A.; Debus, J.; Holland-Letz, T.; Kopka, K.; Haberkorn, U. The Clinical Impact of Additional Late PET/CT Imaging with 68Ga-PSMA-11 (HBED-CC) in the Diagnosis of Prostate Cancer. J. Nucl. Med. 2017, 58, 750–755. [Google Scholar] [CrossRef] [Green Version]
- Alberts, I.; Sachpekidis, C.; Dijkstra, L.; Prenosil, G.; Gourni, E.; Boxler, S.; Gross, T.; Thalmann, G.; Rahbar, K.; Rominger, A.; et al. The role of additional late PSMA-ligand PET/CT in the differentiation between lymph node metastases and ganglia. Eur. J. Pediatr. 2020, 47, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Zlatopolskiy, B.D.; Endepols, H.; Krapf, P.; Guliyev, M.; Urusova, E.A.; Richarz, R.; Hohberg, M.; Dietlein, M.; Drzezga, A.E.; Neumaier, B. Discovery of 18F-JK-PSMA-7, a PET Probe for the Detection of Small PSMA-Positive Lesions. J. Nucl. Med. 2019, 60, 817–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, A.; Lin, R.; Yu, J.; Zhang, F.; Zheng, Q.; Yuan, X.; Sun, Z.; Zhong, Z. The differential diagnostic value of dual-phase 18F-DCFPyL PET/CT in prostate carcinoma. Prostate Cancer Prostatic Dis. 2022, 1–8. [Google Scholar] [CrossRef]
| Characteristics | Parameters (%) |
|---|---|
| Number of patients | 108 |
| Age (y) | |
| Median | 68.5 |
| Range | 49–83 |
| Primary Gleason score | |
| 5 | 4 (4%) |
| 6 | 13 (12%) |
| 7 | 54 (50%) |
| 8 | 18 (17%) |
| 9 | 17 (16%) |
| 10 | 2 (2%) |
| Treatment | |
| Surgery | 40 (37%) |
| Surgery + RTx | 44 (41%) |
| RTx | 22 (20%) |
| HIFU | 2 (2%) |
| Local Recurrence (Per-Patient Detection Rate) | Number of Lesions (Lesion-Based Detection Rate) | False Positive (Per-Patient Detection Rate) | |
|---|---|---|---|
| 60 min | 27 | 31 | 1 |
| 120 min | 28 | 32 | 0 |
| 60 + 120 | 28 | 33 | 1 |
| Gleason 5 (n = 4) | Gleason 6 (n = 4) | Gleason 7 (n = 13) | Gleason 8 (n = 9) | Gleason 9 (n = 3) | |
|---|---|---|---|---|---|
| SUVmax60 | 6.3 | 5.5 | 6.4 | 9.1 | 7.9 |
| SUVmax120 | 5.8 | 8.6 | 7.2 | 13.5 | 10.6 |
| p | ns | ns | 0.0024 | 0.0092 | ns |
| PSA > 0.2 to <0.5 ng/mL (n = 2) | PSA 0.5 to <1 ng/mL (n = 3) | PSA 1.0 to <2.0 ng/mL (n = 8) | PSA ≥ 2 ng/mL (n = 20) | |
|---|---|---|---|---|
| SUVmax60 | 5.1 | 3.2 | 8.0 | 7.3 |
| SUVmax120 | 8.0 | 5.8 | 10.2 | 8.7 |
| p | ns | ns | 0.042 | 0.0038 |
| Prostatectomy (n = 12) | RTx (n = 17) | Prostatectomy + RTx (n = 4) | |
|---|---|---|---|
| SUVmax60 | 7.8 | 6.3 | 7.6 |
| SUVmax120 | 8.6 | 8.6 | 9.6 |
| p | 0.0096 | 0.0075 | ns |
| Gleason 6 (n = 4) | Gleason 7 (n = 71) | Gleason 8 (n = 25) | Gleason 9 (n = 16) | Gleason 10 (n = 3) | |
|---|---|---|---|---|---|
| SUVmax60 | 13.9 | 5.5 | 5.6 | 3.8 | 4.8 |
| SUVmax120 | 22.0 | 7.5 | 13.8 | 5.4 | 3.9 |
| p | ns | <0.000001 | 0.00002 | 0.00085 | ns |
| PSA > 0.2 to <0.5 ng/mL (n = 6) | PSA 0.5 to <1 ng/mL (n = 16) | PSA 1.0 to <2.0 ng/mL (n = 23) | PSA ≥ 2 ng/mL (n = 74) | |
|---|---|---|---|---|
| SUVmax60 | 4.7 | 5.0 | 3.9 | 6.0 |
| SUVmax120 | 7.8 | 6.4 | 6.7 | 9.5 |
| p | ns | 0.0015 | 0.00017 | <0.000001 |
| Gleason 6 (n = 8) | Gleason 7 (n = 12) | Gleason 8 (n = 7) | Gleason 9 (n = 3) | |
|---|---|---|---|---|
| SUVmax60 | 7.2 | 5.7 | 12.9 | 4.8 |
| SUVmax120 | 8.0 | 6.8 | 17.1 | 10.9 |
| p | ns | 0.037 | 0.022 | ns |
| PSA > 0.2 to <0.5 ng/mL (n = 2) | PSA 0.5 to <1 ng/mL (n = 4) | PSA 1.0 to <2.0 ng/mL (n = 3) | PSA ≥ 2 ng/mL (n = 21) | |
|---|---|---|---|---|
| SUVmax60 | 4.9 | 2.8 | 12.8 | 7.4 |
| SUVmax120 | 6.5 | 5.9 | 18.2 | 9.7 |
| p | ns | ns | ns | 0.0038 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunikowska, J.; Pełka, K.; Tayara, O.; Królicki, L. Ga-68-PSMA-11 PET/CT in Patients with Biochemical Recurrence of Prostate Cancer after Primary Treatment with Curative Intent—Impact of Delayed Imaging. J. Clin. Med. 2022, 11, 3311. https://doi.org/10.3390/jcm11123311
Kunikowska J, Pełka K, Tayara O, Królicki L. Ga-68-PSMA-11 PET/CT in Patients with Biochemical Recurrence of Prostate Cancer after Primary Treatment with Curative Intent—Impact of Delayed Imaging. Journal of Clinical Medicine. 2022; 11(12):3311. https://doi.org/10.3390/jcm11123311
Chicago/Turabian StyleKunikowska, Jolanta, Kacper Pełka, Omar Tayara, and Leszek Królicki. 2022. "Ga-68-PSMA-11 PET/CT in Patients with Biochemical Recurrence of Prostate Cancer after Primary Treatment with Curative Intent—Impact of Delayed Imaging" Journal of Clinical Medicine 11, no. 12: 3311. https://doi.org/10.3390/jcm11123311
APA StyleKunikowska, J., Pełka, K., Tayara, O., & Królicki, L. (2022). Ga-68-PSMA-11 PET/CT in Patients with Biochemical Recurrence of Prostate Cancer after Primary Treatment with Curative Intent—Impact of Delayed Imaging. Journal of Clinical Medicine, 11(12), 3311. https://doi.org/10.3390/jcm11123311






