Abstract
Ischemic and hemorrhagic complications are major determinants of survival in acute coronary syndrome (ACS) patients undergoing coronary surgery. We investigated the association of preoperative platelet reactivity to P2Y12 antagonists with ischemic and hemorrhagic complications after Off-Pump Coronary Artery Bypass surgery (OPCAB) in ACS patients who received dual anti-platelet therapy (DAPT) within 5 days prior to surgery. This prospective, observational study with 177 patients compared the incidence of perioperative major bleeding and major adverse cardiac events (MACEs) in relation to the tertile distribution of the % inhibitory response to P2Y12 antagonists, as measured by a thromboelastography platelet mapping assay. The incidences of perioperative major bleeding and MACEs were similar in relation to the tertile distribution of inhibitory response to P2Y12 antagonists. The % inhibitory responses to P2Y12 antagonists between patients who did or did not exhibit MACEs, and with or without major bleeding, were 58 ± 20% and 56 ± 20% (p = 0.578) and 57 ± 19% and 56 ± 21% (p = 0.923), respectively. In ACS patients who received DAPT close to OPCAB, the platelet inhibitory response to P2Y12 antagonists was not associated with ischemic or hemorrhagic complications. OPCAB may obviate the need for routine platelet function testing for ACS patients requiring DAPT and surgical revascularization. Clinical Registration Number: NCT02184884.
1. Introduction
Dual anti-platelet therapy (DAPT) has been used for prophylaxis against recurrent ischemic attacks in acute coronary syndromes (ACS) [1]. This challenges the guidelines recommending P2Y12 antagonist discontinuation before surgery due to heightened bleeding risks [2]. Both ischemic and bleeding complications are major determinants of survival in ACS patients undergoing coronary bypass graft surgery (CABG) [3]. Unfortunately, the ischemic and hemorrhagic responses to DAPT vary among individuals and cannot be predicted by clinical risk factors alone.
Platelet function tests play a valuable role in predicting clinical outcomes in ACS patients undergoing percutaneous coronary intervention (PCI) [4]. A collaborative analysis involving 20,839 PCI patients reported that there may be an optimal therapeutic window of P2Y12 inhibition, within which the anticipated risks of ischemic and bleeding complications are the lowest [5].
Thus, we hypothesized that a similar optimal therapeutic window is applicable to ACS patients undergoing CABG, providing a safe perioperative milieu with regard to ischemic and bleeding complications, and eliminating the need for unnecessary delay from DAPT interruption. However, evidence regarding the role of platelet function tests in CABG is scarce and is mostly limited to its negative predictive value, indicating that surgery may proceed when there is low platelet inhibition [6,7]. Such evidence is even more scarce in ACS patients. For patients on DAPT, off-pump CABG (OPCAB) has been suggested as a rational choice for surgical revascularization, as it evades cardiopulmonary bypass (CPB)-related coagulopathy [8].
This prospective, observational study aimed to investigate the association of preoperative platelet reactivity to adenosine diphosphate (ADP), as assessed by thromboelastography platelet mapping, with ischemic and bleeding complications in ACS patients who received DAPT within 5 days prior to surgery.
2. Materials and Methods
2.1. Patients
A total of 205 patients who underwent multivessel OPCAB between June 2014 and December 2019 and who were taking a daily oral regimen of aspirin 100 mg and clopidogrel 75 mg (or 90 mg of ticagrelor twice daily) for at least 1 week were assessed for eligibility. Patients who discontinued DAPT for more than 5 days pre-surgery (3 days for ticagrelor) were excluded. Exclusion criteria included emergency surgery, bleeding diathesis or hepatic dysfunction, left ventricular ejection fraction < 40%, hemoglobin < 11 g/dL, platelet count < 100,000/μL, creatinine > 1.4 mg/dL, and glycoprotein IIb/IIIa inhibitor or anticoagulants use. Patients with poor graft patency, as confirmed intraoperatively using a transit time flow measurement after completion of grafting, were also excluded. We finally included 177 patients. (Figure 1).
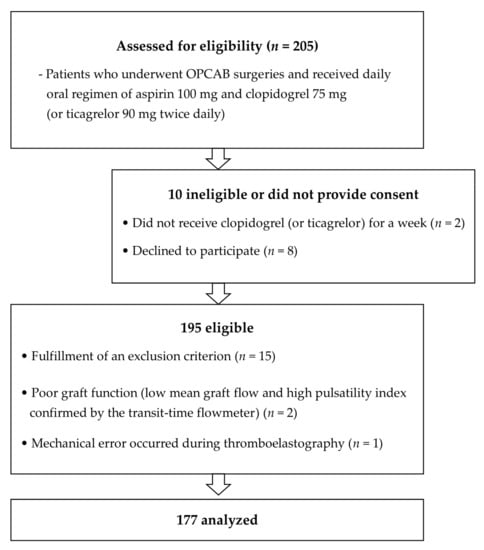
Figure 1.
Patient enrolment into the study. The figure illustrates the inclusion and exclusion criteria applied in the study and the resulting number of patients that were finally analyzed. OPCAB, off-pump coronary surgery.
2.2. Primary Endpoint
The primary endpoint was a comparison of the incidence of perioperative major adverse cardiovascular events (MACEs) and major bleeding in relation to the tertile distribution of the % platelet inhibitory response to P2Y12 antagonists.
A thromboelastography platelet mapping assay was used to measure the platelet reactivity. Major bleeding was defined as class ≥2 (moderate) universal definition of perioperative bleeding (UDPB), which is as follows: delayed sternal closure, postoperative bleeding >800 mL within 12 h, transfusion of ≥2 units of packed erythrocytes (pRBCs), fresh frozen plasma (FFP), platelets or cryoprecipitate transfusion, use of prothrombin complex concentrates or recombinant activated factor VII, or the need for re-exploration [9]. MACEs were defined as in-hospital cardiac death, myocardial infarction, or need for revascularization. Postoperative myocardial infarction was defined according to the universal definition [10]. Troponin-T was evaluated 1-day pre-surgery and at 24-h and 48-h post-surgery.
2.3. Secondary Endpoints
MACEs and major bleeding risk factors, including the % platelet inhibitory response to antiplatelet agents, were evaluated. Additionally, we evaluated transfusion requirements and other major morbidity endpoints. These endpoints included postoperative stroke, acute kidney injury, prolonged ventilation >24 h, deep sternal wound infection, any cardiac reoperation, and pulmonary thromboembolism. Acute kidney injury was diagnosed according to the Kidney Disease: Improving Global Outcomes criteria [11]. Computerized tomography coronary angiography was performed within 2 weeks post-CABG to assess the graft patency and pulmonary thromboembolism occurrence. Other complications were assessed according to the Society of Thoracic Surgeons’ morbidity definition. (https://www.sts.org/quality-safety/performance-measures/descriptions, accessed on 6 April 2014)
2.4. Data Collection
Blood sampling and a thromboelastography® Platelet Mapping™ assay (Haemoscope Corp., Niles, IL, USA) were performed immediately before anesthesia induction [6] by an anesthesiologist not involved in the patient management (S.S). The surgeons and anesthesiologists involved in patient management were blinded to the results.
The perioperative data assessed were patient characteristics, operation time, need for proximal aortocoronary anastomosis, the number of grafts placed, blood loss within the first 24 h post-surgery (intra- and post-operative blood loss), and transfusion requirements for pRBCs, FFP, and platelets during the same period. Perioperative laboratory data, including hemoglobin, platelet counts, prothrombin time, activated partial thromboplastin time, and troponin T were evaluated. The lengths of hospital and intensive care unit stays were also recorded.
2.5. Clinical Management
Institutional standard anesthetic and surgical management and intensive unit care were provided to all patients, as in our previous study [6]. Routine monitoring included pulmonary artery catheter and transesophageal echocardiography. Anesthesia was maintained with sevoflurane and sufentanil. All surgical procedures were performed via median sternotomy by two teams of cardiac surgeons, who each had more than 500 cases of OPCAB surgeries before the study. The target activated clotting time for systemic heparinization was 250–300 s. Proximal aortocoronary anastomosis was performed with the Heartstring device (Maquet Cardiovascular, San Jose, CA, USA). A cell-salvage device was used in all patients and intraoperative blood loss was estimated by the amount of blood collected by it. Salvaged blood was transfused to the patient before leaving the operating theatre. No patient received antifibrinolytics, and pRBCs were transfused at hemoglobin levels < 8 g/dL at the discretion of the attending physician.
When bleeding exceeded 200 mL/h for 2 consecutive hours post-surgery, FFP and/or platelets were transfused, in case of an international normalized ratio >1.3 and/or platelet counts < 50,000/μL. Reoperation was performed when bleeding exceeded 200 mL/h for ≥6 h or ≥400 mL for the first 1 h. In laboratory tests, if there was an increase in the international normalized ratio or a decrease in the platelet count that did not meet the transfusion criteria, and the bleeding exceeded 200 mL/h for 3 h, FFP or platelet transfusion was decided at the discretion of the attending physician. DAPT was restarted within 24 h post-surgery in the absence of major bleeding.
2.6. Statistical Analyses
SPSS v25.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. In patients with ACS, the incidence of perioperative MACEs was assumed to be 20% (unpublished institutional data). We determined that estimating a 10% dropout rate, 195 patients would be required to detect a two-fold increase in the incidence of MACEs in the first tertile of P2Y12 receptor antagonist responsiveness (showing the lowest percentage of platelet inhibition) at α = 0.05 and β = 0.8.
Data are shown as mean (SD), median (IQR), or number of patients (percentage). Normality was assessed using the Shapiro–Wilk test. Continuous variables were compared among the tertiles by one-way analysis of variance with Bonferroni post-hoc tests. Continuous variables were compared between patients with and without postoperative MACEs or major bleeding (UDPB ≥ class 2) with the independent t-test, or Mann–Whitney U-test. Proportions were compared using Fisher’s exact test or χ2 test. Multivariable logistic regression analysis was used to determine if the mean % platelet inhibitory response to P2Y12 antagonists or aspirin was an independent risk factor for major bleeding. Known risk factors of UDPB (EuroSCORE, preoperative hemoglobin, and number of grafts (instead of CPB duration)) [9] and the mean % platelet inhibitory response to P2Y12 antagonists or aspirin were included in the multivariable analysis. Odds ratios (OR) and associated 95% confidence intervals were calculated. p < 0.05 was considered statistically significant.
3. Results
OPCAB was completed in all 177 patients (95% of patients received clopidogrel, 5% received ticagrelor). Of the patients in the study, 29 (16%) were diagnosed with non-ST-elevation myocardial infarction preoperatively, and the rest were unstable angina. Among them, 40% and 75% of patients took P2Y12 antagonists and aspirin, respectively, until the day before surgery. Patients’ characteristics, including the number of patients who stopped P2Y12 antagonists and/or aspirin 1 day before surgery, were all similar in terms of tertile distributions of % platelet inhibitory response to P2Y12 antagonists, except for the % platelet inhibitory response to aspirin (Supplementary Table S1). The mean % platelet inhibitory responses to ADP and arachidonic acid were 56% (20%) and 73% (21%), respectively.
The perioperative blood loss and transfusion requirements were all similar according to the tertile distribution of the % platelet inhibitory response to P2Y12 antagonists, except for the platelet concentrate transfusion (Table 1). Significantly more patients in the third tertile required platelet concentrate transfusion.

Table 1.
Perioperative data in relation to tertile distribution of percentage of platelet inhibitory response to adenosine diphosphate.
The MACEs and major bleeding incidences were 17% and 40%, respectively. The incidence of MACEs and other morbidity endpoints was similar among the tertiles, with the exception of acute kidney injury, which was significantly higher in patients in the second tertile than in those in the first and third tertiles (Table 2). In linear regression analysis, the % platelet inhibitory response to P2Y12 antagonists before OPCAB and peak troponin-T values for 48 h postoperatively showed no significant correlation (R2 = 0.003, p = 0.5).

Table 2.
Changes in troponin T values and postoperative outcomes.
None of the thromboelastography parameters, including the mean % platelet inhibitory response to P2Y12 antagonists, were associated with MACEs or major bleeding (UDPB ≥ class 2) (Table 3). The % platelet inhibitory response to P2Y12 antagonists and aspirin showed no correlation with the amount of postoperative bleeding within 12 h (R = 0.06 and 0.115, p = 0.43 and 0.13, respectively) or 24 h (R = 0.05 and 0.123, p = 0.5 and 0.11, respectively). Even when a partial correlation analysis was used to adjust the confounding effect of platelet transfusion, there was no significant correlation between the % platelet inhibitory response to antiplatelet therapy (P2Y12 antagonists and aspirin) and the amount of postoperative bleeding within 12 h (R = 0.03 and 0.075, p = 0.69 and 0.33, respectively) or 24 h (R = 0.033 and 0.1, p = 0.66 and 0.19, respectively).

Table 3.
Preoperative thromboelastography parameters.
Logistic regression analysis to identify major bleeding risk factors concomitantly analyzed the previously cited risk factors and the variable of interest, % inhibitory response to P2Y12 receptor antagonists. However, none of these variables remained as independent risk factors in the multivariable analysis (Table 4). Compared with a previous study [6], the results did not change even when the % inhibitory response to P2Y12 antagonist was added as a dichotomous variable with a cut-off value of 70% (Supplementary Table S2).

Table 4.
Predictive power of chosen variables.
4. Discussion
This prospective, observational study demonstrated that in ACS patients with recent exposure to DAPT pre-OPCAB, the % platelet inhibitory response to P2Y12 antagonists was not associated with increased incidence of perioperative ischemic or hemorrhagic complications.
The existence of on-treatment response variability as well as variability in platelet function recovery after discontinuation of P2Y12 antagonists stands against the uniform cessation-protocol and suggests the need for objective measurement of platelet inhibitory response to P2Y12 antagonists [4]. Recent studies have suggested the existence of a therapeutic window of platelet reactivity in patients undergoing PCI treated with P2Y12 antagonists where maximum ischemic benefits can be achieved while avoiding excessive bleeding [4]. Such therapeutic windows would be of particular clinical relevance to ACS patients undergoing CABG, as these patients stand at high risk of both ischemic and bleeding complications while confronting the need for discontinuation of P2Y12 inhibitors [12,13]. Studies of patients undergoing CABG showed that an individualized strategy for preoperative clopidogrel cessation, based on preoperative platelet function tests, could reduce both preoperative delay and bleeding diatheses [6,14]. Current guidelines issue Class IIa or IIb recommendations for preoperative platelet function tests [15,16]. However, most evidence stems from on-pump CABG requiring CPB, which inevitably accompanies hemodilution and consumptive coagulopathy.
In that context, OPCAB is an important alternative surgical technique to reduce hemorrhagic complications related to recent P2Y12 antagonist exposure [8,17]. OPCAB may allow surgical revascularization in ACS patients requiring DAPT and could provide ischemic protection without markedly increasing bleeding risk; however, no comprehensive evidence exists. Thus, we investigated the association between the degree of preoperative P2Y12 inhibition and the occurrence of bleeding as well as ischemic complications in ACS patients undergoing OPCAB.
Contrary to expectations, platelet inhibition levels measured using thromboelastography platelet mapping before OPCAB were not associated with the incidence of perioperative MACEs. Moreover, preoperative baseline troponin-T levels and serially assessed postoperative troponin-T levels did not correlate with the degree of platelet inhibition. Additionally, the incidence of postoperative stroke, pulmonary thromboembolism, and cardiac death was relatively low, and those events did not correlate with the degree of platelet inhibitory response to P2Y12 antagonists.
Cogent explanations for the observed results are as follows. DAPT maintenance for up to 5 days prior to OPCAB in all patients (82% of patients took P2Y12 antagonists within 3 days prior to surgery) might have exerted ischemic benefits, not only during the preoperative waiting period but also against the perioperative occurrence of MACEs. Such a benefit could have been strengthened further by the “East Asian paradox” [18], where rates of ischemic events are lower with clopidogrel use despite a higher rate of high on-treatment platelet reactivity.
Furthermore, unlike the accumulating evidence of an association between the extent of platelet inhibition and CABG-related bleeding [6,19,20], the degree of platelet inhibition in ACS patients undergoing OPCAB was not associated with postoperative major bleeding occurrence. Despite inclusion of only patients with ACS in this study, putative reasons for discrepancy are as follows. First, the increased bleeding risk in patients with ACS is attributable to the more aggressive DAPT, rather than the ACS per se [21]. Patients enrolled in the current study did not have major risk factors of bleeding other than receiving DAPT; only 9 patients (5%) met the criteria for severe (class 3) postoperative bleeding in the current study, as opposed to 8.2%, according to the UDPB [9], while none of the patients exhibited massive bleeding (class 4 UDPB: >2000 mL blood loss within 12 h). Additionally, all patients underwent OPCAB, which could have reduced the risk of bleeding by avoiding CPB. Even if the focus is on P2Y12 antagonists, the relatively lower efficacy of P2Y12 antagonists could have affected our results. In a previous study, using the same thromboelastography platelet mapping assay, patients exhibiting the highest % inhibitory response to clopidogrel (third tertile) had significantly more postoperative bleeding than those in the first and second tertiles [6]. The mean % inhibitory response to P2Y12 antagonists in the current study’s third tertile was comparatively lower (79% vs. 87%) [6]. Therefore, in patients at low risk of postoperative bleeding, other than those receiving DAPT, there seems to be no need to postpone OPCAB based on the degree of platelet inhibitory response to P2Y12 antagonists. Nonetheless, as the cause of bleeding after CABG is multifactorial, it is difficult to deduce the role of platelet function testing in patients with other major risk factors of bleeding.
Overall, our findings imply a ceiling-effect of ischemic protection against MACEs by DAPT at a low degree of P2Y12 receptor inhibition (in the presence of aspirin continuation, the mean percent inhibitory response to ADP and arachidonic acid were 56% and 73%, respectively), without inciting serious bleeding complications. Moreover, the current study adds clinical significance as the majority (82%) of patients received P2Y12 antagonists within 3 days prior to surgery (40% of patients until 1 day prior) and not 4 or 5 days prior. Thus, our results strongly imply ischemic and hemorrhagic benefits of OPCAB in ACS patients who received DAPT in proximity to surgery.
This study is subject to the following limitations. First, this is a single-center study of a certain ethnic background with specific inclusion criteria, which limits generalization of the observed results. Secondly, the physicians were not blinded to DAPT exposure, which may have confounded decisions regarding platelet transfusions and the subsequent amount of blood loss. However, as in a previous study [6], the distribution of discontinuation dates was similar in relation to tertile distributions of the % inhibitory response to ADP in this study, reflecting the inter-individual variability of the drug response when the discontinuation dates of the P2Y12 inhibitor were mostly within a close range of 1 and 3 days before surgery (Supplementary Table S1). In addition, the degree of P2Y12 inhibition showed no correlation with the amount of postoperative bleeding even after adjustment for platelet transfusion. Thirdly, discontinuation of P2Y12 antagonists before surgery was at the discretion of the attending cardiologists and surgeons.
In conclusion, in ACS patients who continued to receive DAPT up to 5 days prior to surgery, the degree of platelet inhibitory response to P2Y12 antagonists before OPCAB was not associated with perioperative ischemic or hemorrhagic complications. Considering the low incidences of serious ischemic and bleeding complications in the current study, our results imply that routine platelet function testing may not be necessary to identify the optimal timing of surgery for ACS patients requiring DAPT, whose risk of bleeding is not increased, when undergoing planned OPCAB.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11123285/s1, Table S1: Patient characteristics in relation to tertile distribution of the platelet inhibitory percentage response to adenosine diphosphate; Table S2: Predictive power of chosen variables.
Author Contributions
Conceptualization, S.S., Y.R.S. and J.-K.S.; Methodology, Y.-L.K.; Software, J.-W.S.; Validation, J.-W.S. and J.H.C.; Formal analysis, S.S., Y.R.S., J.-W.S. and J.-K.S.; Investigation, Y.R.S., J.H.C. and J.-K.S.; Data curation, S.S., Y.R.S., J.-W.S., J.H.C. and Y.-L.K.; Writing—original draft preparation, S.S.; Writing—Review and Editing, Y.R.S., Y.-L.K. and J.-K.S.; Supervision, J.-K.S.; Project Administration, S.S. and J.-K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Yonsei University Health System, Seoul, Republic of Korea (4-2014-0172; approval date: 2 May 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eisen, A.; Giugliano, R.P.; Braunwald, E. Updates on Acute Coronary Syndrome: A Review. JAMA Cardiol. 2016, 1, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, V.A.; Saha, S.P.; Oestreich, J.H.; Song, H.K.; Rosengart, T.; Reece, T.B.; Mazer, C.D.; Bridges, C.R.; Despotis, G.J.; Jointer, K.; et al. 2012 update to the Society of Thoracic Surgeons guideline on use of antiplatelet drugs in patients having cardiac and noncardiac operations. Ann. Thorac. Surg. 2012, 94, 1761–1781. [Google Scholar] [CrossRef] [PubMed]
- Morici, N.; Moja, L.; Rosato, V.; Oreglia, J.A.; Sacco, A.; de Marco, F.; Bruschi, G.; Klugmann, S.; La Vecchia, C.; Savonitto, S. Time from adenosine di-phosphate receptor antagonist discontinuation to coronary bypass surgery in patients with acute coronary syndrome: Meta-analysis and meta-regression. Int. J. Cardiol. 2013, 168, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Tantry, U.S.; Bonello, L.; Aradi, D.; Price, M.J.; Jeong, Y.H.; Angiolillo, D.J.; Stone, G.W.; Curzen, N.; Geisler, T.; Ten Berg, J.; et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J. Am. Coll. Cardiol. 2013, 62, 2261–2273. [Google Scholar] [CrossRef] [PubMed]
- Aradi, D.; Kirtane, A.; Bonello, L.; Gurbel, P.A.; Tantry, U.S.; Huber, K.; Freynhofer, M.K.; ten Berg, J.; Janssen, P.; Angiolillo, D.J.; et al. Bleeding and stent thrombosis on P2Y12-inhibitors: Collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur. Heart J. 2015, 36, 1762–1771. [Google Scholar] [CrossRef]
- Kwak, Y.L.; Kim, J.C.; Choi, Y.S.; Yoo, K.J.; Song, Y.; Shim, J.K. Clopidogrel responsiveness regardless of the discontinuation date predicts increased blood loss and transfusion requirement after off-pump coronary artery bypass graft surgery. J. Am. Coll. Cardiol. 2010, 56, 1994–2002. [Google Scholar] [CrossRef]
- Mannacio, V.; Meier, P.; Antignano, A.; Di Tommaso, L.; De Amicis, V.; Vosa, C. Individualized strategy for clopidogrel suspension in patients undergoing off-pump coronary surgery for acute coronary syndrome: A case-control study. J. Thorac. Cardiovasc. Surg. 2014, 148, 1299–1306. [Google Scholar] [CrossRef]
- Shim, J.K.; Choi, Y.S.; Oh, Y.J.; Bang, S.O.; Yoo, K.J.; Kwak, Y.L. Effects of preoperative aspirin and clopidogrel therapy on perioperative blood loss and blood transfusion requirements in patients undergoing off-pump coronary artery bypass graft surgery. J. Thorac. Cardiovasc. Surg. 2007, 134, 59–64. [Google Scholar] [CrossRef][Green Version]
- Dyke, C.; Aronson, S.; Dietrich, W.; Hofmann, A.; Karkouti, K.; Levi, M.; Murphy, G.J.; Sellke, F.W.; Shore-Lesserson, L.; von Heymann, C.; et al. Universal definition of perioperative bleeding in adult cardiac surgery. J. Thorac. Cardiovasc. Surg. 2014, 147, 1458–1463.e1. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit. Care 2013, 17, 204. [Google Scholar] [CrossRef] [PubMed]
- Kremke, M.; Tang, M.; Bak, M.; Kristensen, K.L.; Hindsholm, K.; Andreasen, J.J.; Hjortdal, V.; Jakobsen, C.J. Antiplatelet therapy at the time of coronary artery bypass grafting: A multicentre cohort study. Eur. J. Cardiothorac. Surg. 2013, 44, e133–e140. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.A.; Mehta, S.R.; Peters, R.; Zhao, F.; Lakkis, N.; Gersh, B.J.; Yusuf, S. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: The Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004, 110, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Mahla, E.; Suarez, T.A.; Bliden, K.P.; Rehak, P.; Metzler, H.; Sequeira, A.J.; Cho, P.; Sell, J.; Fan, J.; Antonino, M.J.; et al. Platelet function measurement-based strategy to reduce bleeding and waiting time in clopidogrel-treated patients undergoing coronary artery bypass graft surgery: The timing based on platelet function strategy to reduce clopidogrel-associated bleeding related to CABG (TARGET-CABG) study. Circ. Cardiovasc. Interv. 2012, 5, 261–269. [Google Scholar] [CrossRef]
- Sousa-Uva, M.; Head, S.J.; Milojevic, M.; Collet, J.P.; Landoni, G.; Castella, M.; Dunning, J.; Gudbjartsson, T.; Linker, N.J.; Sandoval, E.; et al. 2017 EACTS Guidelines on perioperative medication in adult cardiac surgery. Eur. J. Cardiothorac. Surg. 2018, 53, 5–33. [Google Scholar] [CrossRef]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2018, 39, 213–260. [Google Scholar] [CrossRef]
- Maltais, S.; Perrault, L.P.; Do, Q.B. Effect of clopidogrel on bleeding and transfusions after off-pump coronary artery bypass graft surgery: Impact of discontinuation prior to surgery. Eur. J. Cardiothorac. Surg. 2008, 34, 127–131. [Google Scholar] [CrossRef]
- Levine, G.N.; Jeong, Y.H.; Goto, S.; Anderson, J.L.; Huo, Y.; Mega, J.L.; Taubert, K.; Smith, S.C., Jr. Expert consensus document: World Heart Federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI. Nat. Rev. Cardiol. 2014, 11, 597–606. [Google Scholar] [CrossRef]
- Malm, C.J.; Hansson, E.C.; Åkesson, J.; Andersson, M.; Hesse, C.; Shams Hakimi, C.; Jeppsson, A. Preoperative platelet function predicts perioperative bleeding complications in ticagrelor-treated cardiac surgery patients: A prospective observational study. Br. J. Anaesth. 2016, 117, 309–315. [Google Scholar] [CrossRef]
- Kapetanakis, E.I.; Medlam, D.A.; Petro, K.R.; Haile, E.; Hill, P.C.; Dullum, M.K.; Bafi, A.S.; Boyce, S.W.; Corso, P.J. Effect of clopidogrel premedication in off-pump cardiac surgery: Are we forfeiting the benefits of reduced hemorrhagic sequelae? Circulation 2006, 113, 1667–1674. [Google Scholar] [CrossRef][Green Version]
- Urban, P.; Mehran, R.; Colleran, R.; Angiolillo, D.J.; Byrne, R.A.; Capodanno, D.; Cuisset, T.; Cutlip, D.; Eerdmans, P.; Eikelboom, J.; et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: A consensus document from the Academic Research Consortium for High Bleeding Risk. Eur. Heart J. 2019, 40, 2632–2653. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).