Interrelations between Gut Microbiota Composition, Nutrient Intake and Diabetes Status in an Adult Japanese Population
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Study Population
2.2. Characteristics Measured
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Subjects
3.2. Differences in the Selected Nutrients Consumed and Proportions of Gut Microbiota Genera between the DM and Non-DM Groups
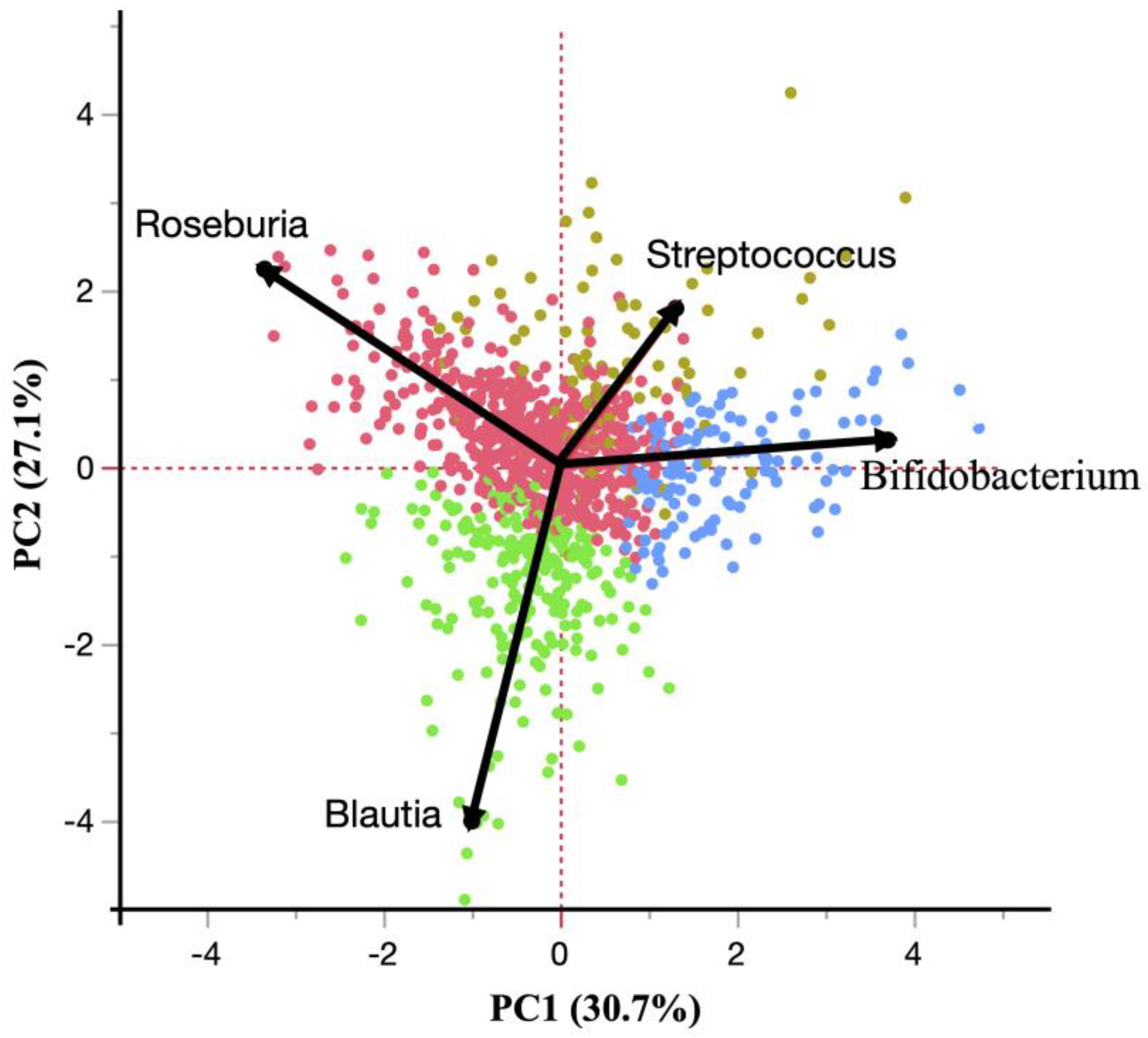
3.3. Correlation between the Selected Nutrients Consumed and Gut Microbiota Genera
3.4. Assessment of Gut Microbiota Genera as Risk Factors for DM
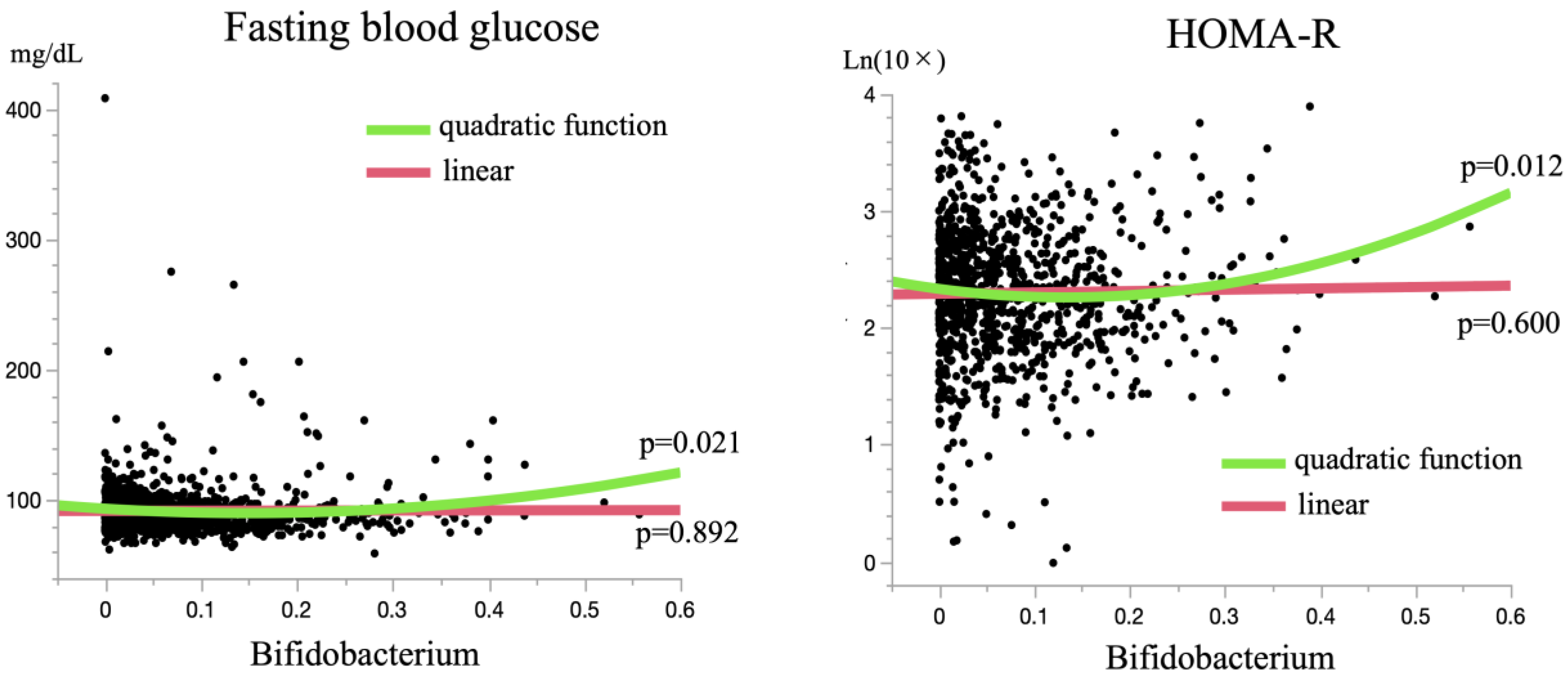
3.5. A Bifidobacterium-Dominant Gut Microbiota Is a Risk Factor for DM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Pathogenesis of type 2 diabetes mellitus. Med. Clin. N. Am. 2004, 88, 787–835. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Microbiota and diabetes: An evolving relationship. Gut 2014, 63, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Xu, J.; Lian, F.; Zhao, L.; Zhao, Y.; Chen, X.; Zhang, X.; Guo, Y.; Zhang, C.; Zhou, Q.; Xue, Z.; et al. Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula. ISME J. 2015, 9, 552–562. [Google Scholar] [CrossRef]
- Candela, M.; Biagi, E.; Soverini, M.; Consolandi, C.; Quercia, S.; Severgnini, M.; Peano, C.; Turroni, S.; Rampelli, S.; Pozzilli, P.; et al. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br. J. Nutr. 2016, 116, 80–93. [Google Scholar] [CrossRef]
- Barengolts, E.; Green, S.J.; Eisenberg, Y.; Akbar, A.; Reddivari, B.; Layden, B.T.; Dugas, L.; Chlipala, G. Gut microbiota varies by opioid use, circulating leptin and oxytocin in African American men with diabetes and high burden of chronic disease. PLoS ONE 2018, 13, e0194171. [Google Scholar] [CrossRef]
- Sedighi, M.; Razavi, S.; Navab-Moghadam, F.; Khamseh, M.E.; Alaei-Shahmiri, F.; Mehrtash, A.; Amirmozafari, N. Comparison of gut microbiota in adult patients with type 2 diabetes and healthy individuals. Microb Pathog. 2017, 111, 362–369. [Google Scholar] [CrossRef]
- Wu, X.; Ma, C.; Han, L.; Nawaz, M.; Gao, F.; Zhang, X.; Yu, P.; Zhao, C.; Li, L.; Zhou, A.; et al. Molecular characterisation of the faecal microbiota in patients with type II diabetes. Curr. Microbiol. 2010, 61, 69–78. [Google Scholar] [CrossRef]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017, 23, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Ogasawara, N.; Funaki, Y.; Mizuno, M.; Iida, A.; Goto, C.; Koikeda, S.; Kasugai, K.; Joh, T. Transglucosidase improves the gut microbiota profile of type 2 diabetes mellitus patients: A randomized double-blind, placebo-controlled study. BMC Gastroenterol. 2013, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Herrema, H.; Niess, J.H. Intestinal microbial metabolites in human metabolism and type 2 diabetes. Diabetologia 2020, 63, 2533–2547. [Google Scholar] [CrossRef]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef]
- De la Cuesta-Zuluaga, J.; Mueller, N.T.; Álvarez-Quintero, R.; Velásquez-Mejía, E.P.; Sierra, J.A.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients 2018, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Jumpertz, R.; Le, D.S.; Turnbaugh, P.J.; Trinidad, C.; Bogardus, C.; Gordon, J.I.; Krakoff, J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr. 2011, 94, 58–65. [Google Scholar] [CrossRef]
- Daimon, M.; Kamba, M.; Murakami, H.; Takahashi, K.; Otaka, H.; Makita, K.; Yanagimachi, M.; Terui, K.; Kageyama, K.; Nigawara, T.; et al. Association Between Pituitary-Adrenal Axis Dominance Over the Renin-Angiotensin-Aldosterone System and Hypertension. J. Clin. Endocrinol. Metab. 2016, 101, 889–897. [Google Scholar] [CrossRef]
- Kamba, A.; Daimon, M.; Murakami, H.; Otaka, H.; Matsuki, K.; Sato, E.; Tanabe, J.; Takayasu, S.; Matsuhashi, Y.; Yanagimachi, M.; et al. Association between Higher Serum Cortisol Levels and Decreased Insulin Secretion in a General Population. PLoS ONE 2016, 11, e0166077. [Google Scholar] [CrossRef]
- Ozato, N.; Saito, S.; Yamaguchi, T.; Katashima, M.; Tokuda, I.; Sawada, K.; Katsuragi, Y.; Kakuta, M.; Imoto, S.; Ihara, K.; et al. Blautia genus associated with visceral fat accumulation in adults 20-76 years of age. NPJ Biofilms Microbiomes 2019, 5, 28. [Google Scholar] [CrossRef]
- Kawada, Y.; Naito, Y.; Andoh, A.; Ozeki, M.; Inoue, R. Effect of storage and DNA extraction method on 16S rRNA-profiled fecal microbiota in Japanese adults. J. Clin. Biochem. Nutr. 2019, 64, 106–111. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Mizutani, S.; Nakajima, T.; Hosoda, F.; Watanabe, H.; Saito, Y.; Shibata, T.; Yachida, S.; Yamada, T. High stability of faecal microbiome composition in guanidine thiocyanate solution at room temperature and robustness during colonoscopy. Gut 2016, 65, 1574–1575. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, K.; Ohno, H.; Murakami, H.; Natsume-Kitatani, Y.; Tanisawa, K.; Hirata, S.; Suzuki, H.; Nagatake, T.; Nishino, T.; Mizuguchi, K.; et al. Method for preparing DNA from feces in guanidine thiocyanate solution affects 16S rRNA-based profiling of human microbiota diversity. Sci. Rep. 2017, 7, 4339. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S. Development and evaluation of dietary assessment methods using biomarkers and diet history questionnaires for individuals. In Research for Evaluation Methods of Nutrition and Dietary Lifestyle Programs Held on Healthy Japan 21; Tanaka, H., Ed.; Summary report; Ministry of Health, Welfare, and Labour: Tokyo, Japan, 2004; pp. 10–44. [Google Scholar]
- Sasaki, S.; Yanagibori, R.; Amano, K. Validity of a self-administered diet history questionnaire for assessment of sodium and potassium—Comparison with single 24-hour urinary excretion. Jpn. Cir. J. 1998, 62, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Comparison of relative validity for food group intake estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef]
- Okuda, M.; Sasaki, S. Assessment of Foods Associated with Sodium and Potassium Intake in Japanese Youths Using the Brief-Type Self-Administered Diet History Questionnaire. Nutrients 2021, 13, 2345. [Google Scholar] [CrossRef]
- Nishida, Y.; Nakamura, H.; Sasaki, S.; Shirahata, T.; Sato, H.; Yogi, S.; Yamada, Y.; Nakae, S.; Tanaka, S.; Katsukawa, F. Evaluation of energy intake by brief-type self-administered diet history questionnaire among male patients with stable/at risk for chronic obstructive pulmonary disease. BMJ Open Respir. Res. 2021, 8, e000807. [Google Scholar] [CrossRef]
- Murakami, K.; Livingstone, M.B.E.; Fujiwara, A.; Sasaki, S. Reproducibility and Relative Validity of the Healthy Eating Index-2015 and Nutrient-Rich Food Index 9.3 Estimated by Comprehensive and Brief Diet History Questionnaires in Japanese Adults. Nutrients 2019, 11, 2540. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- The Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus; Nanjo, K.; Tajima, N.; Kadowaki, T.; Kashiwagi, A.; Araki, E.; Ito, C.; Inagaki, N.; Iwamoto, Y.; Kasuga, M.; et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J. Diabetes Investig. 2010, 1, 212–228. [Google Scholar]
- Sonnenburg, J.L.; Bäckhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Heller, J.J.; Guo, X.; Chen, Z.M.; Fish, K.; Fu, Y.X.; Zhou, L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 2012, 36, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Houten, S.M.; Mataki, C.; Christoffolete, M.A.; Kim, B.W.; Sato, H.; Messaddeq, N.; Harney, J.W.; Ezaki, O.; Kodama, T.; et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006, 439, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Serpa, J.; Caiado, F.; Carvalho, T.; Torre, C.; Gonçalves, L.G.; Casalou, C.; Lamosa, P.; Rodrigues, M.; Zhu, Z.; Lam, Z.E.; et al. Butyrate-rich colonic microenvironment is a relevant selection factor for metabolically adapted tumor cells. J. Biol. Chem. 2010, 285, 39211–39223. [Google Scholar] [CrossRef]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef]
- Firouzi, S.; Majid, H.A.; Ismail, A.; Kamaruddin, N.A.; Barakatun-Nisak, M.Y. Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: A randomized controlled trial. Eur. J. Nutr. 2017, 56, 1535–1550. [Google Scholar] [CrossRef]
- Pedersen, C.; Gallagher, E.; Horton, F.; Ellis, R.J.; Ijaz, U.Z.; Wu, H.; Jaiyeola, E.; Diribe, O.; Duparc, T.; Cani, P.D.; et al. Host-microbiome interactions in human type 2 diabetes following prebiotic fibre (galacto-oligosaccharide) intake. Br. J. Nutr. 2016, 116, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Houghton, D.; Hardy, T.; Stewart, C.; Errington, L.; Day, C.P.; Trenell, M.I.; Avery, L. Systematic review assessing the effectiveness of dietary intervention on gut microbiota in adults with type 2 diabetes. Diabetologia 2018, 61, 1700–1711. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [PubMed]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef]
- Liu, C.; Li, J.; Zhang, Y.; Ohilip, A.; Shi, E.; Chi, X.; Meng, J. Influence of glucose fermentation on CO₂ assimilation to acetate in homoacetogen Blautia coccoides GA-1. J. Ind. Microbiol. Biotechnol. 2015, 42, 1217–1224. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Mueller, N.T.; Differding, M.K.; Zhang, M.; Maruthur, N.M.; Juraschek, S.P.; Miller, E.R., 3rd; Appel, L.J.; Yeh, H.C. Metformin Affects Gut Microbiome Composition and Function and Circulating Short-Chain Fatty Acids: A Randomized Trial. Diabetes Care 2021, 44, 1462–1471. [Google Scholar] [CrossRef]
- Kubota, K.; Kamijima, Y.; Kao Yang, Y.H.; Kimura, S.; Chia-Cheng Lai, E.; Man, K.K.C.; Ryan, P.; Schuemie, M.; Stang, P.; Su, C.C.; et al. Penetration of new antidiabetic medications in East Asian countries and the United States: A cross-national comparative study. PLoS ONE 2018, 13, e0208796. [Google Scholar] [CrossRef]
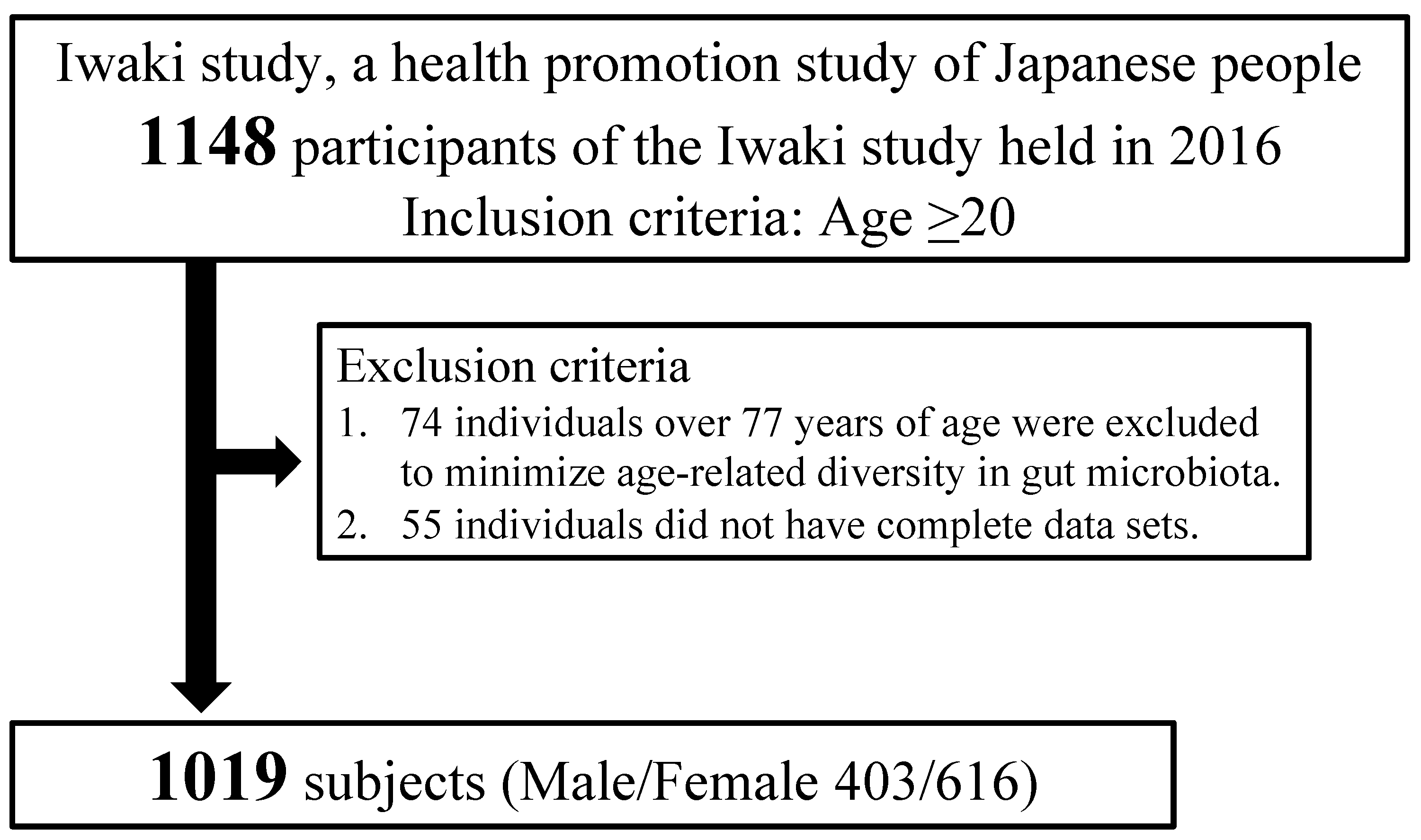
| Characteristics | DM (n = 96) | Non-DM (n = 923) | p |
|---|---|---|---|
| Number (Gender: M/F) | 44/52 | 359/564 | 0.188 |
| Age (years) | 61.47 ± 10.76 | 51.43 ± 14.12 | <0.001 ** |
| Height (cm) | 160.30 ± 9.06 | 161.35 ± 8.90 | 0.270 |
| Body weight (kg) | 64.72 ± 12.36 | 59.34 ± 11.27 | <0.001 ** |
| Body mass index (kg/m2) | 25.15 ± 4.15 | 22.70 ± 3.26 | <0.001 ** |
| Percent body fat | 29.25 ± 9.70 | 25.96 ± 7.96 | <0.001 ** |
| Fasting plasma glucose (mg/dL) | 129 ± 44.43 | 87.85 ± 9.56 | <0.001 ** |
| HbA1c (%) | 7.21 ± 1.31 | 5.71 ± 0.30 | <0.001 ** |
| Fasting serum insulin: IRI (mU/mL) | 7.30 ± 4.26 | 5.06 ± 2.62 | <0.001 ** |
| HOMA-R | 2.31 ± 1.53 | 1.23 ± 0.63 | <0.001 ** |
| HOMA-β | 63.75 ± 166.54 | 81.00 ± 2.13 | <0.001 ** |
| Systolic blood pressure (mmHg) | 133.5 ± 18.45 | 123.10 ± 17.45 | <0.001 ** |
| Diastolic blood pressure (mmHg) | 79.25 ± 10.60 | 75.09 ± 12.07 | 0.001 ** |
| LDL cholesterol (mg/dL) | 123.41 ± 37.18 | 117.21 ± 29.15 | 0.054 |
| Triglyceride (mg/dL) | 128.98 ± 105.11 | 94.98 ± 61.62 | <0.001 ** |
| HDL cholesterol (mg/dL) | 58.13 ± 17.36 | 65.52 ± 17.08 | <0.001 ** |
| Serum albumin (g/dL) | 4.45 ± 0.34 | 4.51 ± 0.29 | 0.056 |
| Serum uric acid (mg/dL) | 5.44 ± 1.37 | 5.05 ± 1.35 | 0.007 ** |
| Serum urea nitrogen (mg/dL) | 15.94 ± 6.79 | 14.00 ± 4.00 | <0.001 ** |
| Serum creatinine (mg/dL) | 0.84 ± 0.97 | 0.71 ± 0.18 | <0.001 ** |
| AST | 26.41 ± 11.00 | 22.86 ± 9.12 | <0.001 ** |
| ALT | 29.06 ± 19.42 | 21.44 ± 13.68 | <0.001 ** |
| γGTP | 39.69 ± 27.80 | 33.33 ± 42.63 | 0.153 |
| Hypertension: n (%) | 69 (71.9) | 304 (32.9) | <0.001 ** |
| Hyperlipidemia: n (%) | 60 (62.5) | 340 (36.8) | <0.001 ** |
| Drinking alcohol: n (%) | 42 (43.8) | 452 (49.0) | 0.330 |
| Smoking (never/ past/ current): n | 59/18/19 | 571/183/168 | 0.917 |
| p (Adjusted) | |||||
|---|---|---|---|---|---|
| DM | Non-DM | Non | Age and Gender | Multiple Factors | |
| Energy (kcal/kg/day) | 31.755 ± 10.93 | 31.998 ± 10.23 | 0.826 | 0.142 | 0.444 |
| Carbohydrate (g/kg/day) | 4.197 ± 1.45 | 4.277 ± 1.43 | 0.599 | 0.095 | 0.720 |
| Protein (g/kg/day) | 1.123 ± 0.48 | 1.198 ± 0.50 | 0.571 | 0.292 | 0.440 |
| Fat (g/kg/day) | 0.903 ± 0.35 | 0.907 ± 0.37 | 0.922 | 0.567 | 0.167 |
| Fiber (g/kg/day) | |||||
| Total | 0.194 ± 0.08 | 0.189 ± 0.09 | 0.601 | 0.107 | 0.882 |
| Water soluble | 0.048 ± 0.02 | 0.047 ± 0.02 | 0.783 | 0.100 | 0.954 |
| Water insoluble | 0.139 ± 0.05 | 0.136 ± 0.06 | 0.587 | 0.108 | 0.872 |
| Bifidobacterium | 0.098 ± 0.11 | 0.078 ± 0.08 | 0.024 * | <0.001 * | <0.001 * |
| Collinsella | 0.044 ± 0.05 | 0.040 ± 0.05 | 0.435 | 0.1648 | 0.339 |
| Bacteroides | 0.088 ± 0.10 | 0.111 ± 0.08 | 0.010 * | 0.048* | 0.057 |
| Prevotella | 0.060 ± 0.12 | 0.049 ± 0.10 | 0.315 | 0.8522 | 0.794 |
| Alistipes | 0.018 ± 0.03 | 0.016 ± 0.02 | 0.359 | 0.4956 | 0.413 |
| Gemmiger | 0.025 ± 0.02 | 0.025 ± 0.03 | 0.858 | 0.8266 | 0.884 |
| Streptococcus | 0.037 ± 0.06 | 0.019 ± 0.03 | <0.001 ** | 0.001 ** | 0.005 ** |
| Roseburia | 0.038 ± 0.04 | 0.046 ± 0.05 | 0.131 | 0.022 * | 0.020 * |
| Anaerostipes | 0.042 ± 0.05 | 0.058 ± 0.06 | 0.017 * | 0.1357 | 0.096 |
| Fusicatenibacter | 0.017 ± 0.02 | 0.021 ± 0.02 | 0.096 | 0.1891 | 0.202 |
| Blautia | 0.057 ± 0.04 | 0.075 ± 0.04 | <0.001 ** | 0.012 * | 0.016 * |
| Ruminococcus 2 | 0.045 ± 0.04 | 0.051 ± 0.06 | 0.341 | 0.6327 | 0.392 |
| Ruminococcus 1 | 0.034 ± 0.04 | 0.032 ± 0.05 | 0.684 | 0.7635 | 0.961 |
| Faecalibacterium | 0.072 ± 0.05 | 0.079 ± 0.06 | 0.231 | 0.1791 | 0.315 |
| Lachnospiracea_incertae_sedis | 0.019 ± 0.01 | 0.020 ± 0.01 | 0.707 | 0.4297 | 0.586 |
| Bifidobacterium | Streptococcus | Roseburia | Blautia | |||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| Energy (kcal/kg/day) | −0.017 | 0.627 | 0.075 | 0.027 * | 0.043 | 0.213 | −0.019 | 0.583 |
| Carbohydrate (g/kg/day) | 0.004 | 0.902 | 0.062 | 0.063 | 0.091 | 0.007 ** | −0.022 | 0.522 |
| Protein (g/kg/day) | −0.023 | 0.493 | 0.047 | 0.164 | 0.010 | 0.765 | −0.032 | 0.353 |
| Fat (g/kg/day) | 0.015 | 0.653 | 0.041 | 0.215 | −0.008 | 0.822 | −0.020 | 0.557 |
| Fiber (g/kg/day) | ||||||||
| Total | −0.028 | 0.420 | 0.015 | 0.674 | 0.092 | 0.010 * | −0.049 | 0.167 |
| Water soluble | −0.024 | 0.486 | 0.004 | 0.900 | 0.091 | 0.010 * | −0.035 | 0.315 |
| Water insoluble | −0.028 | 0.453 | 0.020 | 0.579 | 0.069 | 0.010 * | −0.049 | 0.171 |
| Univariate | Multiple Factors Adjusted | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p | OR | 95%CI | p | |
| Bifidobacterium (per 0.1) | 1.28 | 1.03–1.59 | 0.026 * | 1.68 | 1.33–2.13 | <0.001 * |
| Streptococcus (per 0.1) | 2.27 | 1.51–3.40 | <0.001 ** | 1.47 | 0.93–2.32 | 0.107 |
| Roseburia (per 0.1) | 0.68 | 0.41–1.12 | 0.132 | 0.54 | 0.30–0.96 | 0.027 * |
| Blautia (per 0.1) | 0.31 | 0.17–0.58 | <0.001 ** | 0.45 | 0.24–0.86 | 0.011* |
| Univariate | Age and Gender Adjusted | Multiple Factors Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | |
| Blautia dominant | Ref | - | - | Ref | - | - | Ref | - | - |
| Roseburia dominant | 1.72 | 0.30–1.11 | 0.101 | 1.44 | 0.74–2.80 | 0.278 | 1.54 | 0.78–3.04 | 0.218 |
| Streptococcus dominant | 4.01 | 1.85–8.70 | <0.001 ** | 2.28 | 1.02–5.10 | 0.044 * | 2.10 | 0.90–4.87 | 0.084 |
| Bifidobacterium dominant | 2.57 | 1.18–5.62 | 0.018 ** | 3.43 | 1.52–7.75 | 0.003 ** | 3.97 | 1.68–9.35 | 0.002 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamura, A.; Murabayashi, M.; Nishiya, Y.; Mizushiri, S.; Hamaura, K.; Ito, R.; Ono, S.; Terada, A.; Murakami, H.; Tanabe, J.; et al. Interrelations between Gut Microbiota Composition, Nutrient Intake and Diabetes Status in an Adult Japanese Population. J. Clin. Med. 2022, 11, 3216. https://doi.org/10.3390/jcm11113216
Tamura A, Murabayashi M, Nishiya Y, Mizushiri S, Hamaura K, Ito R, Ono S, Terada A, Murakami H, Tanabe J, et al. Interrelations between Gut Microbiota Composition, Nutrient Intake and Diabetes Status in an Adult Japanese Population. Journal of Clinical Medicine. 2022; 11(11):3216. https://doi.org/10.3390/jcm11113216
Chicago/Turabian StyleTamura, Ayumi, Masaya Murabayashi, Yuki Nishiya, Satoru Mizushiri, Kiho Hamaura, Ryoma Ito, Shoma Ono, Akihide Terada, Hiroshi Murakami, Jutaro Tanabe, and et al. 2022. "Interrelations between Gut Microbiota Composition, Nutrient Intake and Diabetes Status in an Adult Japanese Population" Journal of Clinical Medicine 11, no. 11: 3216. https://doi.org/10.3390/jcm11113216
APA StyleTamura, A., Murabayashi, M., Nishiya, Y., Mizushiri, S., Hamaura, K., Ito, R., Ono, S., Terada, A., Murakami, H., Tanabe, J., Yanagimachi, M., Tokuda, I., Sawada, K., Ihara, K., & Daimon, M. (2022). Interrelations between Gut Microbiota Composition, Nutrient Intake and Diabetes Status in an Adult Japanese Population. Journal of Clinical Medicine, 11(11), 3216. https://doi.org/10.3390/jcm11113216






