Human Amniotic Suspension Allograft Improves Pain and Function in Knee Osteoarthritis: A Prospective Not Randomized Clinical Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Amniotic Suspension Allograft Preparation
2.2. Statistical Analysis
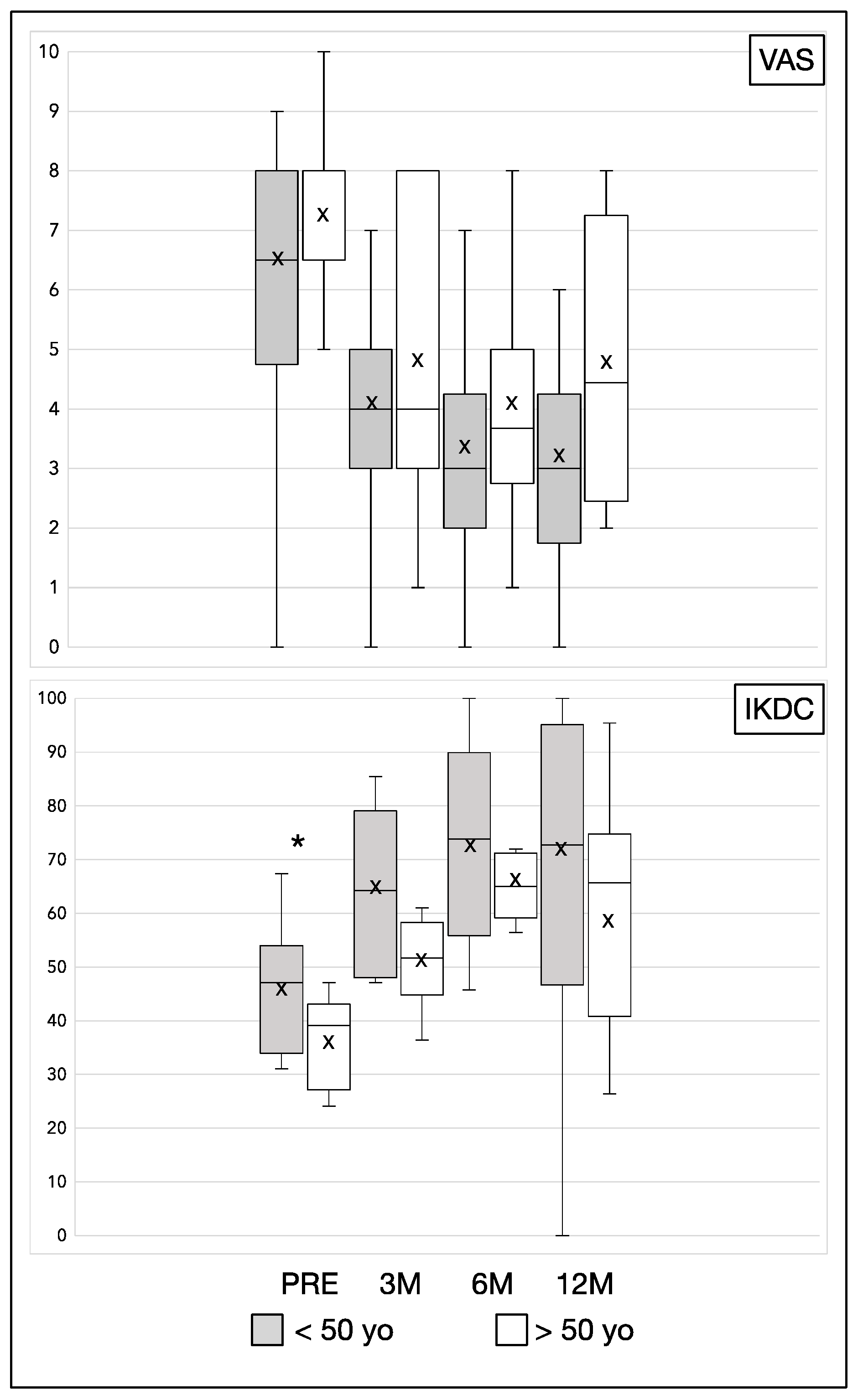
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sellam, J.; Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farinelli, L.; Aquili, A.; Mattioli-Belmonte, M.; Manzotti, S.; D’Angelo, F.; Ciccullo, C.; Gigante, A. Synovial mast cells from knee and hip osteoarthritis: Histological study and clinical correlations. J. Exp. Orthop. 2022, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Arden, N.K.; Perry, T.A.; Bannuru, R.R.; Bruyère, O.; Cooper, C.; Haugen, I.K.; Hochberg, M.C.; McAlindon, T.E.; Mobasheri, A.; Reginster, J.-Y. Non-surgical management of knee osteoarthritis: Comparison of ESCEO and OARSI 2019 guidelines. Nat. Rev. Rheumatol. 2021, 17, 59–66. [Google Scholar] [CrossRef]
- Caplan, A.I.; Correa, D. The MSC: An injury drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Murray, I.R.; Corselli, M.; Petrigliano, F.A.; Soo, C.; Péault, B. Recent insights into the identity of mesenchymal stem cells: Implications for orthopaedic applications. Bone Jt. J. 2014, 96B, 291–298. [Google Scholar] [CrossRef]
- De Francesco, F.; Gravina, P.; Busato, A.; Farinelli, L.; Soranzo, C.; Vidal, L.; Zingaretti, N.; Zavan, B.; Sbarbati, A.; Riccio, M.; et al. Stem cells in autologous microfragmented adipose: Current perspectives in osteoarthritis disease. Int. J. Mol. Sci. 2021, 22, 10197. [Google Scholar] [CrossRef]
- Screpis, D.; Natali, S.; Farinelli, L.; Piovan, G.; Iacono, V.; de Girolamo, L.; Viganò, M.; Zorzi, C. Autologous Microfragmented Adipose Tissue for the Treatment of Knee Osteoarthritis: Real-World Data at Two Years Follow-Up. J. Clin. Med. 2022, 11, 1268. [Google Scholar] [CrossRef]
- Senesi, L.; De Francesco, F.; Farinelli, L.; Manzotti, S.; Gagliardi, G.; Papalia, G.F.; Riccio, M.; Gigante, A. Mechanical and enzymatic procedures to isolate the stromal vascular fraction from adipose tissue: Preliminary results. Front. Cell Dev. Biol. 2019, 7, 88. [Google Scholar] [CrossRef]
- Natali, S.; Screpis, D.; Farinelli, L.; Iacono, V.; Vacca, V.; Gigante, A.; Zorzi, C. The use of intra-articular injection of autologous micro-fragmented adipose tissue as pain treatment for ankle osteoarthritis: A prospective not randomized clinical study. Int. Orthop. 2021, 45, 2239–2244. [Google Scholar] [CrossRef]
- Friel, N.A.; de Girolamo, L.; Gomoll, A.H.; Mowry, K.C.; Vines, J.B.; Farr, J. Amniotic Fluid, Cells, and Membrane Application. Oper. Tech. Sports Med. 2017, 25, 20–24. [Google Scholar] [CrossRef]
- Le Blanc, K.; Tammik, C.; Rosendahl, K.; Zetterberg, E.; Ringdén, O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003, 31, 890–896. [Google Scholar] [CrossRef]
- Sawhney, C.P. Amniotic membrane as a biological dressing in the management of burns. Burns 1989, 15, 339–342. [Google Scholar] [CrossRef]
- Mermet, I.; Pottier, N.; Sainthillier, J.M.; Malugani, C.; Cairey-Remonnay, S.; Maddens, S.; Riethmuller, D.; Tiberghien, P.; Humbert, P.; Aubin, F. Use of amniotic membrane transplantation in the treatment of venous leg ulcers. Wound Repair Regen. 2007, 15, 459–464. [Google Scholar] [CrossRef]
- McIntyre, J.A.; Jones, I.A.; Danilkovich, A.; Vangsness, C.T.J. The Placenta: Applications in Orthopaedic Sports Medicine. Am. J. Sports Med. 2018, 46, 234–247. [Google Scholar] [CrossRef]
- McQuilling, J.P.; Vines, J.B.; Kimmerling, K.A.; Mowry, K.C. Proteomic Comparison of Amnion and Chorion and Evaluation of the Effects of Processing on Placental Membranes. Wounds Compend. Clin. Res. Pract. 2017, 29, E36–E40. [Google Scholar]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [Green Version]
- Montagner, G.; Trojan, D.; Cogliati, E.; Manea, F.; Vantini, A.; Paolin, A. Stability analysis of the antibiotic cocktail used by Treviso Tissue Bank Foundation for tissues decontamination. Cell Tissue Bank. 2018, 19, 721–726. [Google Scholar] [CrossRef]
- Serafini, A.; Riello, E.; Trojan, D.; Cogliati, E.; Palù, G.; Manganelli, R.; Paolin, A. Evaluation of new antibiotic cocktails against contaminating bacteria found in allograft tissues. Cell Tissue Bank. 2016, 17, 619–628. [Google Scholar] [CrossRef] [Green Version]
- Vines, J.B.; Aliprantis, A.O.; Gomoll, A.H.; Farr, J. Cryopreserved Amniotic Suspension for the Treatment of Knee Osteoarthritis. J. Knee Surg. 2016, 29, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Farr, J.; Gomoll, A.H.; Yanke, A.B.; Strauss, E.J.; Mowry, K.C. A Randomized Controlled Single-Blind Study Demonstrating Superiority of Amniotic Suspension Allograft Injection Over Hyaluronic Acid and Saline Control for Modification of Knee Osteoarthritis Symptoms. J. Knee Surg. 2019, 32, 1143–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomoll, A.H.; Farr, J.; Cole, B.J.; Flanigan, D.C.; Lattermann, C.; Mandelbaum, B.R.; Strickland, S.M.; Zaslav, K.R.; Kimmerling, K.A.; Mowry, K.C. Safety and Efficacy of an Amniotic Suspension Allograft Injection Over 12 Months in a Single-Blinded, Randomized Controlled Trial for Symptomatic Osteoarthritis of the Knee. Arthrosc. J. Arthrosc. Relat. Surg. 2021, 37, 2246–2257. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, R.; Tighe, S. Injectable Amniotic Membrane/Umbilical Cord Particulate for Knee Osteoarthritis: A Prospective, Single-Center Pilot Study. Pain Med. 2019, 20, 2283–2291. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Guermazi, A.; Lo, G.H.; Grainger, A.J.; Conaghan, P.G.; Boudreau, R.M.; Roemer, F.W. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthr. Cartil. 2011, 19, 990–1002. [Google Scholar] [CrossRef] [Green Version]
- Adinolfi, M.; Akle, C.A.; McColl, I.; Fensom, A.H.; Tansley, L.; Connolly, P.; Hsi, B.L.; Faulk, W.P.; Travers, P.; Bodmer, W.F. Expression of HLA antigens, beta 2-microglobulin and enzymes by human amniotic epithelial cells. Nature 1982, 295, 325–327. [Google Scholar] [CrossRef]
- Akle, C.A.; Adinolfi, M.; Welsh, K.I.; Leibowitz, S. McColl, I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet 1981, 2, 1003–1005. [Google Scholar] [CrossRef]
- Strong, D.M.; Friedlaender, G.E.; Tomford, W.W.; Springfield, D.S.; Shives, T.C.; Burchardt, H.; Enneking, W.F.; Mankin, H.J. Immunologic responses in human recipients of osseous and osteochondral allografts. Clin. Orthop. Relat. Res. 1996, 326, 107–114. [Google Scholar] [CrossRef]
- Beggs, K.J.; Lyubimov, A.; Borneman, J.N.; Bartholomew, A.; Moseley, A.; Dodds, R.; Archambault, M.P.; Smith, A.K.; McIntosh, K.R. Immunologic consequences of multiple, high-dose administration of allogeneic mesenchymal stem cells to baboons. Cell Transplant. 2006, 15, 711–721. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age > 18 years old | Systemic cardiovascular and coagulation disorders or anticoagulant therapy |
| Degenerative joint changes (KL 1–3) | Degenerative joint changes (KL 4)/surgical MRI findings |
| Failure of previous conservative treatment (antiinflammatory, physical therapy, intra-articular steroid, viscosupplementation and platelet-rich plasma) | Intra-articular steroid or viscosupplementation injections performed within the last three months. |
| History of chronic (≥4 months) pain or knee swelling with limitation of daily activities | Rheumatic diseases and septic knee arthritis |
| Characteristics | Knee OA (N = 25) |
|---|---|
| Age (years) [Range] | 45.09 (15.31) [22–76] |
| Sex (F/M) | 14/11 |
| Body-Mass-Index (BMI), mean (SD) [range] | 22.86 (3.71) [17.9–36.5] |
| Employment status (worker/retiree) | 18/7 |
| Smoker, ex-smoker or non-smoker | 5/16/4 |
| Previous surgery on affected knee (Meniscus surgery, osteotomy, cartilage procedure, arthroscopic debridement) | 9/16 |
| Radiographic stage (Kellgren Lawrence) | |
| Grade I | 5 |
| Grade II | 15 |
| Grade III | 5 |
| Disease duration, years (SD) | 4.4 (2.3) |
| Variable | Follow Up | Values | z Value | p | |
|---|---|---|---|---|---|
| IKDC Mean (SD) | PRE | 42.47 (11.44) | PRE versus 3 MO | −2.65 | 0.008 |
| 3 MO | 61.07 (15.3) | PRE versus 6 MO | −4.01 | 0.000 | |
| 6 MO | 71.97 (16.85) | PRE versus 12 MO | −3.18 | 0.001 | |
| 12 MO | 66.80 (25.43) | 3 MO versus 6 MO | −3.47 | 0.000 | |
| 6 MO versus 12 MO | −1.68 | 0.93 | |||
| VAS Mean (SD) | PRE | 6.95 (1.70) | PRE versus 3 MO | −3.52 | 0.000 |
| 3 MO | 4.45 (1.95) | PRE versus 6 MO | −3.82 | 0.000 | |
| 6 MO | 3.67 (1.74) | PRE versus 12 MO | −3.41 | 0.000 | |
| 12 MO | 3.90 (2.19) | 3 MO versus 6 MO | −2.70 | 0.007 | |
| 6 MO versus 12 MO | −0.53 | 0.60 |
| Variable | Follow up | ≤50 Years Old (N = 13) | >50 Years Old (N = 12) | p |
|---|---|---|---|---|
| IKDC Mean (SD) | PRE | 46.76 (11.59) | 36.28 (8.31) | 0.03 |
| 3 MO | 66.22 (13.80) | 52.7 (14.53) | 0.07 | |
| 6 MO | 74.7 (19.01) | 67.54 (12.46) | 0.48 | |
| 12 MO | 72.1 (26.11) | 58.2 (23.30) | 0.22 | |
| VAS Mean (SD) | PRE | 6.62 (1.61) | 7.44 (1.81) | 0.22 |
| 3 MO | 4.15 (1.34) | 4.89 (2.62) | 0.49 | |
| 6 MO | 3.46 (1.45) | 4.00 (2.20) | 0.44 | |
| 12 MO | 3.31 (1.55) | 4.88 (2.80) | 0.23 |
| Variable | Follow up | Previous Knee Surgery (N = 10) | Not Previous Knee Surgery (N = 15) | p |
|---|---|---|---|---|
| IKDC Mean (SD) | PRE | 38.83 (8.55) | 43.83 (12.82) | 0.36 |
| 3 MO | 63.87 (16.11) | 58.1 (14.68) | 0.65 | |
| 6 MO | 73.54 (16.10) | 68.97 (16.33) | 0.81 | |
| 12 MO | 69.53 (23.49) | 62.78 (26.24) | 0.71 | |
| VAS Mean (SD) | PRE | 7.13 (1.81) | 6.86 (1.70) | 0.91 |
| 3 MO | 4.00 (1.51) | 4.71 (2.16) | 0.38 | |
| 6 MO | 3.29 (1.70) | 3.86 (1.79) | 0.42 | |
| 12 MO | 3.00 (1.41) | 4.36 (2.41) | 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natali, S.; Farinelli, L.; Screpis, D.; Trojan, D.; Montagner, G.; Favaretto, F.; Zorzi, C. Human Amniotic Suspension Allograft Improves Pain and Function in Knee Osteoarthritis: A Prospective Not Randomized Clinical Pilot Study. J. Clin. Med. 2022, 11, 3295. https://doi.org/10.3390/jcm11123295
Natali S, Farinelli L, Screpis D, Trojan D, Montagner G, Favaretto F, Zorzi C. Human Amniotic Suspension Allograft Improves Pain and Function in Knee Osteoarthritis: A Prospective Not Randomized Clinical Pilot Study. Journal of Clinical Medicine. 2022; 11(12):3295. https://doi.org/10.3390/jcm11123295
Chicago/Turabian StyleNatali, Simone, Luca Farinelli, Daniele Screpis, Diletta Trojan, Giulia Montagner, Francesca Favaretto, and Claudio Zorzi. 2022. "Human Amniotic Suspension Allograft Improves Pain and Function in Knee Osteoarthritis: A Prospective Not Randomized Clinical Pilot Study" Journal of Clinical Medicine 11, no. 12: 3295. https://doi.org/10.3390/jcm11123295
APA StyleNatali, S., Farinelli, L., Screpis, D., Trojan, D., Montagner, G., Favaretto, F., & Zorzi, C. (2022). Human Amniotic Suspension Allograft Improves Pain and Function in Knee Osteoarthritis: A Prospective Not Randomized Clinical Pilot Study. Journal of Clinical Medicine, 11(12), 3295. https://doi.org/10.3390/jcm11123295






