1. Introduction
Overcrowding of the emergency departments (EDs) is a widely discussed issue involving all EDs worldwide, caused by exit-blocks, decreasing numbers of ED beds and increasing need for acute care and, eventually, resulting in increased mortality rates, costs and prolonged length of stays (LOS) in the EDs. Consequently, this impairs the quality and safety of acute care [
1,
2,
3,
4].
The EDs face a rather heterogenous population of patients with both urgent and non-urgent medical conditions. Frail elderly patients are one of the most substantial and frequent visitors of the EDs, and their clinical presentation differs from the younger patient population: due to delayed, diminished or atypical clinical presentations and symptoms, the risk stratification of these patients is considered remarkably challenging. Additionally, due to age-related organ function declines, the patient population tend to have a higher risk for negative outcomes during their stay in the ED. For earlier mentioned reasons, the perspective of the aging population and consequential increases in elderly patients seeking care from the EDs is concerning [
5,
6].
The risk stratification in the EDs rely principally on vital-sign based track-and-trigger score systems, such as National Early Warning Score (NEWS) system. However, they can be insufficient in assessing the patients, especially elderly, with normal vital signs but with high risk of critical illness [
7]. Therefore, tools for reflecting the underlying pathogenetic pathways of existing comorbidities as well as different acutellnesses are needed to improve the patient flow of the overcrowded EDs. The improved patient flow [
8,
9], would ideally result in more safe discharges and leave the hospital beds to the patients that require most clinical attention. Consequently, this could provide the EDs with increased resources and reduced costs, not to mention the advantages from the aspect of elderly hospitalization [
10].
Prognostic biomarkers have been suggested as a potential tool for the clinical decision-making in the emergency setting [
11]. One of the novel biomarkers, soluble urokinase plasminogen activator receptor (suPAR), is a nonspecific inflammatory biomarker, which is released in blood plasma when the urokinase plasminogen activator receptor (uPAR) is cleaved from the cell membrane of immunoactive cells such as monocytes, activated T-lymphocytes and macrophages in response to inflammatory stimuli. The plasma concentration of suPAR increases in both acute and chronic inflammatory states such as infectious diseases, sepsis, autoimmune diseases, malignancies, cardiovascular diseases and organ dysfunctions such as liver and kidney failure, when, in contrast, stays rather low in primary healthy individuals [
12,
13,
14]. Furthermore, suPAR values in the general population increase with advancing age: a former study suggests that patients aged 74–89 years had significantly higher suPAR values than individuals between 24–66 years [
15].
SuPAR has shown to have excellent prognostic value in both healthy individuals and in individuals with comorbidities [
16,
17,
18,
19]. In critically ill patients, suPAR levels are associated with increased risk of mortality, hospital admission, readmission rates as well as further complications [
14,
20,
21,
22,
23]. Furthermore, suPAR values are studied to be strong predictors of mortality when adjusted with NEWS scoring, age and sex in ED patient population, and, interestingly, in hospitalized COVID-19 patients [
24,
25].
In contrast, low suPAR values have been observed to support the decision of discharge from the ED without increasing the risk for negative outcomes [
26].
The EDs need additional tools for the risk assessment of their patients to improve their patient flow and avoid overcrowding. SuPAR is well understood when it comes to its characteristics and prognostic values. However, considering that aging increases suPAR levels, the optimal clinical setting for its use in the risk stratification of elderly ED patients is unclear. For that reason, in addition to evaluating the risk-predicting value of suPAR in the ED setting, this study aimed to determine the optimal cut-off values for the utilization of suPAR, concentrating on the elderly patient population.
2. Materials and Methods
2.1. Patient Population and Data Collection
This study was a prospective cohort study conceived in two Finnish hospital regions (Helsinki and Mikkeli). The included study population consists of unselected acute medical patients that sought care from the two study EDs between 4 March 2020 to 11 May 2020 (Mikkeli) or between 1 May 2020 to 31 May 2020 (Helsinki, Meilahti). The patient populations of the two hospitals were similar and consisted of patients from all medical specialties (internal medicine, surgery, trauma etc.).
The data were collected from the two hospital areas’ electronic health record systems (Uranus in Helsinki, Effica in Mikkeli). To be included in the study, the patient’s index admission was required to involve routine venous blood sampling and given consent (in Meilahti).
2.2. Biomarker Measurements
Plasma suPAR levels were incorporated as part of the standard blood sampling at the EDs. The actual measurement was carried out using suPARnostic® Turbilatex assay (ViroGates A/S, Birkerød, Denmark) on a Cobas c501 clinical chemistry analyser (Roche Diagnostics Ltd., Espoo, Finland). The analyzing process was performed according to the manufacturer’s instructions. The other laboratory markers (C-reactive protein, creatinine, troponin T) were measured following regional standards. The suPAR values were available for the ED physicians in the same time frame as the other laboratory test results.
2.3. Statistics
The results are presented as numbers [N (%)] for categorical variables and as median [interquartile range (IQR)] for continuous variables. The patients were divided in two groups by age: (1) ≥75 years (=elderly) and (2) <75 years (=younger). For comparison of these groups, Fisher’s exact test or Pearson’s chi-squared test was used for categorical variables and Mann-Whitney U-test or Student’s t-test for continuous variables. Multivariable logistic regression analysis was used to determine independent risk factors for 30-day mortality, the results of which are presented as odds ratios (OR) with 95% confidence intervals (CI). We compared models with age group and suPAR interaction to ones without using likelihood-ratio tests (LRTs). Some unevenly and widely distributed values are presented on a logarithmic scale. NEWS scoring was excluded from the multivariable analysis due to missing data. A p-value less than 0.05 was considered statistically significant. The data was analyzed with SPSS Statistics Software 27.0 (IBM, Armonk, NY, USA).
2.4. Outcomes
The primary outcomes of this study were the all-cause mortality within 30 days of index admission and the number of discharges from the ED within 24 h of index admission. Secondary outcomes were hospital admissions, 7-day and 30-day readmissions and LOSs in the ED and in the hospital. All the outcomes were assessed in the whole population and separately in the elderly and in the younger.
3. Results
3.1. Whole Study Population and Age Groups
A total amount of 1858 (Mikkeli 1747 and Helsinki 111) patients were included in the study. Median age of the study population was 70 years (IQR 56–79) and 961 (52%) were women. 88 patients (5%) died within 30 days of index admission. Median length of stay (LOS) in the ED was 254 min (IQR 176–364), and 2 days (IQR 1–5) in the hospital.
The elderly constituted 36% (669/1858) of the patients with a female proportion of 48%. The rest 64% (1190/1858) of the patients were younger with a female proportion of 48%, respectively. The elderly had higher 30-day mortality compared with the younger (8% vs. 2%, p = 0.001)
The elderly were discharged from ED significantly less frequently during the first 24 h compared with the younger (30% vs. 54%, p < 0.001). Similar difference between the age groups was seen in hospital readmissions within 7 days of discharge (10% vs. 6%, p = 0.001). On the contrary, the amount of hospital admissions was higher in the elderly (68%) than in the younger (46%).
SuPAR values were available for 1845 (99.3%) patients. Median suPAR level in the whole study population was 4.1 ng/mL (IQR 3.3–6.0), 3.7 ng/mL (IQR 3.0–5.0) in the younger, and 5.4 ng/mL (IQR 4.1–7.7) in the elderly, respectively. Statistically significant differences between the age groups were additionally seen in the higher median glomerular filtration rates (GFRs) of the younger and in the higher median NEWS scores as well as median plasma levels of C-reactive protein (CRP) and troponine T (TnT) of the elderly.
For more detailed characteristics of the study groups, see
Table 1.
3.2. SuPAR, Discharges and Mortality
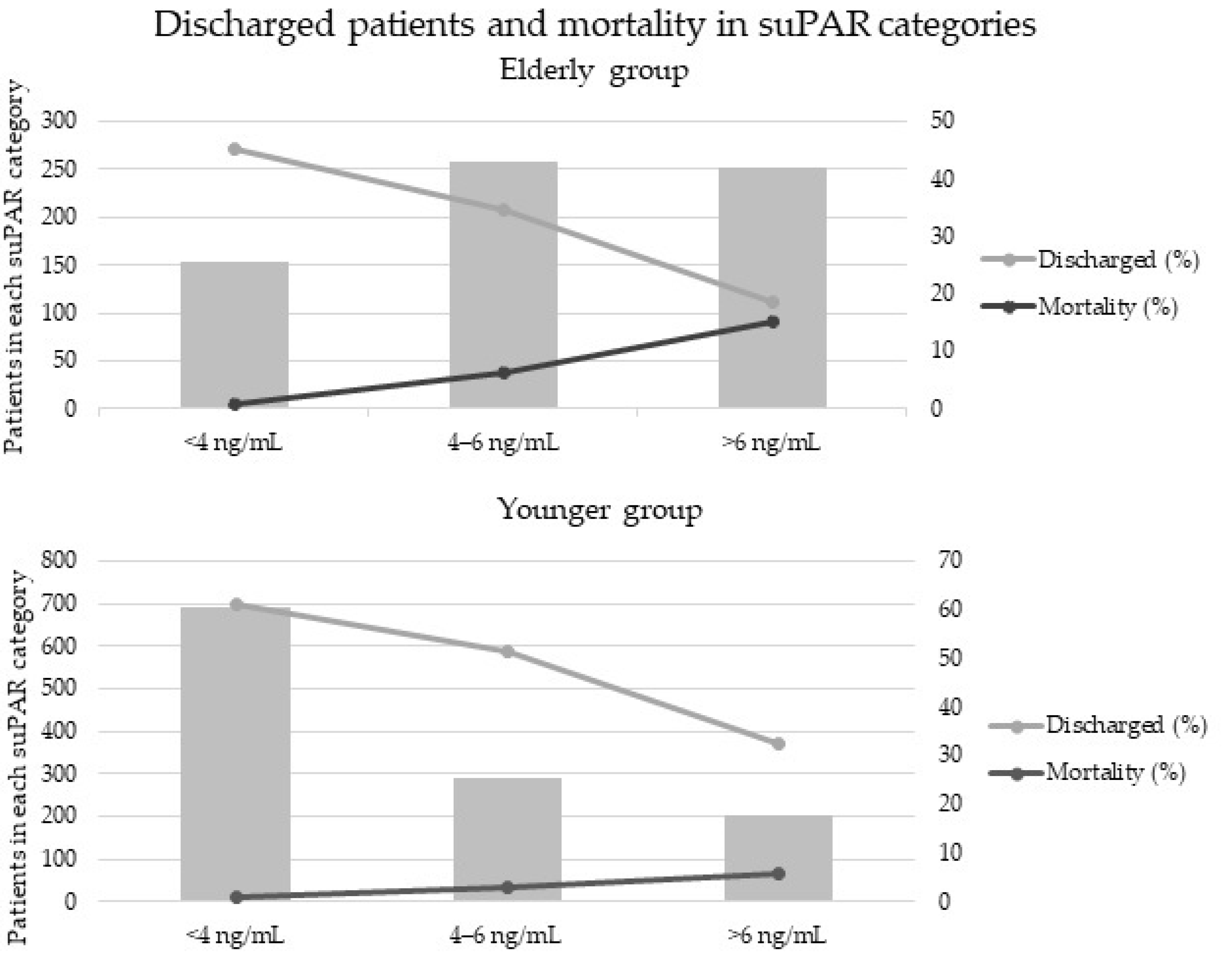
The age groups were divided into three additional groups according to their suPAR levels: (1) suPAR <4 ng/mL, (2) suPAR 4–6 ng/mL, (3) suPAR >6 ng/mL. As
Figure 1 shows, increasing suPAR levels were associated with decreasing proportion of discharged patients, the percentage of discharged patients within each suPAR category being: (1) 61% (2) 51% (3) 32% in the younger and (1) 45% (2) 34% (3) 19% in the elderly. Additionally, mortality rate increased along the suPAR level category: (1) 0.9% (2) 3% (3) 6% in the younger and (1) 0.7% (2) 6% (3) 15% in the elderly.
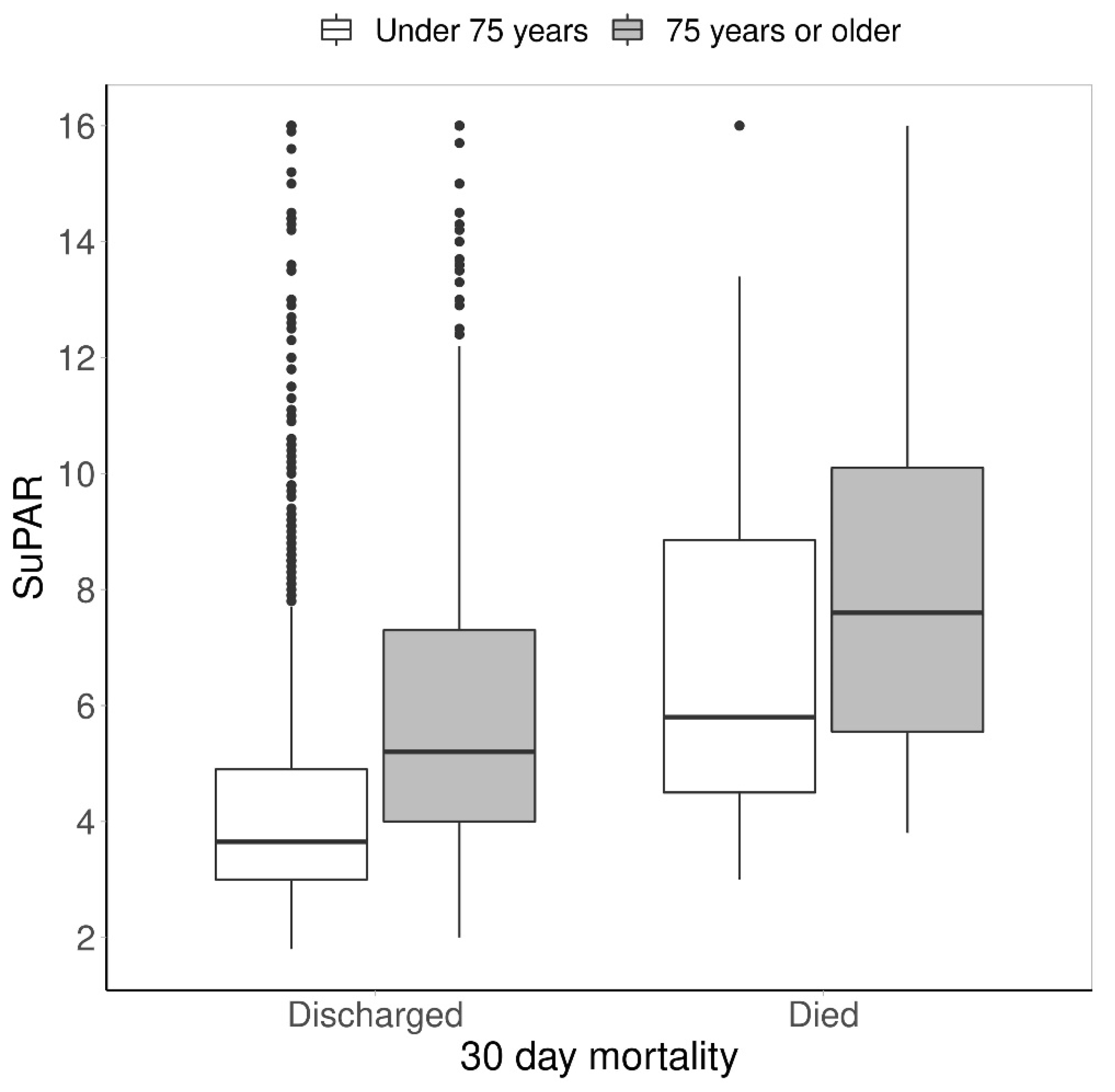
SuPAR levels were additionally observed between the discharged patients and the patients who died within 30 days of index admission. The differences were investigated in the two age groups. The median suPAR levels of the younger group who died within 30 days [5.8 ng/mL (IQR 4.1–9.7) were significantly higher than the levels of the younger discharged group [3.5 ng/mL (IQR 2.9–4.4)]. A similar trend was seen in the elderly group [7.6 ng/mL (IQR 5.5–10.1) vs. 4.5 ng/mL (IQR 3.7–5.8)]. Median suPAR values were higher in the elderly group, both in the discharged group and mortality group (See
Figure 2).
3.3. Different SuPAR Cut-Offs in the ≥75 Years Group
To evaluate the predictive value of suPAR levels in the elderly population, the study’s outcomes were also assessed with different ranges using three separate suPAR cut-off values (0–4 ng/mL, 0–5 ng/mL and 0–6 ng/mL) in the elderly group separately (
Table 2).
First, in the suPAR 0–4 ng/mL group, there were 153/23% elderly patients. In this group, 45% were discharged within 24 h, whereas 47% were admitted to hospital. One patient (0.6%) died within 30 days of index admission. The median LOS was 264 min (170–391) in the ED and 2 days (1.0–4.0) in the hospital.
Second, the suPAR 0–5 ng/mL group consisted of 289/43% elderly patients. In this group, 42% were discharged within 24 h of index admission and 57% admitted to hospital. Nine patients (3%) died within 30 days of index admission. The median LOS was 261 min (175–384) in the ED and 2 days (1.0–4.0) in the hospital.
Finally, in the suPAR 0–6 ng/mL group, there were 409/61% elderly patients, of which 23% were discharged within 24 h and 37% admitted to hospital. A total of 17 (4%) patients died within 30 days of index admission. The median LOS was 255 min (174–363) in the ED and 2.0 days (1.0–5.0) in the hospital.
3.4. Determination of Predictors for 30-Day Mortality—Unadjusted and Adjusted with Other Risk-Predicting Factors
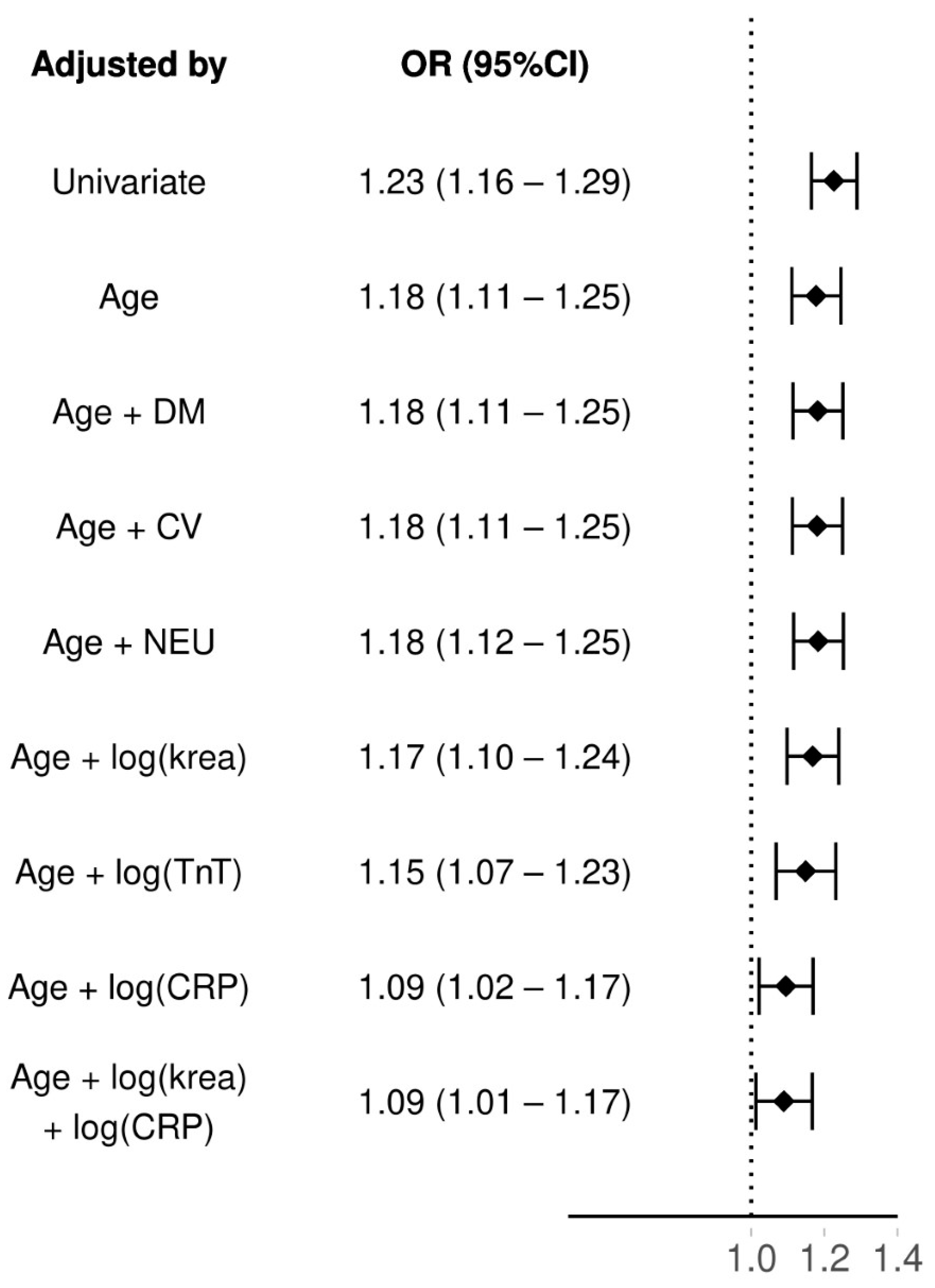
The results for regression models can be found from
Figure 3. SuPAR had an odds ratio (OR) of 1.23 (95% CI: 1.16–1.29) as a 30-day mortality predictor. When adjusting suPAR by age its OR slightly dropped: 1.18 (95%CI: 1.11–1.25). As age was correlated with suPAR, we kept it as a predictor and further adjusted the model with neurological and cardiovascular comorbidities, diabetes mellitus and logarithmized plasma levels of creatinine (krea) and troponine T (TnT). All of these had an association of equivalent level as when only adjusting suPAR with age. Only C-reactive protein (CRP) lowered the OR of suPAR considerably, when also adjusting with age the OR dropped to 1.09 (95%CI: 1.02–1.17). However, adding creatinine to the model with both age and CRP did not lower the OR of suPAR further (OR 1.09 95% CI: 1.01–1.17). Adding an interaction between the age and suPAR did not significantly increase fit of the model (LRT age as group
p = 0.72, age as continous
p = 0.63).
4. Discussion
The EDs are overcrowding and the current methods for the risk stratification are insufficient, especially in the elderly patient population. Thus, new methods for the assessment of these patients are needed. This study aimed to evaluate suPAR, a nonspecific prognostic biomarker, as a tool of this kind in the ED patient population. Additionally, the study analysed, for the first time according to our knowledge, the prognostic role and risk-predicting value of suPAR in the elderly population. According to LRT, adding interaction between suPAR and age did not improve any of the models significantly.
As a previous study working with the same research data has concluded [
27], this study confirms that suPAR has prognostic value in predicting both negative and positive outcomes: patients with increased suPAR levels are more likely to die within 30 days of index admission, and patients with low suPAR levels are more likely to be discharged from the ED and survive within 30 days of index admission, regardless of age. Vice versa, the suPAR levels among patients who died within 30 days were significantly higher than the levels of the discharged patients. Additionally, our regression analysis indicates that suPAR acts as a predictor for 30-day mortality both independently and when adjusted with age, NEWS scoring, CRP and comorbidities such as diabetes mellitus, cardiovascular diseases and neurological diseases. When suPAR is simultaneously adjusted with three factors, the predictive value weakens (OR 1.09 (1.01–1.17).
Moreover, the study results suggest that suPAR levels are positively associated with age and the median suPAR level among the elderly population (5.4 ng/mL) is significantly higher than in the whole population (4.1 ng/mL) and in the younger population (3.7 ng/mL). Additionally, according to
Figure 2, the median suPAR levels increase with age, regardless of whether the patient dies or is discharged from the ED.
However, despite the higher median suPAR levels, the study data suggests that the utilization of 6 ng/mL cut-off value would lead to excessive mortality rates in the elderly population (2.5%) and would thus impair the safety-related properties of low suPAR levels. (
Table 2). The incidence of 30-day mortality was highest in the suPAR 0–6 ng/mL group when compared to the 0–4 ng/mL group and the 0–5 ng/mL group. Between the groups, an increase of this kind was additionally seen in both the number of discharges (8.0% increase from 0–4 ng/mL group to 0–5 ng/mL group, 5.1% increase from 0–5 ng/mL group to 0–6 ng/mL group) and the amount of 30-day readmissions (4.3% increase, 3.2% increase). The median length of stay in the ED or in the hospital did not significantly differ between the groups.
For that reason, a cut-off value of 4 ng/mL would successfully work as a predictor for both positive and negative outcomes in all patients, regardless of age. On the other hand, in the elderly, an elevation of the cut-off value from 4 ng/mL to 5 ng/mL resulted in a significant increase in the proportion of discharges (10.3% vs. 18.3%) but only one death within 30 days of index admission.
SuPAR is a nonspecific biomarker, and elevated suPAR values can be caused by chronic non-acute as well as acute diseases. The aim of this study was to determine if suPAR can predict negative outcomes in an unselected patient population with various chronic illnesses, especially in the elderly. According to the study results, suPAR predicts mortality in this group, regardless of age. However, due to its unspecificity, suPAR is not a diagnostic tool. For that reason, suPAR should be used more as a directional prognostic tool alongside other clinical features and assessment methods such as clinical examination, scoring systems and other laboratory markers. Judging by previous study and the data presented in this manuscript, suPAR could thus be used in the decision to either admit or discharge the ED patient.
Limitations
As with the majority of studies, this study is subject to limitations. First, the ED physicians were conscious of the patients’ suPAR results in Mikkeli but not in Helsinki, and therefore the evaluation of the effects on the outcomes is not possible. Second, the smoking habits of the included patients were not taken into account, regardless of knowing that regular smokers have approximately 1 ng/mL higher suPAR levels than non-smokers [
28,
29]. Third, as drawn blood samples and given consent in Meilahti were required for the inclusion, the study excluded the patients with minor clinical issues, mental issues or nurse visits, for example. Additionally, the patients that were not able to give a consent in Melahti were excluded from the study.
5. Conclusions
The study results suggest that suPAR levels were clearly elevated in the ED patients, the elderly patients displaying the highest levels. However, age and suPAR were not associated with 30-day mortality. High suPAR concentrations were associated with higher mortality and lesser probability to be discharged from the ED. Furthermore, as a nonspecific prognostic biomarker utilized in the ED, suPAR successfully predicts all-cause 30-day mortality in all age groups. SuPAR maintains its predictive value when it is used with other commonly used risk assessment tools. Low suPAR values can work as a support in discharging patients from the ED without increasing the risk of negative outcomes.
For all the patients arriving at the ED, the safest cut-off value for suPAR would be 4 ng/mL. On the other hand, a cut-off value of 5 ng/mL should be considered as a potential alternative in the elderly population. The cut-off value of 6 ng/mL should not be utilized.
Our study confirmed that suPAR could successfully act as an addition to the risk assessment of elderly patients and the patients of which the current risk stratification methods fail to identify, especially as these patients are one of the most time- and resource-consuming patients of the ED.
Author Contributions
S.S. and R.M.H. contributed equally. Conceptualization, M.C., J.K., H.H. and S.S.; Methodology, M.C., J.K., H.H. and S.S.; Validation, M.C., H.H. and J.K.; Formal Analysis, R.M.H. and M.H.; Investigation, S.S., H.H. and R.M.H.; Resources, ViroGates (Analyzation equipment), Data Curation, Writing—Original Draft Preparation, R.M.H. and S.S.; Writing—Review & Editing, J.K., H.H., M.H., M.C., S.S. and R.M.H.; Visualization, M.H. and R.M.H.; Supervision, H.H.; J.K. and M.C.; Project Administration, M.C. and H.H.; Funding Acquisition, M.C. and H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Department of Emergency Medicine and Services, Helsinki University Hospital (HUS), Haartmaninkatu 4, PL 340, 00029 HUS. Analyzation equipment for suPAR measurements were received from ViroGates. Open access funding provided by University of Helsinki.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethical Committee of the Helsinki University Hospital (the part of the study conducted in Helsinki, ref. No. § 33 HUS/141/2020 & § 32 HUS/3346/2019) and by the South Savo social- and healthcare authority (the part of the study conducted in Mikkeli, approval 684/13.02.03/2019). The study didn’t affect the treatment of the included patients.
Informed Consent Statement
According to the Ethical Committee’s instructions, informed consent was obtained from all subjects in Meilahti hospital.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
All authors would like to thank ISLAB and HUSLAB for effortless co-operation during a somewhat challenging time with the pandemic. We additionally acknowledge Mitja Lääperi for assistance in the statistical analysis.
Conflicts of Interest
H.H. has received a lecture fee from ViroGates AS. Other authors have no conflict of interest.
References
- Pines, J.M.; Hilton, J.A.; Weber, E.J.; Alkemade, A.J.; Al Shabanah, H.; Anderson, P.D.; Bernhard, M.; Bertini, A.; Gries, A.; Ferrandiz, S.; et al. International perspectives on emergency department crowding. Acad. Emerg. Med. 2011, 18, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.C.; Hsia, R.Y.; Weiss, R.E.; Zingmond, D.; Liang, L.J.; Han, W.; McCreath, H.; Asch, S.M. Effect of emergency department crowding on outcomes of admitted patients. Ann. Emerg. Med. 2013, 61, 605–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, E.J.; Pouch, S.M.; Larson, E.L. The relationship between emergency department crowding and patient outcomes: A systematic review. J. Nurs. Scholarsh. 2014, 46, 106–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, S.; Knowles, E.; Boyle, A. Exit block in emergency departments: A rapid evidence review. Emerg. Med. J. 2017, 34, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Kemp, K.; Mertanen, R.; Lääperi, M.; Niemi-Murola, L.; Lehtonen, L.; Castren, M. Nonspecific complaints in the emergency department—A systematic review. Scand. J. Trauma Resusc. Emerg. Med. 2020, 28, 6. [Google Scholar] [CrossRef] [PubMed]
- Häseler-Ouart, K.; Arefian, H.; Hartmann, M.; Kwetkat, A. Geriatric assessment for older adults admitted to the emergency department: A systematic review and meta-analysis. Exp. Gerontol. 2021, 144, 111184. [Google Scholar] [CrossRef]
- LaMantia, M.A.; Stewart, P.W.; Platts-Mills, T.F.; Biese, K.J.; Forbach, C.; Zamora, E.; McCall, B.K.; Shofer, F.S.; Cairns, C.B.; Busby-Whitehead, J.; et al. Predictive value of initial triage vital signs for critically ill older adults. West. J. Emerg. Med. 2013, 14, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Hoot, N.R.; Aronsky, D. Systematic Review of Emergency Department Crowding: Causes, Effects, and Solutions. Ann. Emerg. Med. 2008, 52, 126–136. [Google Scholar] [CrossRef]
- Oredsson, S.; Jonsson, H.; Rognes, J.; Lind, L.; Göransson, K.E.; Ehrenberg, A.; Asplund, K.; Castrén, M.; Farrohknia, N. A systematic review of triage-related interventions to improve patient flow in emergency departments. Scand. J. Trauma Resusc. Emerg. Med. 2011, 19, 43. [Google Scholar] [CrossRef] [Green Version]
- Creditor, M.C. Hazards of Hospitalization of the Elderly. Ann. Intern. Med. 1993, 118, 219–223. [Google Scholar] [CrossRef]
- Schuetz, P.; Hausfater, P.; Amin, D.; Amin, A.; Haubitz, S.; Faessler, L.; Kutz, A.; Conca, A.; Reutlinger, B.; Canavaggio, P.; et al. Biomarkers from distinct biological pathways improve early risk stratification in medical emergency patients: The multinational, prospective, observational TRIAGE study. Crit. Care 2015, 19, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thunø, M.; MacHo, B.; Eugen-Olsen, J. SuPAR: The molecular crystal ball. Dis. Markers 2009, 27, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Haupt, T.H.; Rasmussen, L.J.H.; Kallemose, T.; Ladelund, S.; Andersen, O.; Pisinger, C.; Eugen-Olsen, J. Healthy lifestyles reduce suPAR and mortality in a Danish general population study. Immun. Ageing 2019, 16, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velissaris, D.; Dimopoulos, G.; Parissis, J.; Alexiou, Z.; Antonakos, N.; Babalis, D.; Gerakari, S.; Kaldis, V.; Koutoukas, P.; Leventogiannis, K.; et al. Prognostic Role of Soluble Urokinase Plasminogen Activator Receptor at the Emergency Department: A Position Paper by the Hellenic Sepsis Study Group. Infect. Dis. Ther. 2020, 9, 407–416. [Google Scholar] [CrossRef]
- Wlazel, R.N.; Szwabe, K.; Guligowska, A.; Kostka, T. Soluble urokinase plasminogen activator receptor level in individuals of advanced age. Sci. Rep. 2020, 10, 15462. [Google Scholar] [CrossRef]
- Curovic, V.R.; Theilade, S.; Winther, S.A.; Tofte, N.; Eugen-Olsen, J.; Persson, F.; Hansen, T.W.; Jeppesen, J.; Rossing, P. Soluble urokinase plasminogen activator receptor predicts cardiovascular events, kidney function decline, and mortality in patients with type 1 diabetes. Diabetes Care 2019, 42, 1112–1119. [Google Scholar] [CrossRef]
- Hayek, S.S.; Divers, J.; Raad, M.; Xu, J.; Bowden, D.W.; Tracy, M.; Reiser, J.; Freedman, B.I. Predicting mortality in African Americans with type 2 diabetes mellitus: Soluble urokinase plasminogen activator receptor, coronary artery calcium, and high-sensitivity c-reactive protein. J. Am. Heart Assoc. 2018, 7, e008194. [Google Scholar] [CrossRef]
- Håkansson, K.E.J.; Ulrik, C.S.; Godtfredsen, N.S.; Kallemose, T.; Andersen, O.; Eugen-Olsen, J.; Marsaa, K.; Rasmussen, L.J.H. High suPAR and low blood eosinophil count are risk factors for hospital readmission and mortality in patients with COPD. Int. J. COPD 2020, 15, 733–743. [Google Scholar] [CrossRef] [Green Version]
- Velissaris, D.; Zareifopoulos, N.; Koniari, I.; Karamouzos, V.; Bousis, D.; Gerakaris, A.; Platanaki, C.; Kounis, N. Soluble Urokinase Plasminogen Activator Receptor as a Diagnostic and Prognostic Biomarker in Cardiac Disease. J. Clin. Med. Res. 2021, 13, 133–142. [Google Scholar] [CrossRef]
- Haupt, T.H.; Petersen, J.; Ellekilde, G.; Klausen, H.H.; Thorball, C.W.; Eugen-Olsen, J.; Andersen, O. Plasma suPAR levels are associated with mortality, admission time, and Charlson Comorbidity Index in the acutely admitted medical patient: A prospective observational study. Crit. Care 2012, 16, R130. [Google Scholar] [CrossRef] [Green Version]
- Nayak, R.; Allingstrup, M.; Phanareth, K.; Kofoed-Enevoldsen, A. suPAR as a biomarker for risk of readmission and mortality in the acute medical setting. Dan. Med. J. 2015, 62, A5146. [Google Scholar] [PubMed]
- Rasmussen, L.J.H.; Ladelund, S.; Haupt, T.H.; Ellekilde, G.; Poulsen, J.H.; Iversen, K.; Eugen-Olsen, J.; Andersen, O. Soluble urokinase plasminogen activator receptor (suPAR) in acute care: A strong marker of disease presence and severity, readmission and mortality. A retrospective cohort study. Emerg. Med. J. 2016, 33, 769–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, T.P.; Albanna, W.; Weiss, M.; Veldeman, M.; Conzen, C.; Nikoubashman, O.; Blume, C.; Kluger, D.S.; Clusmann, H.; Loosen, S.H.; et al. The Role of Soluble Urokinase Plasminogen Activator Receptor (suPAR) in the Context of Aneurysmal Subarachnoid Hemorrhage (aSAH)—A Prospective Observational Study. Front. Neurol. 2022, 13, 841024. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.J.H.; Ladelund, S.; Haupt, T.H.; Ellekilde, G.E.; Eugen-Olsen, J.; Andersen, O. Combining national early warning score with soluble urokinase plasminogen activator receptor (SUPAR) improves risk prediction in acute medical patients: A registry-based cohort study. Crit. Care Med. 2018, 46, 1961–1968. [Google Scholar] [CrossRef]
- Kakar, A.; Rani, P.; Batra, T.; Hasan, R.; Choudhury, S. A Comparative Analysis of Serial Measurements of Soluble Urokinase-type Plasminogen Activator Receptor (suPAR) and C-Reactive Protein in Patients with Moderate COVID-19: A Single Center Study from India. medRxiv 2022. [Google Scholar]
- Schultz, M.; Rasmussen, L.J.H.; Høi-Hansen, T.; Kjøller, E.; Jensen, B.N.; Lind, M.N.; Ravn, L.; Kallemose, T.; Lange, T.; Køber, L.; et al. Early discharge from the emergency department based on soluble urokinase plasminogen activator receptor (suPAR) levels: A TRIAGE III substudy. Dis. Markers 2019, 2019, 3403549. [Google Scholar] [CrossRef]
- Santeri, S.; Peter, A.A.; Kristiina, N.; Jesper, E.O.; Harri, H. suPAR cut-offs for stratification of low, medium, and high-risk acute medical patients in the emergency department. BMC Emerg. Med. 2021, 21, 149. [Google Scholar] [CrossRef]
- Eugen-Olsen, J.; Ladelund, S.; Sørensen, L.T. Plasma suPAR is lowered by smoking cessation: A randomized controlled study. Eur. J. Clin. Investig. 2016, 46, 305–311. [Google Scholar] [CrossRef]
- Haupt, T.H.; Kallemose, T.; Ladelund, S.; Rasmussen, L.J.H.; Thorball, C.W.; Andersen, O.; Pisinger, C.; Eugen-Olsen, J. Risk factors associated with serum levels of the inflammatory biomarker soluble urokinase plasminogen activator receptor in a general population. Biomark. Insights 2014, 9, 91–100. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).