Predictors and Consequences of Sac Shrinkage after Endovascular Infrarenal Aortic Aneurysm Repair
Abstract
:1. Introduction
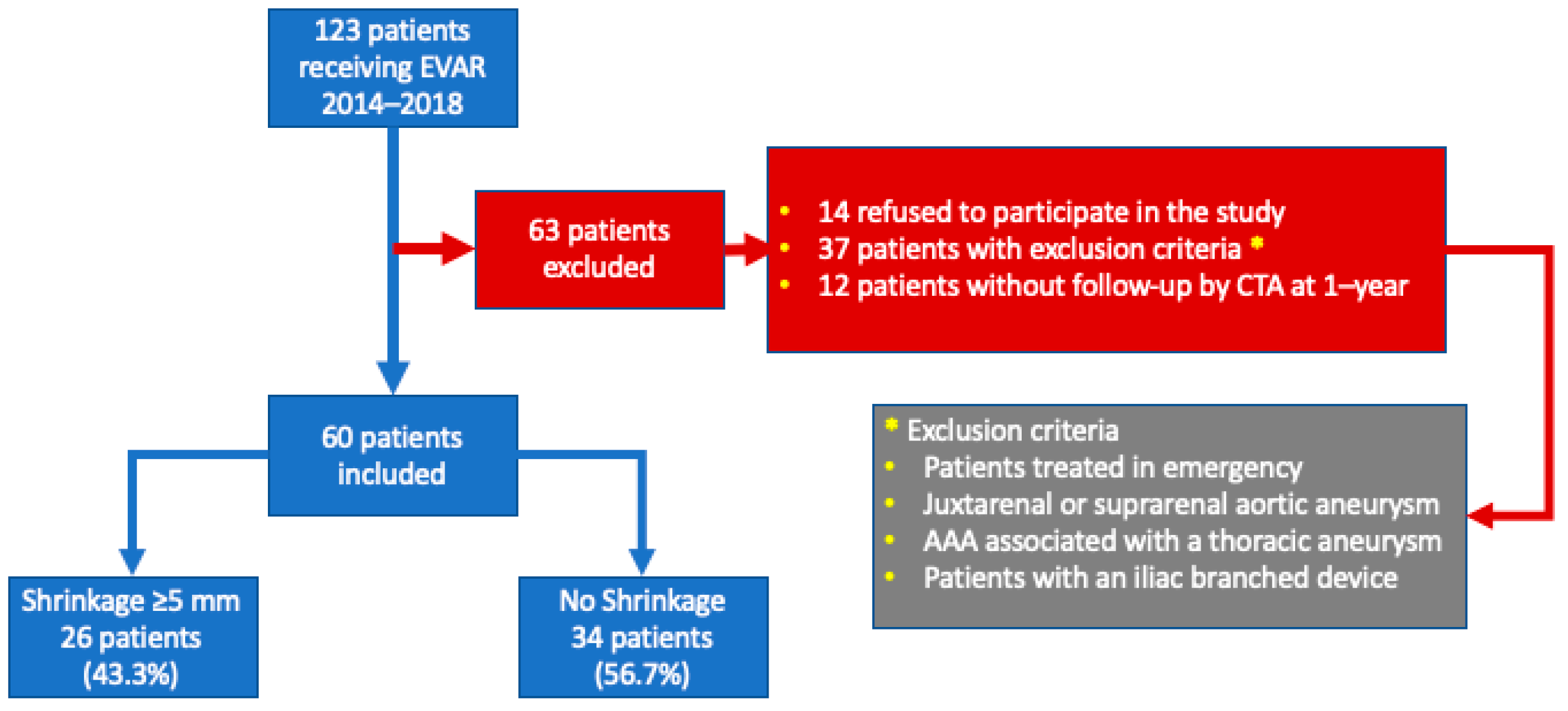
2. Materials and Methods
2.1. Exclusion Criteria
2.2. Data Management
2.3. Endpoints
2.4. Statistical Analysis
3. Results
3.1. Population Demographics and Baseline Characteristics
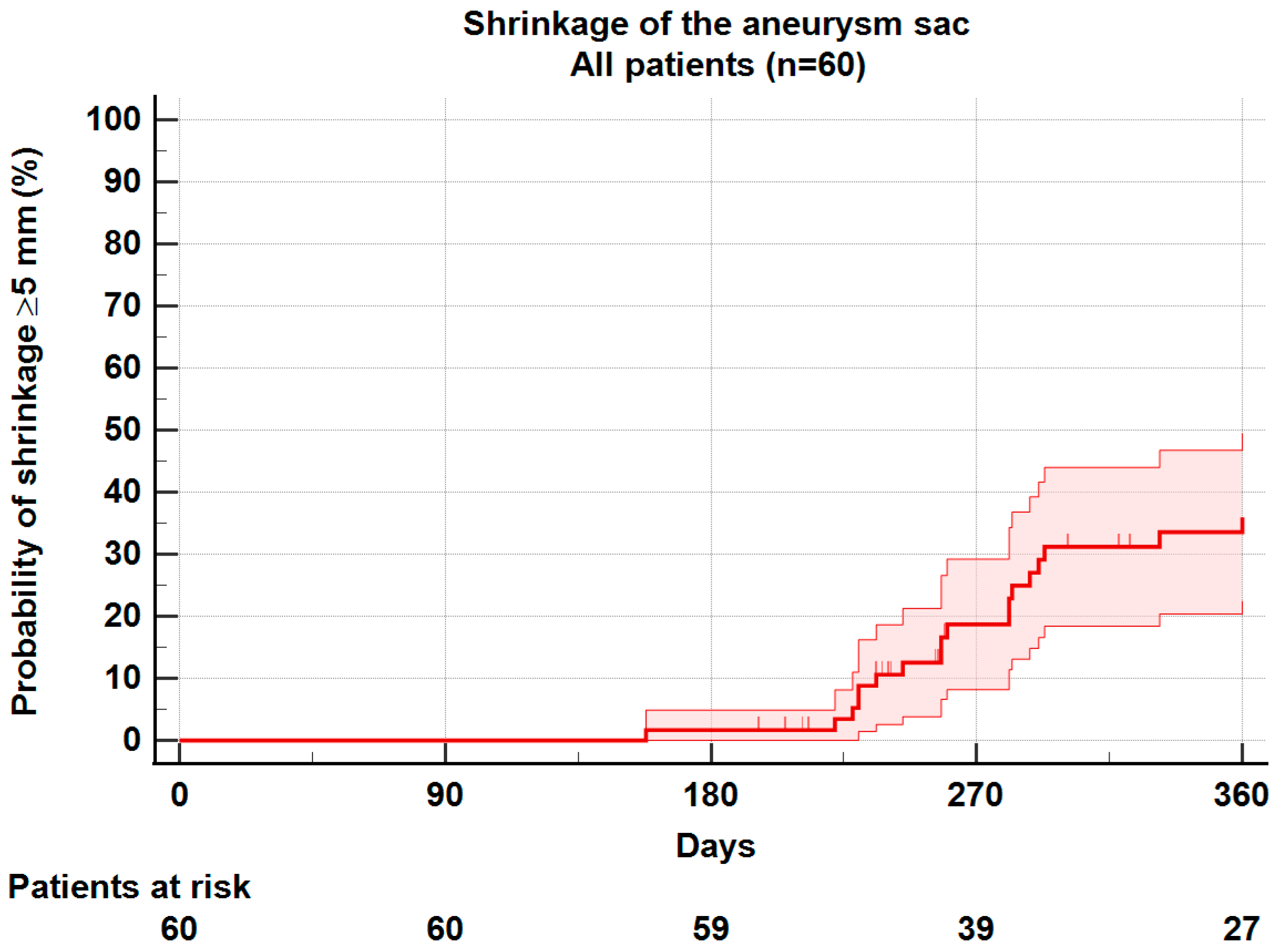
3.2. Sac Shrinkage
3.3. Demographics and Comorbidities
3.4. Aneurysm Morphology
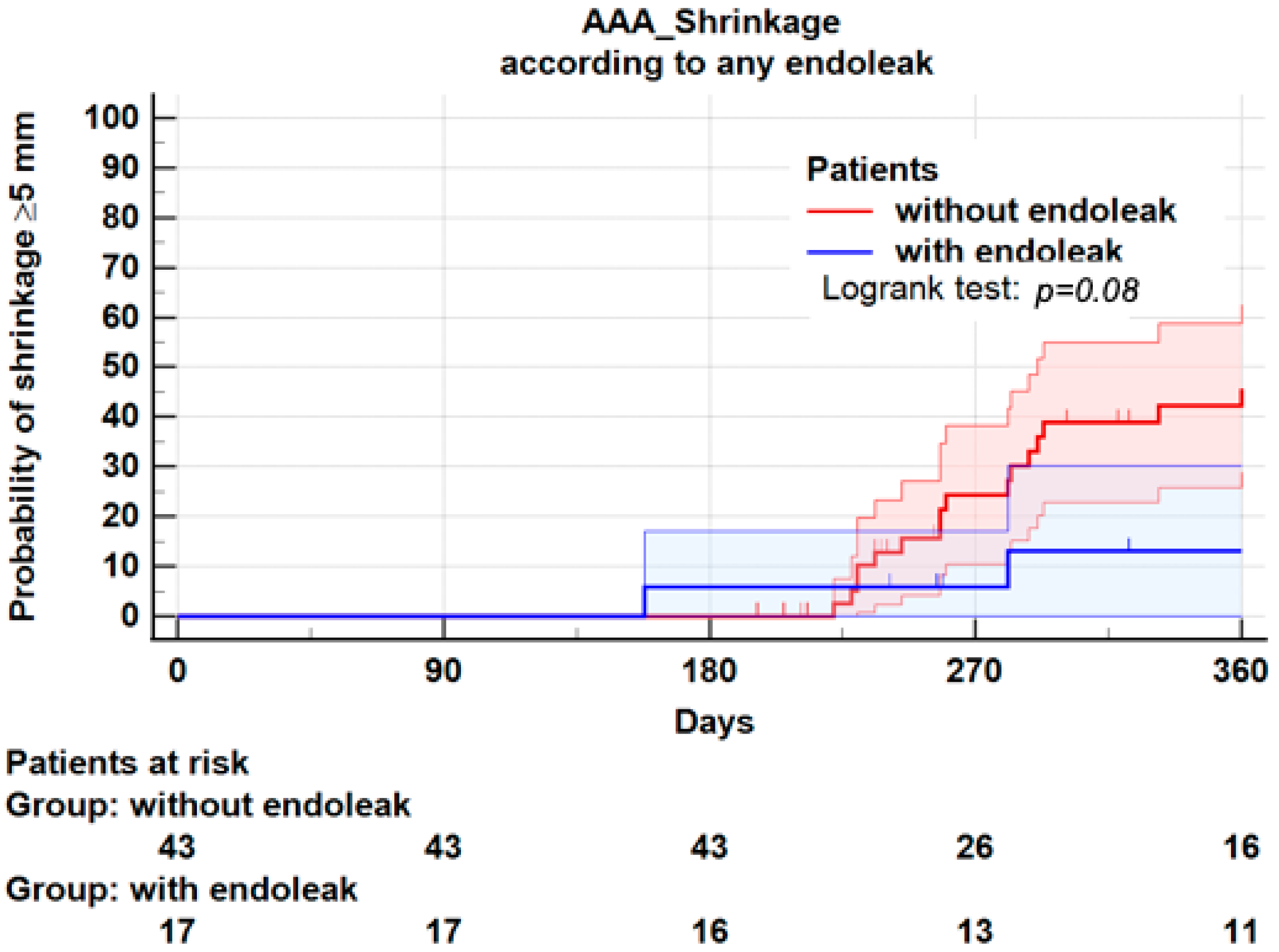
3.5. Endoleaks
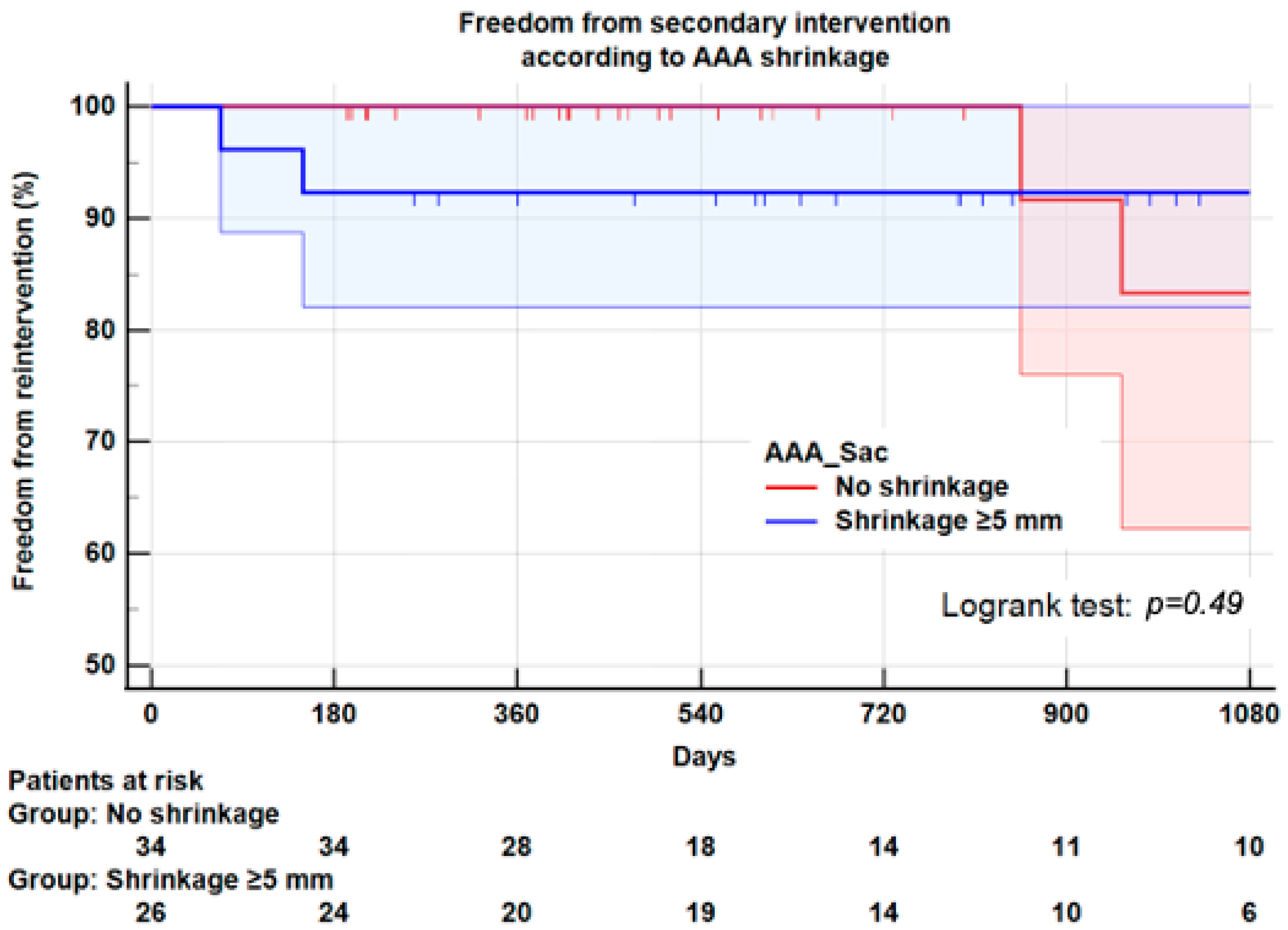
3.6. Reintervention
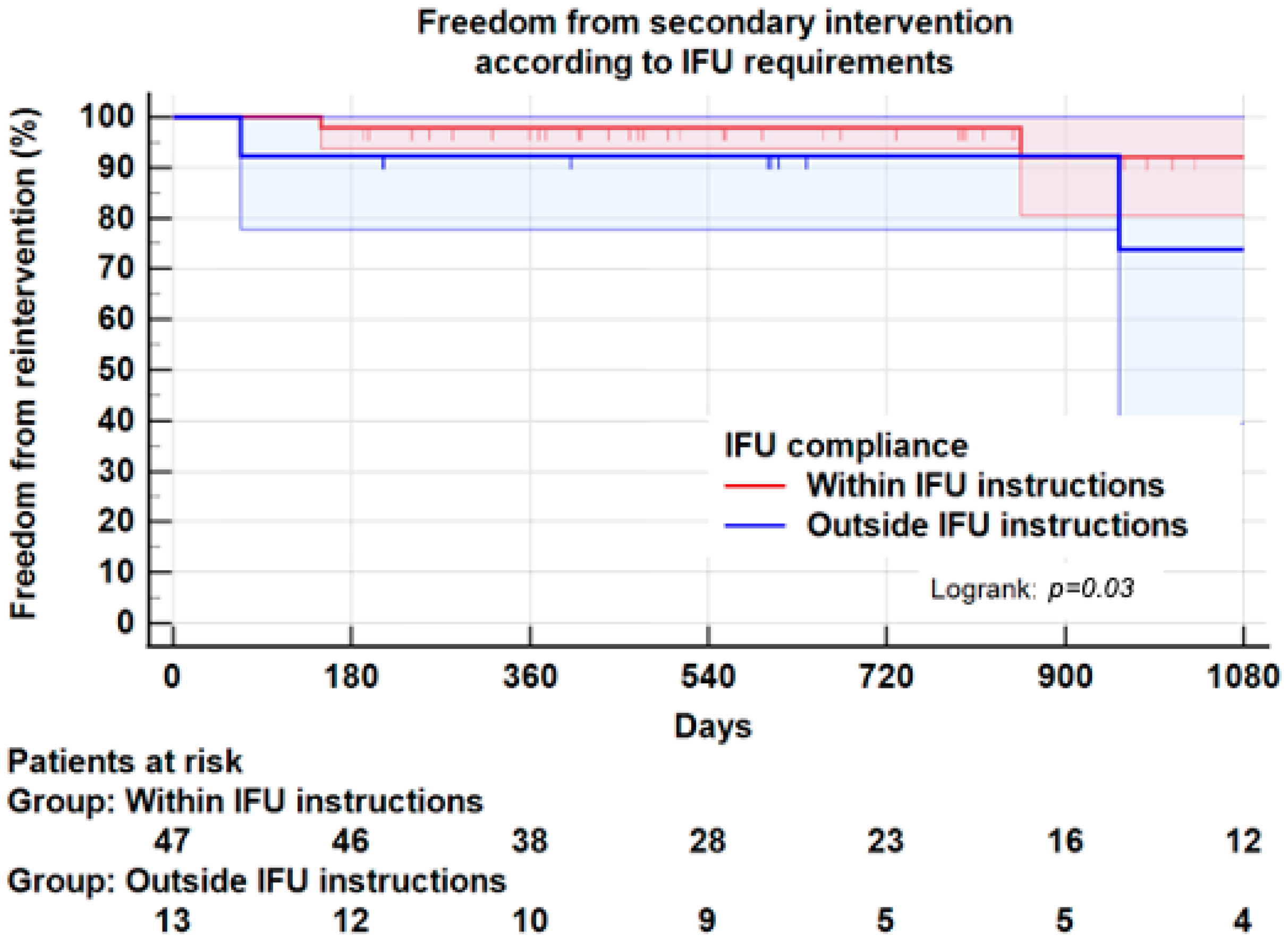
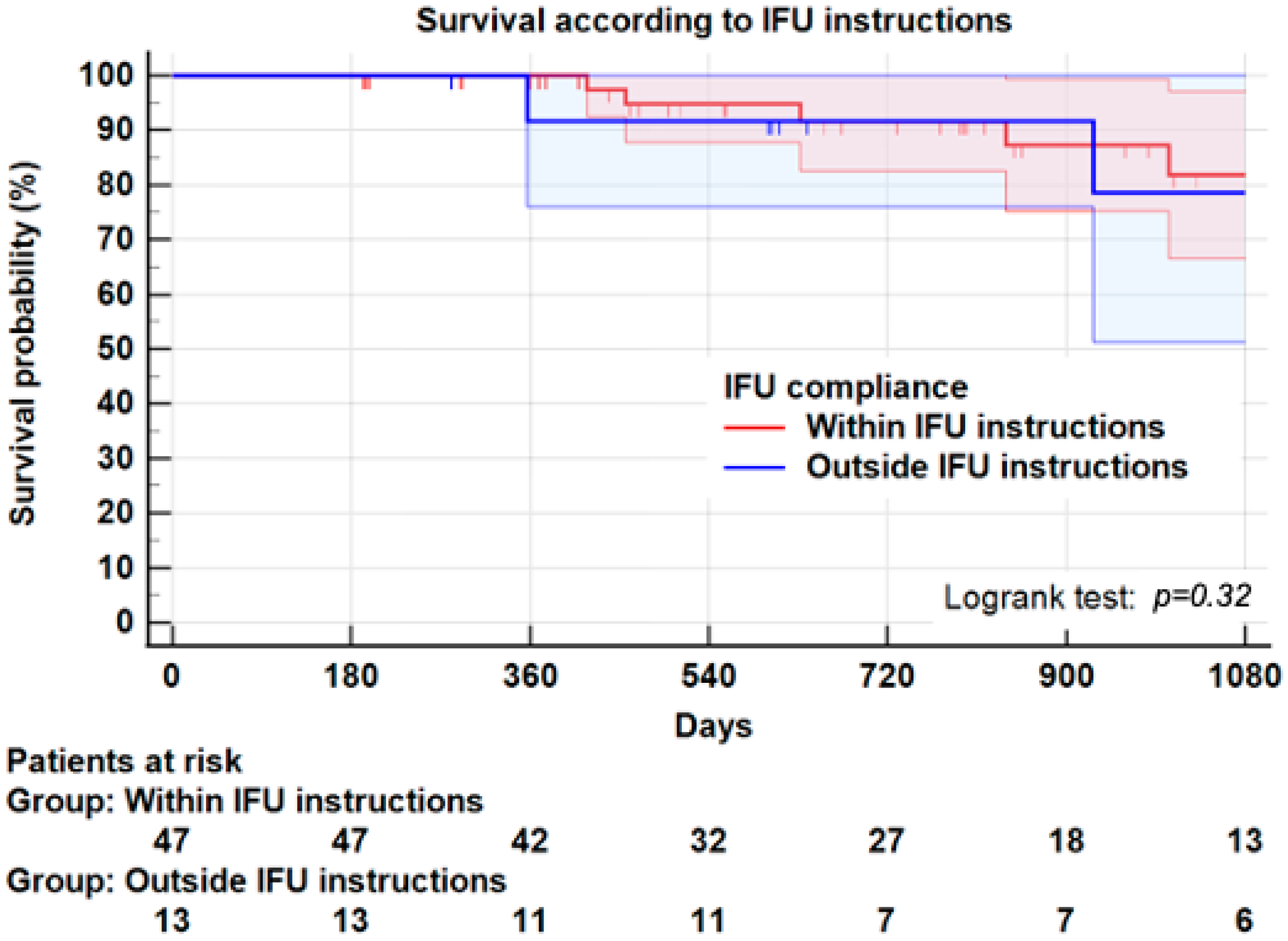
3.7. Instructions for Use
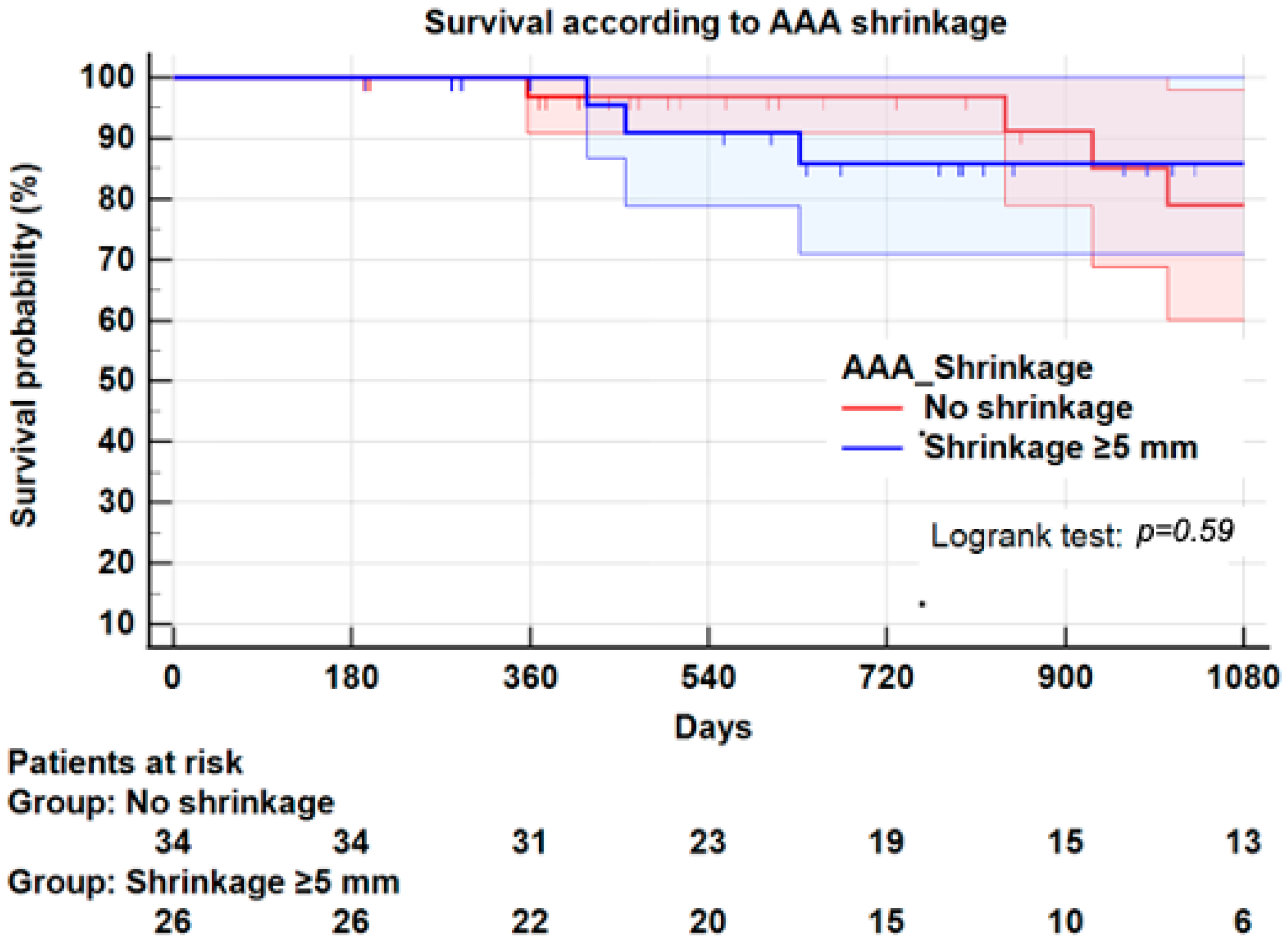
3.8. Survival
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parodi, J.C.; Palmaz, J.C.; Barone, H.D. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann. Vasc. Surg. 1991, 5, 491–499. [Google Scholar] [CrossRef]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EVAR Trial Participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): Randomised controlled trial. Lancet 2005, 365, 2179–2186. [Google Scholar] [CrossRef]
- Blankensteijn, J.D.; de Jong, S.E.; Prinssen, M.; van der Ham, A.C.; Buth, J.; van Sterkenburg, S.M.; Verhagen, H.J.; Buskens, E.; Grobbee, D.E.; Dutch Randomized Endovascular Aneurysm Management (DREAM) Trial Group. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N. Engl. J. Med. 2005, 352, 2398–2405. [Google Scholar] [CrossRef] [Green Version]
- Chaikof, E.L.; Blankensteijn, J.D.; Harris, P.L.; White, G.H.; Zarins, C.K.; Bernhard, V.M.; Matsumura, J.S.; May, J.; Veith, F.J.; Fillinger, M.F.; et al. Reporting standards for endovascular aortic aneurysm repair. J. Vasc. Surg. 2002, 35, 1048–1060. [Google Scholar] [CrossRef] [Green Version]
- Bastos Goncalves, F.; Baderkhan, H.; Verhagen, H.J.; Wanhainen, A.; Bjorck, M.; Stolker, R.J.; Hoeks, S.E.; Mani, K. Early sac shrinkage predicts a low risk of late complications after endovascular aortic aneurysm repair. Br. J. Surg. 2014, 101, 802–810. [Google Scholar] [CrossRef] [Green Version]
- Houbballah, R.; Majewski, M.; Becquemin, J.P. Significant sac retraction after endovascular aneurysm repair is a robust indicator of durable treatment success. J. Vasc. Surg. 2010, 52, 878–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deery, S.E.; Ergul, E.A.; Schermerhorn, M.L.; Siracuse, J.J.; Schanzer, A.; Goodney, P.P.; Cambria, R.P.; Patel, V.I.; Vascular Study Group of New England. Aneurysm sac expansion is independently associated with late mortality in patients treated with endovascular aneurysm repair. J. Vasc. Surg. 2018, 67, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, T.F.X.; Deery, S.E.; Boitano, L.T.; Siracuse, J.J.; Schermerhorn, M.L.; Scali, S.T.; Schanzer, A.; Lancaster, R.T.; Patel, V.I. Aneurysm sac failure to regress after endovascular aneurysm repair is associated with lower long-term survival. J. Vasc. Surg. 2019, 69, 414–422. [Google Scholar] [CrossRef]
- Fairman, R.M.; Nolte, L.; Snyder, S.A.; Chuter, T.A.; Greenberg, R.K.; Zenith, I. Factors predictive of early or late aneurysm sac size change following endovascular repair. J. Vasc. Surg. 2006, 43, 649–656. [Google Scholar] [CrossRef] [Green Version]
- Schanzer, A.; Greenberg, R.K.; Hevelone, N.; Robinson, W.P.; Eslami, M.H.; Goldberg, R.J.; Messina, L. Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation 2011, 123, 2848–2855. [Google Scholar] [CrossRef]
- Keith, C.J.; Jr Passman, M.A.; Gaffud, M.J.; Novak, Z.; Pearce, B.J.; Matthews, T.C.; Patterson, M.A.; Jordan, W.D. Comparison of outcomes following endovascular repair of abdominal aortic aneurysms based on size threshold. J. Vasc. Surg. 2013, 58, 1458–1466. [Google Scholar] [CrossRef]
- Peppelenbosch, N.; Buth, J.; Harris, P.L.; van Marrewijk, C.; Fransen, G.; Collaborators, E. Diameter of abdominal aortic aneurysm and outcome of endovascular aneurysm repair: Does size matter? A report from EUROSTAR. J. Vasc. Surg. 2004, 39, 288–297. [Google Scholar] [CrossRef] [Green Version]
- van Keulen, J.W.; Moll, F.L.; Tolenaar, J.L.; Verhagen, H.J.; van Herwaarden, J.A. Validation of a new standardized method to measure proximal aneurysm neck angulation. J. Vasc. Surg. 2010, 51, 821–828. [Google Scholar] [CrossRef] [Green Version]
- Rouby, A.F.; Kuntz, S.; Delay, C.; Thaveau, F.; Georg, Y.; Lejay, A.; Chakfe, N. Volume Change after Endovascular Treatment of Common Iliac Arteries ≥ 17 mm Diameter: Assessment of Type 1b Endoleak Risk Factors. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Poldermans, D.; Bax, J.J.; Boersma, E.; De Hert, S.; Eeckhout, E.; Fowkes, G.; Gorenek, B.; Hennerici, M.G.; Iung, B.; Kelm, M.; et al. Guidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery: The Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery of the European Society of Cardiology (ESC) and endorsed by the European Society of Anaesthesiology (ESA). Eur. J. Anaesthesiol. 2010, 27, 92–137. [Google Scholar] [CrossRef] [Green Version]
- Staitieh, B.S.; Ioachimescu, O.C. Interpretation of pulmonary function tests: Beyond the basics. J. Investig. Med. 2017, 65, 301–310. [Google Scholar] [CrossRef]
- Porrini, E.; Ruggenenti, P.; Luis-Lima, S.; Carrara, F.; Jiménez, A.; de Vries, A.P.J.; Torres, A.; Gaspari, F.; Remuzzi, G. Estimated GFR: Time for a critical appraisal. Nat. Rev. Nephrol. 2019, 15, 177–190. [Google Scholar] [CrossRef]
- Schanzer, A.; Oderich, G.S. Management of Abdominal Aortic Aneurysms. N. Engl. J. Med. 2021, 385, 1690–1698. [Google Scholar] [CrossRef]
- Teijink, J.A.W.; Power, A.H.; Böckler, D.; Peeters, P.; van Sterkenburg, S.; Bouwman, L.H.; Verhagen, H.J.; Bosiers, M.; Riambau, V.; Becquemin, J.P.; et al. Editor’s Choice—Five Year Outcomes of the Endurant Stent Graft for Endovascular Abdominal Aortic Aneurysm Repair in the ENGAGE Registry. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2019, 58, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Soler, R.J.; Bartoli, M.A.; Mancini, J.; Lerussi, G.; Thevenin, B.; Sarlon-Bartoli, G.; Magnan, P.E. Aneurysm sac shrinkage after endovascular repair: Predictive factors and long-term follow-up. Ann. Vasc. Surg. 2015, 29, 770–779. [Google Scholar] [CrossRef]
- Cieri, E.; De Rango, P.; Isernia, G.; Simonte, G.; Verzini, F.; Parlani, G.; Ciucci, A.; Cao, P. Effect of stentgraft model on aneurysm shrinkage in 1450 endovascular aortic repairs. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Rhee, R.Y.; Eskandari, M.K.; Zajko, A.B.; Makaroun, M.S. Long-term fate of the aneurysmal sac after endoluminal exclusion of abdominal aortic aneurysms. J. Vasc. Surg. 2000, 32, 689–696. [Google Scholar] [CrossRef] [Green Version]
- Zarins, C.K.; Crabtree, T.; Bloch, D.A.; Arko, F.R.; Ouriel, K.; White, R.A. Endovascular aneurysm repair at 5 years: Does aneurysm diameter predict outcome? J. Vasc. Surg. 2006, 44, 920–929; discussion 9–31. [Google Scholar] [CrossRef] [Green Version]
- Sadek, M.; Dexter, D.J.; Rockman, C.B.; Hoang, H.; Mussa, F.F.; Cayne, N.S.; Jacobowitz, G.R.; Veith, F.J.; Adelman, M.A.; Maldonado, T.S. Preoperative relative abdominal aortic aneurysm thrombus burden predicts endoleak and sac enlargement after endovascular anerysm repair. Ann. Vasc. Surg. 2013, 27, 1036–1041. [Google Scholar] [CrossRef]
- Lalys, F.; Daoudal, A.; Gindre, J.; Goksu, C.; Lucas, A.; Kaladji, A. Influencing factors of sac shrinkage after endovascular aneurysm repair. J. Vasc. Surg. 2017, 65, 1830–1838. [Google Scholar] [CrossRef] [Green Version]
- Kazi, M.; Thyberg, J.; Religa, P.; Roy, J.; Eriksson, P.; Hedin, U.; Swedenborg, J. Influence of intraluminal thrombus on structural and cellular composition of abdominal aortic aneurysm wall. J. Vasc. Surg. 2003, 38, 1283–1292. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, A.; Morita, H.; Hayashi, N.; Nomura, Y.; Hoshina, K.; Shigematsu, K.; Ohtsu, H.; Miyata, T.; Komuro, I. Inverse Correlation Between Calcium Accumulation and the Expansion Rate of Abdominal Aortic Aneurysms. Circ. J. 2016, 80, 332–339. [Google Scholar] [CrossRef] [Green Version]
- Lindholt, J.S. Aneurysmal wall calcification predicts natural history of small abdominal aortic aneurysms. Atherosclerosis 2008, 197, 673–678. [Google Scholar] [CrossRef]
- Bailey, M.A.; Sohrabi, S.; Flood, K.; Griffin, K.J.; Rashid, S.T.; Johnson, A.B.; Baxter, P.D.; Patel, J.V.; Scott, D.J. Calcium channel blockers enhance sac shrinkage after endovascular aneurysm repair. J. Vasc. Surg. 2012, 55, 1593–1599. [Google Scholar] [CrossRef] [Green Version]
- Twine, C.P.; Williams, I.M. Systematic review and meta-analysis of the effects of statin therapy on abdominal aortic aneurysms. Br. J. Surg. 2011, 98, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Evans, J.; Bell, P.R.; Thompson, M.M. HMG-CoA reductase inhibitors (statins) decrease MMP-3 and MMP-9 concentrations in abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 2005, 30, 259–262. [Google Scholar] [CrossRef] [Green Version]
- Bertges, D.J.; Chow, K.; Wyers, M.C.; Landsittel, D.; Frydrych, A.V.; Tan, W.A.; Rhee, R.Y.; Fillinger, M.F.; Fairman, R.M.; Stavropoulos, W.; et al. Abdominal aortic aneurysm size regression after endovascular repair is endograft dependent. J. Vasc. Surg. 2003, 37, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Broker, H.S.; Foteh, K.I.; Murphy, E.H.; Davis, C.M.; Clagett, G.P.; Modrall, J.G.; Buckley, C.J.; Arko, F.R. Device-specific aneurysm sac morphology after endovascular aneurysm repair: Evaluation of contemporary graft materials. J. Vasc. Surg. 2008, 47, 702–706; discussion 7. [Google Scholar] [CrossRef] [Green Version]
- Wales, L.; Dunckley, M.; Bohm, N.; Kwok, T.; Bratby, M.; Morgan, R.; Thompson, M.; Loftus, I. Device-specific outcomes following endovascular aortic aneurysm repair. Eur. J. Vasc. Endovasc. Surg. 2008, 36, 661–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benveniste, G.L.; Tjahjono, R.; Chen, O.; Verhagen, H.J.M.; Bockler, D.; Varcoe, R.L. Long-term Results of 180 Consecutive Patients with Abdominal Aortic Aneurysm Treated with the Endurant Stent Graft System. Ann. Vasc. Surg. 2020, 67, 265–273. [Google Scholar] [CrossRef]
- Ward, T.J.; Cohen, S.; Fischman, A.M.; Kim, E.; Nowakowski, F.S.; Ellozy, S.H.; Faries, P.L.; Marin, M.L.; Lookstein, R.A. Preoperative inferior mesenteric artery embolization before endovascular aneurysm repair: Decreased incidence of type II endoleak and aneurysm sac enlargement with 24-month follow-up. J. Vasc. Interv. Radiol. 2013, 24, 49–55. [Google Scholar] [CrossRef]
- Vaillant, M.; Barral, P.A.; Mancini, J.; De Masi, M.; Bal, L.; Piquet, P.; Gaudry, M. Preoperative Inferior Mesenteric Artery Embolization is a Cost-effective Technique that May Reduce the Rate of Aneurysm Sac Diameter Enlargement and Reintervention after EVAR. Ann. Vasc. Surg. 2019, 60, 85–94. [Google Scholar] [CrossRef]
| Patient Characteristics | ||
|---|---|---|
| Mean Age, years (±SD) | 75.3 (±8.3) | |
| N | % | |
| ASA score | ||
| ASA 2 | 16 | 26.7 |
| ASA 3 | 42 | 70.0 |
| ASA 4 | 2 | 3.3 |
| Male gender | 49 | 81.7 |
| Smoking history | 42 | 70.0 |
| Cardiac disease | 28 | 46.7 |
| Hypertension | 49 | 81.7 |
| Diabetes mellitus | 9 | 15.0 |
| Dyslipidaemia | 28 | 46.7 |
| Chronic renal insufficiency * | 13 | 21.7 |
| COPD | 20 | 33.3 |
| Antiplatelet therapy | 28 | 46.7 |
| Statins | 35 | 58.3 |
| Aneurysm Characteristics | Median | IQR |
| Aneurysm maximum diameter (mm) | 57 | (54–61) |
| Neck length (mm) | 28 | (17–36) |
| Neck maximum diameter (mm) | 25 | (24–28) |
| Neck angle (degree) | 27 | (21–46) |
| Iliac arteries maximum diameter (mm) | 18 | (15–20) |
| Thrombus Index (TI) of the sac † | 0.5 | (0–0.5) |
| Other Characteristics | N | % or IQR |
| Inferior Mesenteric Artery patency | 45/60 | 75.0% |
| Number of patent lumbar arteries (±SD) | 5 | (4–7) |
| Patient Characteristics | |||
|---|---|---|---|
| No Shrinkage N = 34 | Shrinkage N = 26 | p-Value | |
| Mean Age, years (±SD) | 76.9 ± 7 | 73.2 ± 9 | 0.08 |
| N (%) | N (%) | ||
| Male gender | 26 (76.5) | 23 (88.5) | 0.32 |
| Smoking history | 24 (70.6) | 18 (69.2) | 0.90 |
| Hypertension | 24 (70.6) | 25 (96.2) | 0.01 |
| Diabetes mellitus | 4 (11.8) | 5 (19.2) | 0.48 |
| Dyslipidaemia | 13 (38.2) | 15 (57.7) | 0.19 |
| Chronic renal insufficiency * | 8 (23.5) | 5 (19.2) | 0.76 |
| COPD | 12 (35.3) | 8 (30.8) | 0.78 |
| Antiaggregant therapy | 17 (50.0) | 11 (42.3) | 0.61 |
| Statins | 19 (55.9) | 16 (61.5) | 0.79 |
| Aneurysm Characteristics | Median (IQR) | Median (IQR) | |
| Aneurysm maximum diameter (mm) | 56 (52–60) | 58 (55–62) | 0.14 |
| Neck length (mm) | 29 (17–36) | 25 (16–35) | 0.55 |
| Neck maximum diameter (mm) | 26 (24–29) | 25(22–28) | 0.41 |
| Neck angle (degree) | 28 (23–46) | 25 (16–45) | 0.53 |
| Neck thrombus (% of circumference) | 25 (0–50) | 0 (0–43) | 0.21 |
| Thrombus Index (TI) of the sac † | 0.50 (0–0.50) | 0.50 (0–0.50) | 0.78 |
| Iliac arteries maximum diameter (mm) | 18 (16–20) | 17 (14–20) | 0.26 |
| Number of patent lumbar arteries (±SD) | 5 (4–6) | 6 (4–7) | 0.96 |
| Other Characteristics | N (%) | N (%) | |
| Inferior Mesenteric Artery patency | 26 (76.5) | 19 (73.1) | 0.77 |
| Failure to comply with at least one IFU instruction (n = 13 patients) †† | 7 (20.6) | 6 (23.1) | 0.89 |
| 1 (2.9) | 1 (3.8) | 0.85 |
| 2 (5.9) | 2 (7.7) | 0.78 |
| 4 (11.8) | 4 (15.4) | 0.72 |
| 2 (5.9) | 2 (7.7) | 0.78 |
| Endoleaks | |||
| All endoleaks | 13 (38.2) | 4 (15.4) | 0.08 |
| Endoleaks Types I–III | 4 (11.8) | 2 (7.7) | 0.60 |
| Endoleaks Type II | 9 (26.5) | 2 (7.7) | 0.08 |
| Reinterventions | |||
| All Ever secondary interventions | 3 (8.8) | 3 (11.5) | 0.73 |
| Index EVAR within IFU N = 48 | Index EVAR outside IFU N = 13 | p-Value | |
|---|---|---|---|
| Characteristics | N (%) | N (%) | |
| All endoleaks | 12 (25.0) | 5 (41.7) | 0.25 |
| Endoleaks Type I–III | 2 (4.2) | 4 (33.3) | 0.01 |
| Endoleaks Type II | 10 (20.8) | 1 (8.3) | 0.32 |
| Reintervention * | 3 (6.3) | 3 (25.0) | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vedani, S.M.; Petitprez, S.; Weinz, E.; Corpataux, J.-M.; Déglise, S.; Deslarzes-Dubuis, C.; Côté, E.; Ricco, J.-B.; Saucy, F. Predictors and Consequences of Sac Shrinkage after Endovascular Infrarenal Aortic Aneurysm Repair. J. Clin. Med. 2022, 11, 3232. https://doi.org/10.3390/jcm11113232
Vedani SM, Petitprez S, Weinz E, Corpataux J-M, Déglise S, Deslarzes-Dubuis C, Côté E, Ricco J-B, Saucy F. Predictors and Consequences of Sac Shrinkage after Endovascular Infrarenal Aortic Aneurysm Repair. Journal of Clinical Medicine. 2022; 11(11):3232. https://doi.org/10.3390/jcm11113232
Chicago/Turabian StyleVedani, Sébastien Michel, Séverine Petitprez, Eva Weinz, Jean-Marc Corpataux, Sébastien Déglise, Céline Deslarzes-Dubuis, Elisabeth Côté, Jean-Baptiste Ricco, and François Saucy. 2022. "Predictors and Consequences of Sac Shrinkage after Endovascular Infrarenal Aortic Aneurysm Repair" Journal of Clinical Medicine 11, no. 11: 3232. https://doi.org/10.3390/jcm11113232






