Efficacy of a Novel Intra-Articular Administration of Platelet-Rich Plasma One-Week Prior to Hyaluronic Acid versus Platelet-Rich Plasma Alone in Knee Osteoarthritis: A Prospective, Randomized, Double-Blind, Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. PRP Preparation
2.4. Intra-Articular Injection
2.5. Outcome Measurements
2.5.1. Primary Outcome
2.5.2. Secondary Outcomes
2.6. Sample Size
2.7. Statistical Analysis
3. Results
3.1. The Clinical and Demographic Characteristics of Study Subject
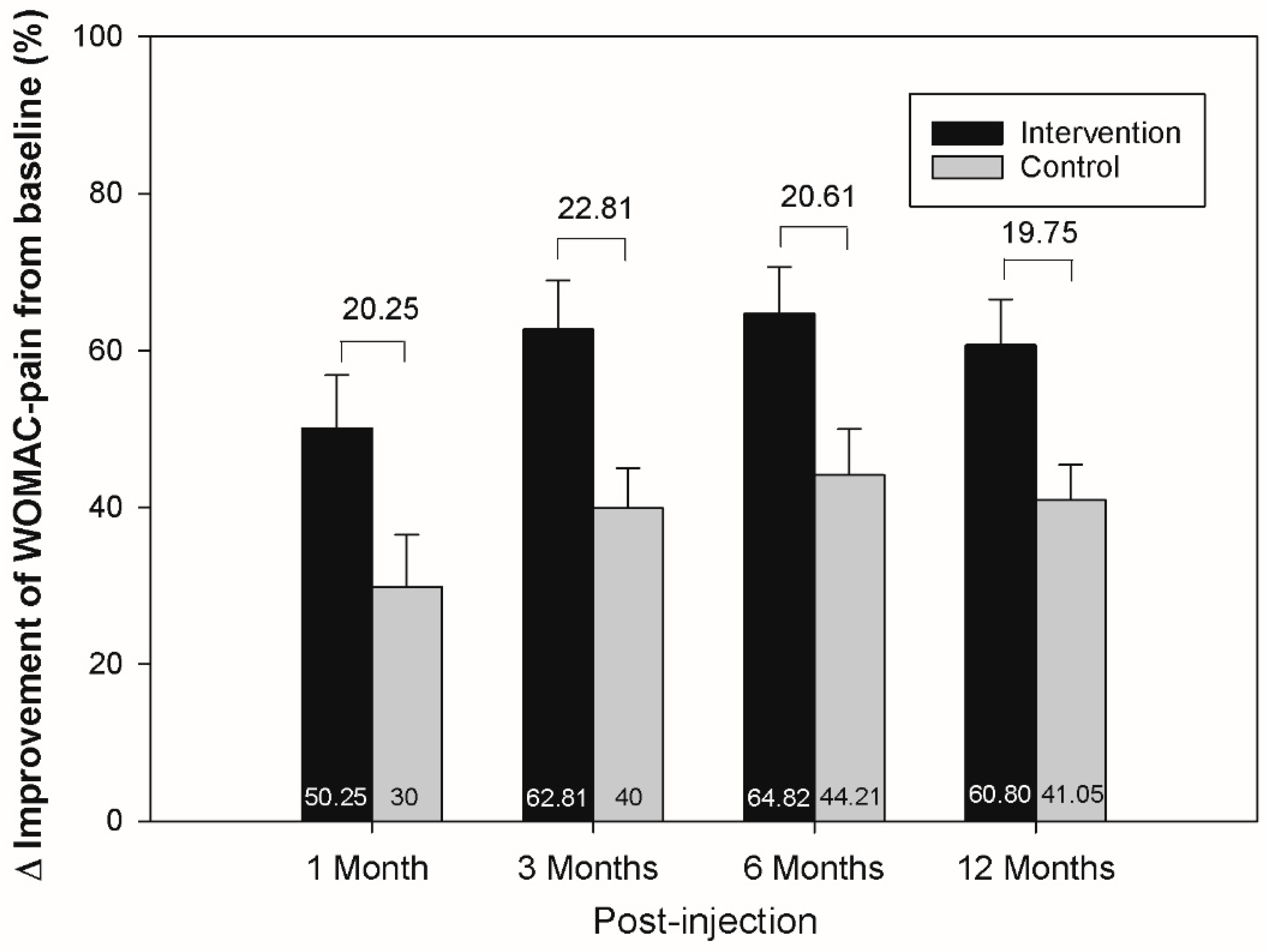
3.2. The Effect of WOMAC
3.3. The Effect of Balance and the Risk of Falls
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, H.S.; Farrag, S.E.; Okasha, A.E.; Kadry, A.O.; Ata, T.B.; Monir, A.A.; Shady, I. Clinical outcomes are associated with changes in ultrasonographic structural appearance after platelet-rich plasma treatment for knee osteoarthritis. Int. J. Rheum. Dis. 2018, 21, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, V.K.; Fryer, J.L.; Zhai, G.; Winzenberg, T.M.; Hosmer, D.; Jones, G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr. Cartil. 2005, 13, 769–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangha, O. Epidemiology of rheumatic diseases. Rheumatology 2000, 39 (Suppl. 2), 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arden, N.; Nevitt, M.C. Osteoarthritis: Epidemiology. Best Pract. Res. Clin. Rheumatol. 2006, 20, 3–25. [Google Scholar] [CrossRef]
- Messier, S.P.; Glasser, J.L.; Ettinger, W.H., Jr.; Craven, T.E.; Miller, M.E. Declines in strength and balance in older adults with chronic knee pain: A 30-month longitudinal, observational study. Arthritis Rheum. 2002, 47, 141–148. [Google Scholar] [CrossRef]
- Barrett, D.S.; Cobb, A.G.; Bentley, G. Joint proprioception in normal, osteoarthritic and replaced knees. J. Bone Jt. Surg. Br. Vol. 1991, 73, 53–56. [Google Scholar] [CrossRef]
- Pandya, N.K.; Draganich, L.F.; Mauer, A.; Piotrowski, G.A.; Pottenger, L. Osteoarthritis of the knees increases the propensity to trip on an obstacle. Clin. Orthop. Relat. Res. 2005, 431, 150–156. [Google Scholar] [CrossRef]
- Vaishya, R.; Pariyo, G.B.; Agarwal, A.K.; Vijay, V. Non-operative management of osteoarthritis of the knee joint. J. Clin. Orthop. Trauma 2016, 7, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Abate, M.; Pulcini, D.; Di Iorio, A.; Schiavone, C. Viscosupplementation with intra-articular hyaluronic acid for treatment of osteoarthritis in the elderly. Curr. Pharm. Des. 2010, 16, 631–640. [Google Scholar] [CrossRef]
- Goldberg, V.M.; Buckwalter, J.A. Hyaluronans in the treatment of osteoarthritis of the knee: Evidence for disease-modifying activity. Osteoarthr. Cartil. 2005, 13, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Julovi, S.M.; Yasuda, T.; Shimizu, M.; Hiramitsu, T.; Nakamura, T. Inhibition of interleukin-1beta-stimulated production of matrix metalloproteinases by hyaluronan via CD44 in human articular cartilage. Arthritis Rheum. 2004, 50, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D.; Bedi, A.; Karlsson, J.; Sancheti, P.; Schemitsch, E. Product Differences in Intra-articular Hyaluronic Acids for Osteoarthritis of the Knee. Am. J. Sports Med. 2016, 44, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Rutjes, A.W.; Jüni, P.; da Costa, B.R.; Trelle, S.; Nüesch, E.; Reichenbach, S. Viscosupplementation for osteoarthritis of the knee: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 157, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Abate, M. Knee osteoarthritis: Hyaluronic acid, platelet-rich plasma or both in association? Expert Opin. Biol. Ther. 2014, 14, 635–649. [Google Scholar] [CrossRef]
- Sánchez, M.; Anitua, E.; Azofra, J.; Aguirre, J.J.; Andia, I. Intra-articular injection of an autologous preparation rich in growth factors for the treatment of knee OA: A retrospective cohort study. Clin. Exp. Rheumatol. 2008, 26, 910–913. [Google Scholar]
- Chang, K.V.; Hung, C.Y.; Aliwarga, F.; Wang, T.G.; Han, D.S.; Chen, W.S. Comparative effectiveness of platelet-rich plasma injections for treating knee joint cartilage degenerative pathology: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2014, 95, 562–575. [Google Scholar] [CrossRef]
- Shen, L.; Yuan, T.; Chen, S.; Xie, X.; Zhang, C. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: Systematic review and meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2017, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Iio, K.; Furukawa, K.I.; Tsuda, E.; Yamamoto, Y.; Maeda, S.; Naraoka, T.; Kimura, Y.; Ishibashi, Y. Hyaluronic acid induces the release of growth factors from platelet-rich plasma. AsiaPac. J. Sports Med. Arthrosc. Rehabil. Technol. 2016, 4, 27–32. [Google Scholar] [CrossRef]
- Chen, W.H.; Lo, W.C.; Hsu, W.C.; Wei, H.J.; Liu, H.Y.; Lee, C.H.; Tina Chen, S.Y.; Shieh, Y.H.; Williams, D.F.; Deng, W.P. Synergistic anabolic actions of hyaluronic acid and platelet-rich plasma on cartilage regeneration in osteoarthritis therapy. Biomaterials 2014, 35, 9599–9607. [Google Scholar] [CrossRef]
- Abate, M.; Verna, S.; Schiavone, C.; Di Gregorio, P.; Salini, V. Efficacy and safety profile of a compound composed of platelet-rich plasma and hyaluronic acid in the treatment for knee osteoarthritis (preliminary results). Eur. J. Orthop. Surg. Traumatol. 2015, 25, 1321–1326. [Google Scholar] [CrossRef]
- Lana, J.F.; Weglein, A.; Sampson, S.E.; Vicente, E.F.; Huber, S.C.; Souza, C.V.; Ambach, M.A.; Vincent, H.; Urban-Paffaro, A.; Onodera, C.M.; et al. Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. J. Stem Cells Regen. Med. 2016, 12, 69–78. [Google Scholar] [PubMed]
- Guo, Y.; Yu, H.; Yuan, L. Treatment of knee osteoarthritis with platelet-rich plasma plus hyaluronic acid in comparison with platelet-rich plasma only. Int. J. Clin. Exp. Med. 2016, 9, 12085–12090. [Google Scholar]
- Jacob, G.; Shetty, V.; Shetty, S. A study assessing intra-articular PRP vs PRP with HMW HA vs PRP with LMW HA in early knee osteoarthritis. J. Arthrosc. Jt. Surg. 2017, 4, 65–71. [Google Scholar] [CrossRef]
- Nasser, E.-T. Treatment of knee osteoarthritis with platelet-rich plasma in comparison with platelet-rich plasma plus hyaluronic acid: A short-term double-blind randomized clinical study. Egypt. Orthop. J. 2018, 53, 31–37. [Google Scholar] [CrossRef]
- Palco, M.; Fenga, D.; Basile, G.C.; Rizzo, P.; Cavalieri, B.; Leonetti, D.; Alito, A.; Bruschetta, A.; Traina, F. Platelet-Rich Plasma Combined with Hyaluronic Acid versus Leucocyte and Platelet-Rich Plasma in the Conservative Treatment of Knee Osteoarthritis. A Retrospective Study. Medicina 2021, 57, 232. [Google Scholar] [CrossRef]
- Sun, S.F.; Lin, G.C.; Hsu, C.W.; Lin, H.S.; Liou, I.S.; Wu, S.Y. Comparing efficacy of intraarticular single crosslinked Hyaluronan (HYAJOINT Plus) and platelet-rich plasma (PRP) versus PRP alone for treating knee osteoarthritis. Sci. Rep. 2021, 11, 140. [Google Scholar] [CrossRef]
- Wu, Y.T.; Hsu, K.C.; Li, T.Y.; Chang, C.K.; Chen, L.C. Effects of Platelet-Rich Plasma on Pain and Muscle Strength in Patients with Knee Osteoarthritis. Am. J. Phys. Med. Rehabil. 2018, 97, 248–254. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, C.M.; Huang, C.F.; Hsu, W.C.; Lee, C.H.; Ou, K.L.; Dubey, N.K.; Deng, W.P. Functional Recovery in Osteoarthritic Chondrocytes Through Hyaluronic Acid and Platelet-Rich Plasma-Inhibited Infrapatellar Fat Pad Adipocytes. Am. J. Sports Med. 2016, 44, 2696–2705. [Google Scholar] [CrossRef]
- Chen, S.R.; Shen, Y.P.; Ho, T.Y.; Li, T.Y.; Su, Y.C.; Chou, Y.C.; Chen, L.C.; Wu, Y.T. One-Year Efficacy of Platelet-Rich Plasma for Moderate-to-Severe Carpal Tunnel Syndrome: A Prospective, Randomized, Double-Blind, Controlled Trial. Arch. Phys. Med. Rehabil. 2021, 102, 951–958. [Google Scholar] [CrossRef]
- Hermans, J.; Bierma-Zeinstra, S.M.; Bos, P.K.; Verhaar, J.A.; Reijman, M. The most accurate approach for intra-articular needle placement in the knee joint: A systematic review. Semin. Arthritis Rheum. 2011, 41, 106–115. [Google Scholar] [CrossRef]
- Martin, D.P.; Engelberg, R.; Agel, J.; Swiontkowski, M.F. Comparison of the Musculoskeletal Function Assessment questionnaire with the Short Form-36, the Western Ontario and McMaster Universities Osteoarthritis Index, and the Sickness Impact Profile health-status measures. J. Bone Jt. Surg. 1997, 79, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Li, T.Y.; Wu, Y.T.; Chen, L.C.; Cheng, S.N.; Pan, R.Y.; Chen, Y.C. An exploratory comparison of single intra-articular injection of platelet-rich plasma vs hyaluronic acid in treatment of haemophilic arthropathy of the knee. Haemophilia 2019, 25, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Salaffi, F.; Leardini, G.; Canesi, B.; Mannoni, A.; Fioravanti, A.; Caporali, R.; Lapadula, G.; Punzi, L. Reliability and validity of the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index in Italian patients with osteoarthritis of the knee. Osteoarthr. Cartil. 2003, 11, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Angst, F.; Aeschlimann, A.; Michel, B.A.; Stucki, G. Minimal clinically important rehabilitation effects in patients with osteoarthritis of the lower extremities. J. Rheumatol. 2002, 29, 131–138. [Google Scholar] [PubMed]
- Tubach, F.; Ravaud, P.; Baron, G.; Falissard, B.; Logeart, I.; Bellamy, N.; Bombardier, C.; Felson, D.; Hochberg, M.; van der Heijde, D.; et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: The minimal clinically important improvement. Ann. Rheum. Dis. 2005, 64, 29–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, H.M.; de Campos, T.F.; Santos, M.B.; Cardoso, J.R.; de Camargo Garcia, M.; Cohen, M.J.G. Influence of knee position on the postural stability index registered by the Biodex Stability System. Gait Posture 2008, 28, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, R.; Arnold, B. Intertester and intratester reliability of a dynamic balance protocol using the Biodex Stability System. J. Sport Rehabil. 1998, 7, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Sundman, E.A.; Cole, B.J.; Karas, V.; Della Valle, C.; Tetreault, M.W.; Mohammed, H.O.; Fortier, L.A. The anti-inflammatory and matrix restorative mechanisms of platelet-rich plasma in osteoarthritis. Am. J. Sports Med. 2014, 42, 35–41. [Google Scholar] [CrossRef]
- Anitua, E.; Sanchez, M.; De la Fuente, M.; Zalduendo, M.M.; Orive, G. Plasma rich in growth factors (PRGF-Endoret) stimulates tendon and synovial fibroblasts migration and improves the biological properties of hyaluronic acid. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1657–1665. [Google Scholar] [CrossRef]
- Anitua, E.; Sanchez, M.; Nurden, A.T.; Zalduendo, M.M.; de la Fuente, M.; Azofra, J.; Andia, I. Platelet-released growth factors enhance the secretion of hyaluronic acid and induce hepatocyte growth factor production by synovial fibroblasts from arthritic patients. Rheumatology 2007, 46, 1769–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, F.; D’Este, M.; Vadalà, G.; Cattani, C.; Papalia, R.; Alini, M.; Denaro, V. Platelet Rich Plasma and Hyaluronic Acid Blend for the Treatment of Osteoarthritis: Rheological and Biological Evaluation. PLoS ONE 2016, 11, e0157048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.I.; Kim, J.H.; Kwak, H.H.; Woo, H.M.; Han, J.H.; Yayon, A.; Jung, Y.C.; Cho, J.M.; Kang, B.J. A placebo-controlled study comparing the efficacy of intra-articular injections of hyaluronic acid and a novel hyaluronic acid-platelet-rich plasma conjugate in a canine model of osteoarthritis. J. Orthop. Surg. Res. 2019, 14, 314. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.S.; Wu, C.M.; Dubey, N.K.; Lo, W.C.; Tsai, F.C.; Tung, T.D.X.; Hung, W.C.; Hsu, W.C.; Chen, W.H.; Deng, W.P. Mechanistic insight into hyaluronic acid and platelet-rich plasma-mediated anti-inflammatory and anti-apoptotic activities in osteoarthritic mice. Aging 2018, 10, 4152–4165. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Huang, H.; Liang, G.; Zeng, L.F.; Yang, W.; Liu, J. Effects and safety of the combination of platelet-rich plasma (PRP) and hyaluronic acid (HA) in the treatment of knee osteoarthritis: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2020, 21, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baria, M.R.; Vasileff, W.K.; Borchers, J.; DiBartola, A.; Flanigan, D.C.; Plunkett, E.; Magnussen, R.A. Treating Knee Osteoarthritis with Platelet-Rich Plasma and Hyaluronic Acid Combination Therapy: A Systematic Review. Am. J. Sports Med. 2022, 50, 273–281. [Google Scholar] [CrossRef]
- Dallari, D.; Stagni, C.; Rani, N.; Sabbioni, G.; Pelotti, P.; Torricelli, P.; Tschon, M.; Giavaresi, G. Ultrasound-Guided Injection of Platelet-Rich Plasma and Hyaluronic Acid, Separately and in Combination, for Hip Osteoarthritis: A Randomized Controlled Study. Am. J. Sports Med. 2016, 44, 664–671. [Google Scholar] [CrossRef]
- Patel, S.; Dhillon, M.S.; Bansal, T. PRP and HA for Hip Osteoarthritis: Letter to the Editor. Am. J. Sports Med. 2016, 44, NP44. [Google Scholar] [CrossRef]
- Le, A.D.K.; Enweze, L.; DeBaun, M.R.; Dragoo, J.L. Platelet-Rich Plasma. Clin. Sports Med. 2019, 38, 17–44. [Google Scholar] [CrossRef]
- Foster, T.E.; Puskas, B.L.; Mandelbaum, B.R.; Gerhardt, M.B.; Rodeo, S.A. Platelet-rich plasma: From basic science to clinical applications. Am. J. Sports Med. 2009, 37, 2259–2272. [Google Scholar] [CrossRef]
- Sun, S.F.; Hsu, C.W.; Hwang, C.W.; Hsu, P.T.; Wang, J.L.; Tsai, S.L.; Chou, Y.J.; Hsu, Y.W.; Huang, C.M.; Wang, Y.L. Hyaluronate improves pain, physical function and balance in the geriatric osteoarthritic knee: A 6-month follow-up study using clinical tests. Osteoarthr. Cartil. 2006, 14, 696–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalaj, N.; Abu Osman, N.A.; Mokhtar, A.H.; George, J.; Abas, W.A. Effect of intra-articular hyaluronic injection on postural stability and risk of fall in patients with bilateral knee osteoarthritis. Sci. World J. 2014, 2014, 815184. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Xu, P.; Huang, G.; Liu, L. Clinical therapy of hyaluronic acid combined with platelet-rich plasma for the treatment of knee osteoarthritis. Exp. Ther. Med. 2018, 16, 2119–2125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; He, Z.; Shu, L.; Li, X.; Ma, M.; Ye, C. Intra-Articular Platelet-Rich Plasma Combined with Hyaluronic Acid Injection for Knee Osteoarthritis Is Superior to Platelet-Rich Plasma or Hyaluronic Acid Alone in Inhibiting Inflammation and Improving Pain and Function. Arthrosc. J. Arthrosc. Relat. Surg. 2021, 37, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Khalaj, N.; Abu Osman, N.A.; Mokhtar, A.H.; Mehdikhani, M.; Wan Abas, W.A. Balance and risk of fall in individuals with bilateral mild and moderate knee osteoarthritis. PLoS ONE 2014, 9, e92270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinman, R.S.; Bennell, K.L.; Metcalf, B.R.; Crossley, K.M. Balance impairments in individuals with symptomatic knee osteoarthritis: A comparison with matched controls using clinical tests. Rheumatology 2002, 41, 1388–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, B.S.; Doherty, S.A.; Mockett, S.; Doherty, M. Effect of pain reduction on postural sway, proprioception, and quadriceps strength in subjects with knee osteoarthritis. Ann. Rheum. Dis. 2002, 61, 422–428. [Google Scholar] [CrossRef]
- Maia, P.A.V.; Cossich, V.R.A.; Salles-Neto, J.I.; Aguiar, D.P.; de Sousa, E.B. Viscosupplementation improves pain, function and muscle strength, but not proprioception, in patients with knee osteoarthritis: A prospective randomized trial. Clinics 2019, 74, e1207. [Google Scholar] [CrossRef] [Green Version]
- Ju, S.B.; Park, G.D.; Kim, S.S. Effects of proprioceptive circuit exercise on knee joint pain and muscle function in patients with knee osteoarthritis. J. Phys. Ther. Sci. 2015, 27, 2439–2441. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.Y.; Li, T.Y.; Lam, K.H.S.; Chou, Y.C.; Hueng, D.Y.; Chen, L.C.; Wu, Y.T. The long-term analgesic effectiveness of platelet-rich plasma injection for carpal tunnel syndrome: A cross-sectional cohort study. Pain Med. 2022. [Google Scholar] [CrossRef]

| Intervention (PRP + HA) Group (n = 22) | Control (PRP + NS) Group (n = 23) | ap Value | |
|---|---|---|---|
| Gender, n (%) | 0.766 | ||
| Female | 18 (81.8) | 18 (78.3) | |
| Male | 4 (18.2) | 5 (21.7) | |
| Age (year) ± SE (range) | 62.2 ± 1.5 (50–74) | 61.3 ± 1.4 (50–75) | 0.569 |
| BMI (kg/m2) | 26.3 ± 0.9 | 25.5 ± 0.7 | 0.683 |
| DM (%) | 1 (4.5) | 3 (13.0) | 0.608 |
| Hypertension (%) | 11 (50.0) | 5 (21.7) | 0.065 |
| Lesion site, n (%) | 0.449 | ||
| Left | 9 (40.9) | 12 (52.2) | |
| Right | 13 (59.1) | 11 (47.8) | |
| Duration (month) ± SE (range) | 34.6 ± 6.3 (6–120) | 31.3 ± 7.3 (6–120) | 0.506 |
| Ahlback Stage | 0.896 | ||
| I | 12 (54.5) | 13 (56.5) | |
| II | 5 (22.7) | 6 (26.1) | |
| III | 5 (22.7) | 4 (17.4) | |
| VAS (SE) | 5.5 ± 0.2 | 5.7 ± 0.2 | 0.404 |
| Lequesne index (SE) | 11.9 ± 0.7 | 10.9 ± 0.5 | 0.308 |
| Intervention (PRP + HA) Group (n = 22) Mean ± SE Mean Difference ± SE | a p value | Control (PRP + NS) Group (n = 23) Mean ± SE Mean Difference ± SE | ap Value | bp Value | |||
|---|---|---|---|---|---|---|---|
| WOMAC (pain) | 19.9 ± 1.4 | 19.0 ± 1.1 | 0.873 | ||||
| Month 1 | 10.0 ± 1.4 | −10.0 ± 1.5 | <0.001 | 13.3 ± 1.1 | −5.7 ± 1.3 | 0.001 | 0.062 |
| Month 3 | 7.4 ± 1.2 | −12.5 ± 1.5 | <0.001 | 11.4 ± 1.0 | −7.6 ± 1.1 | <0.001 | 0.017 |
| Month 6 | 7.1 ± 1.2 | −12.9 ± 1.4 | <0.001 | 10.6 ± 1.1 | −8.4 ± 1.2 | <0.001 | 0.020 |
| Month 12 | 7.8 ± 1.3 | −12.1 ± 1.4 | <0.001 | 10.8 ± 1.1 | −7.8 ± 0.9 | <0.001 | 0.049 |
| WOMAC (stiffness) | 8.5 ± 0.5 | 7.4 ± 0.4 | 0.107 | ||||
| Month 1 | 5.1 ± 0.7 | −3.4 ± 0.7 | 0.001 | 4.9 ± 0.5 | −2.4 ± 0.6 | 0.002 | 0.551 |
| Month 3 | 3.4 ± 0.6 | −5.1 ± 0.7 | <0.001 | 4.1 ± 0.6 | −3.2 ± 0.7 | 0.001 | 0.083 |
| Month 6 | 2.9 ± 0.5 | −5.6 ± 0.7 | <0.001 | 4.4 ± 0.5 | −3.0 ± 0.5 | <0.001 | 0.006 |
| Month 12 | 2.5 ± 0.5 | −6.0 ± 0.6 | <0.001 | 3.7 ± 0.5 | −3.7 ± 0.5 | <0.001 | 0.006 |
| WOMAC (function) | 67.1 ± 5.1 | 60.5 ± 4.2 | 0.207 | ||||
| Month 1 | 40.9 ± 5.5 | −26.3 ± 5.4 | 0.001 | 39.8 ± 3.4 | −20.7 ± 2.8 | <0.001 | 0.910 |
| Month 3 | 29.6 ± 4.6 | −37.6 ± 5.2 | <0.001 | 36.8 ± 3.5 | −23.7 ± 3.3 | <0.001 | 0.112 |
| Month 6 | 25.4 ± 3.9 | −41.7 ± 4.2 | <0.001 | 28.4 ± 3.0 | −32.1 ± 3.7 | <0.001 | 0.102 |
| Month 12 | 25.4 ± 3.8 | −41.8 ± 4.5 | <0.001 | 31.1 ± 3.4 | −29.4 ± 4.2 | <0.001 | 0.080 |
| WOMAC (total) | 95.5 ± 6.5 | 86.9 ± 5.1 | 0.237 | ||||
| Month 1 | 55.9 ± 7.3 | −39.6 ± 7.3 | <0.001 | 58.1 ± 4.6 | −28.8 ± 3.8 | <0.001 | 0.433 |
| Month 3 | 40.3 ± 6.1 | −55.2 ± 6.9 | <0.001 | 52.4 ± 4.6 | −34.5 ± 4.2 | <0.001 | 0.040 |
| Month 6 | 35.3 ± 5.3 | −60.2 ± 5.7 | <0.001 | 43.3 ± 4.1 | −43.6 ± 4.7 | <0.001 | 0.051 |
| Month 12 | 35.6 ± 5.4 | −59.9 ± 5.9 | <0.001 | 45.6 ± 4.3 | −41.3 ± 4.5 | <0.001 | 0.021 |
| Intervention (PRP + HA) Group (n = 22) | Control (PRP + NS) Group (n = 23) | ap Value | |
|---|---|---|---|
| n (%) | n (%) | ||
| WOMAC (pain) | |||
| Month 1 | 17 (77.3) | 17 (73.9) | 0.793 |
| Month 3 | 20 (90.9) | 17 (73.9) | 0.243 |
| Month 6 | 21 (95.5) | 20 (87.0) | 0.608 |
| Month 12 | 22 (100) | 21 (91.3) | 0.489 |
| WOMAC (function) | |||
| Month 1 | 14 (63.6) | 16 (69.6) | 0.673 |
| Month 3 | 19 (86.4) | 19 (82.6) | 0.728 |
| Month 6 | 20 (90.9) | 22 (95.6) | 0.608 |
| Month 12 | 21 (95.5) | 20 (87.0) | 0.608 |
| Intervention (PRP + HA) Group (n = 22) Mean ± SE Mean Difference ± SE | ap Value | Control (PRP + NS) Group (n = 23) Mean ± SE Mean Difference ± SE | ap Value | bp Value | |||
|---|---|---|---|---|---|---|---|
| Balance-OSI | 0.69 ± 0.04 | 0.65 ± 0.08 | 0.255 | ||||
| Month 1 | 0.54 ± 0.04 | −0.15 ± 0.03 | <0.001 | 0.58 ± 0.07 | −0.07 ± 0.06 | 0.046 | 0.240 |
| Month 3 | 0.44 ± 0.03 | −0.25 ± 0.03 | <0.001 | 0.51 ± 0.05 | −0.15 ± 0.07 | 0.056 | 0.020 |
| Month 6 | 0.43 ± 0.04 | −0.26 ± 0.03 | <0.001 | 0.52 ± 0.05 | −0.14 ± 0.06 | 0.027 | 0.030 |
| Month 12 | 0.40 ± 0.03 | −0.29 ± 0.03 | <0.001 | 0.47 ± 0.05 | −0.18 ± 0.08 | 0.025 | 0.019 |
| Balance-APSI | 0.52 ± 0.03 | 0.52 ± 0.07 | 0.413 | ||||
| Month 1 | 0.38 ± 0.03 | −0.14 ± 0.02 | <0.001 | 0.41 ± 0.05 | −0.11 ± 0.05 | 0.037 | 0.108 |
| Month 3 | 0.35 ± 0.03 | −0.17 ± 0.03 | <0.001 | 0.39 ± 0.04 | −0.13 ± 0.07 | 0.135 | 0.114 |
| Month 6 | 0.33 ± 0.03 | −0.19 ± 0.03 | <0.001 | 0.39 ± 0.04 | −0.12 ± 0.06 | 0.087 | 0.073 |
| Month 12 | 0.32 ± 0.02 | −0.20 ± 0.03 | <0.001 | 0.39 ± 0.05 | −0.13 ± 0.06 | 0.074 | 0.097 |
| Balance-MLSI | 0.36 ± 0.03 | 0.32 ± 0.05 | 0.127 | ||||
| Month 1 | 0.29 ± 0.04 | −0.07 ± 0.03 | 0.025 | 0.30 ± 0.06 | −0.03 ± 0.07 | 0.064 | 0.600 |
| Month 3 | 0.22 ± 0.02 | −0.15 ± 0.03 | <0.001 | 0.28 ± 0.05 | −0.04 ± 0.06 | 0.384 | 0.023 |
| Month 6 | 0.25 ± 0.03 | −0.11 ± 0.03 | 0.001 | 0.30 ± 0.03 | −0.03 ± 0.05 | 0.765 | 0.042 |
| Month 12 | 0.22 ± 0.03 | −0.14 ± 0.04 | 0.001 | 0.25 ± 0.03 | −0.07 ± 0.06 | 0.262 | 0.143 |
| Risk fall-6 level | 2.3 ± 0.2 | 2.1 ± 0.2 | 0.532 | ||||
| Month 1 | 1.9 ± 0.2 | −0.4 ± 0.1 | 0.001 | 1.9 ± 0.2 | −0.2 ± 0.1 | 0.030 | 0.180 |
| Month 3 | 1.6 ± 0.1 | −0.7 ± 0.1 | <0.001 | 1.7 ± 0.2 | −0.4 ± 0.1 | 0.004 | 0.149 |
| Month 6 | 1.5 ± 0.1 | −0.8 ± 0.1 | <0.001 | 1.6 ± 0.2 | −0.5 ± 0.2 | 0.005 | 0.334 |
| Month 12 | 1.4 ± 0.1 | −0.9 ± 0.2 | <0.001 | 1.4 ± 0.2 | −0.8 ± 0.1 | <0.001 | 0.829 |
| Risk fall-8 level | 1.3 ± 0.1 | 1.1 ± 0.1 | 0.407 | ||||
| Month 1 | 1.1 ± 0.1 | −0.1 ± 0.04 | 0.004 | 1.0 ± 0.1 | −0.1 ± 0.04 | 0.020 | 1.000 |
| Month 3 | 1.0 ± 0.1 | −0.3 ± 0.07 | 0.002 | 1.0 ± 0.1 | −0.1 ± 0.05 | 0.005 | 0.184 |
| Month 6 | 1.0 ± 0.1 | −0.2 ± 0.07 | 0.003 | 1.0 ± 0.1 | −0.1 ± 0.05 | 0.005 | 0.439 |
| Month 12 | 0.9 ± 0.1 | −0.4 ± 0.10 | 0.001 | 0.9 ± 0.1 | −0.3 ± 0.05 | <0.001 | 0.323 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-T.; Li, T.-Y.; Lee, K.-C.; Lam, K.H.S.; Chang, C.-Y.; Chang, C.-K.; Chen, L.-C. Efficacy of a Novel Intra-Articular Administration of Platelet-Rich Plasma One-Week Prior to Hyaluronic Acid versus Platelet-Rich Plasma Alone in Knee Osteoarthritis: A Prospective, Randomized, Double-Blind, Controlled Trial. J. Clin. Med. 2022, 11, 3241. https://doi.org/10.3390/jcm11113241
Wu Y-T, Li T-Y, Lee K-C, Lam KHS, Chang C-Y, Chang C-K, Chen L-C. Efficacy of a Novel Intra-Articular Administration of Platelet-Rich Plasma One-Week Prior to Hyaluronic Acid versus Platelet-Rich Plasma Alone in Knee Osteoarthritis: A Prospective, Randomized, Double-Blind, Controlled Trial. Journal of Clinical Medicine. 2022; 11(11):3241. https://doi.org/10.3390/jcm11113241
Chicago/Turabian StyleWu, Yung-Tsan, Tsung-Ying Li, Kuei-Chen Lee, King Hei Stanley Lam, Chih-Ya Chang, Cheng-Kuang Chang, and Liang-Cheng Chen. 2022. "Efficacy of a Novel Intra-Articular Administration of Platelet-Rich Plasma One-Week Prior to Hyaluronic Acid versus Platelet-Rich Plasma Alone in Knee Osteoarthritis: A Prospective, Randomized, Double-Blind, Controlled Trial" Journal of Clinical Medicine 11, no. 11: 3241. https://doi.org/10.3390/jcm11113241
APA StyleWu, Y.-T., Li, T.-Y., Lee, K.-C., Lam, K. H. S., Chang, C.-Y., Chang, C.-K., & Chen, L.-C. (2022). Efficacy of a Novel Intra-Articular Administration of Platelet-Rich Plasma One-Week Prior to Hyaluronic Acid versus Platelet-Rich Plasma Alone in Knee Osteoarthritis: A Prospective, Randomized, Double-Blind, Controlled Trial. Journal of Clinical Medicine, 11(11), 3241. https://doi.org/10.3390/jcm11113241







