Angle of Uterine Flexion and Adenomyosis
Abstract
:1. Introduction
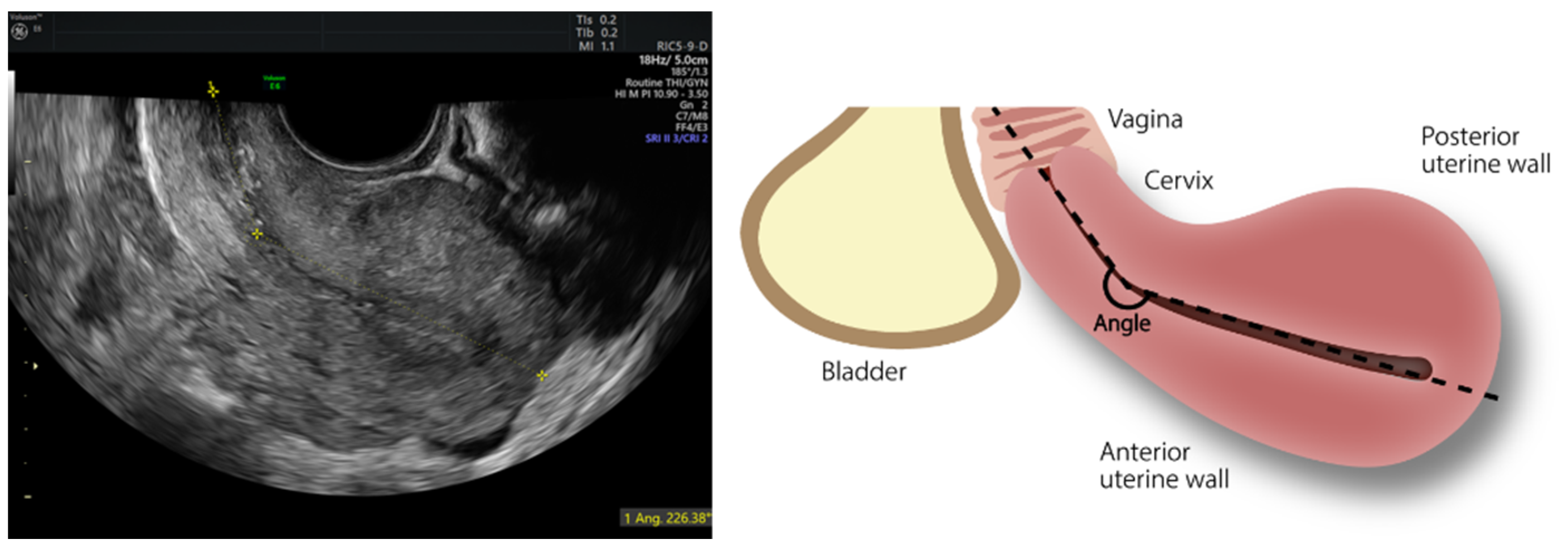
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Struble, J.; Reid, S.; Bedaiwy, M.A. Adenomyosis: A Clinical Review of a Challenging Gynecologic Condition. J. Minim. Invasive Gynecol. 2016, 23, 164–185. [Google Scholar] [CrossRef]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Daguati, R.; Abbiati, A.; Fedele, L. Adenomyosis: Epidemiological factors. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 465–477. [Google Scholar] [CrossRef]
- Filip, G.; Balzano, A.; Cagnacci, A. Histological evaluation of the prevalence of adenomyosis, myomas and of their concomitance. Minerva Ginecol. 2019, 71, 177–181. [Google Scholar] [CrossRef]
- Exacoustos, C.; Zupi, E. A new era in diagnosing adenomyosis is coming. Fertil Steril. 2018, 110, 858. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, R.K.; Horrow, M.M.; Smith, R.J.; Springer, J. Adenomyosis: A Sonographic Diagnosis. RadioGraphics 2018, 38, 1576–1589. [Google Scholar] [CrossRef]
- Bazot, M.; Daraï, E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil. Steril. 2018, 109, 389–397. [Google Scholar] [CrossRef] [Green Version]
- Zhai, J.; Vannuccini, S.; Petraglia, F.; Giudice, L.C. Adenomyosis: Mechanisms and Pathogenesis. Semin. Reprod. Med. 2020, 38, 129–143. [Google Scholar] [CrossRef]
- Grandi, G.; Ferrari, S.; Xholli, A.; Cannoletta, M.; Palma, F.; Volpe, A.; Cagnacci, A. Prevalence of menstrual pain in young women: What is dysmenorrhea? J. Pain Res. 2012, 5, 169–174. [Google Scholar] [CrossRef]
- Cagnacci, A.; Grandi, G.; Cannoletta, M.; Xholli, A.; Piacenti, I.; Volpe, A. Intensity of menstrual pain and estimated angle of uterine flexion. Acta Obstet. Gynecol. Scand. 2014, 93, 58–63. [Google Scholar] [CrossRef]
- van den Bosch, T.; Dueholm, M.; Leone, F.P.G.; Rasmussen, C.; Votino, A.; Van Schoubroeck, D.; Landolfo, C.; Installé, A.J.F.; Guerriero, S.; Exacoustos, C.; et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: A consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet. Gynecol. 2015, 46, 284–298. [Google Scholar] [CrossRef]
- Harmsen, M.J.; van den Bosch, T.; de Leeuw, R.A.; Dueholm, M.; Exacoustos, C.; Valentin, L.; Hehenkamp, W.; Groenman, F.; De Bruyn, C.; Rasmussen, C.; et al. Consensus on revised definitions of morphological uterus sonographic assessment (MUSA) features of adenomyosis: Results of a modified Delphi procedure. Ultrasound Obstet. Gynecol. 2021; accepted . [Google Scholar] [CrossRef]
- Reid, S.; Lu, C.; Condous, G. Can we improve the prediction of pouch of Douglas obliteration in women with suspected endometriosis using ultrasound-based models? A multicenter prospective observational study. Acta Obstet. Gynecol. Scand. 2015, 94, 1297–1306. [Google Scholar] [CrossRef]
- Haylen, B.T.; Mcnally, G.; Ramsay, P.; Birrell, W.; Logan, V. A standardised ultrasonic diagnosis and an accurate prevalence for the retroverted uterus in general gynaecology patients. Aust. N. Zeal. J. Obstet. Gynaecol. 2007, 47, 326–328. [Google Scholar] [CrossRef]
- Fauconnier, A.; Dubuisson, J.B.; Foulot, H.; Deyrolles, C.; Sarrot, F.; Laveyssière, M.-N.; Jansé-Marec, J.; Bréart, G. Mobile uterine retroversion is associated with dyspareunia and dysmenorrhea in an unselected population of women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 127, 252–256. [Google Scholar] [CrossRef]
- Leyendecker, G.; Bilgicyildirim, A.; Inacker, M.; Stalf, T.; Huppert, P.; Mall, G.; Böttcher, B.; Wildt, L. Adenomyosis and endometriosis. Re-visiting their association and further insights into the mechanisms of auto-traumatisation. An MRI study. Arch. Gynecol. Obstet. 2015, 291, 917–932. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, M.; Togashi, K.; Kido, A.; Nakai, A.; Fujiwara, T.; Koyama, T.; Fujii, S. Dysmenorrhea: Evaluation with cine-mode-display MR imaging--initial experience. Radiology 2005, 235, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Xholli, A.; Simoncini, G.; Vujosevic, S.; Trombetta, G.; Chiodini, A.; Ferraro, M.; Cagnacci, A. Menstrual Pain and Elasticity of Uterine Cervix. J. Clin. Med. 2021, 10, 1110. [Google Scholar] [CrossRef]
- Leyendecker, G.; Kunz, G.; Herbertz, M.; Beil, D.; Huppert, P.; Mall, G.; Kissler, S.; Noe, M.; Wildt, L. Uterine peristaltic activity and the development of endometriosis. Ann. N. Y. Acad. Sci. 2004, 1034, 338–355. [Google Scholar] [CrossRef]
- Ott, J.; Nouri, K.; Demmel, M.; Zafraani, S.; Greilberger, U.; Huber, J.; Mayerhofer, K. Fourteen-Year Experience with laparoscopic ventrosuspension in patients with retroverted and retroflected uterus and pelvic pain syndromes. J. Minim. Invasive Gynecol. 2010, 17, 749–753. [Google Scholar] [CrossRef]
- Osada, H. Uterine adenomyosis and adenomyoma: The surgical approach. Fertil. Steril. 2018, 109, 406–417. [Google Scholar] [CrossRef] [Green Version]
- Gargiulo, T.; Leo, L.; Gomel, V. Laparoscopic uterine suspension using three-stitch technique. J. Am. Assoc. Gynecol. Laparosc. 2000, 7, 233–236. [Google Scholar] [CrossRef]

| Mean ± SD | N/Tot | No Adenomyosis | Adenomyosis | p Value | |
|---|---|---|---|---|---|
| (n = 44) | (n = 76) | ||||
| Age (yrs.) | 36.7 ± 6.8 | 35.5 ± 6.8 | 37.4 ± 6.7 | 0.139 | |
| BMI (kg/m2) | 22.7 ± 4.6 | 21.5 ± 9.7 | 23.3 ± 5.2 | 0.189 | |
| Age at menarche (yrs.) | 12.4 ± 1.5 | 12.6 ± 1.5 | 12.3 ± 1.5 | 0.293 | |
| Pregnancy | 17/120 (14.2%) | 4/44 (9.1%) | 13/76 (17.1%) | 0.227 | |
| Abortions | 5/120 (4.2%) | 2/44 (4.54%) | 3/76 (3.94%) | 0.956 | |
| Menstrual pain (n) | 90/120 (75%) | 36/44 (81.8%) | 54/76 (71.0%) | 0.190 | |
| (VAS) | 5.51 ± 3.60 | 5.81 ± 3.61 | 5.32 ± 3.63 | 0.476 | |
| Intermenstrual pain (n) | 60/120 (50%) | 17/44 (38.6%) | 43/76 (56.6%) | 0.058 | |
| (VAS) | 3.57 ± 3.69 | 2.57 ± 3.34 | 4.04 ± 3.79 | 0.034 | |
| Pain at Intercourse (n) | 68/120 (56.7%) | 25/44 (56.8%) | 43/76 (56.6%) | 0.983 | |
| (VAS) | 3.75 ± 3.48 | 3.64 ± 3.64 | 3.82 ± 3.41 | 0.786 | |
| Heavy Menstrual Periods | 21/120 (17.5%) | 9/44 (20.4%) | 12/76 (15.8%) | 0.524 | |
| Hormone therapy | 44/120 (36.7%) | 12/44 (27.2%) | 32/76 (42.1%) | 0.104 | |
| Uterine volume (cm3) | 62.2 ± 37.5 | 53.0 ± 22.2 | 67.5 ± 43.1 | 0.040 | |
| Angle of flexion (°) | 146.1 ± 50.7 | 127.2 ± 38.2 | 157.5 ± 54.1 | 0.001 | |
| <150 | 90/120 (75.0%) | 39/44 (88.6%) | 51/76 (64.5%) | 0.004 | |
| 150–210 | 8/120 (6.7%) | 2/44 (4.5%) | 6/76 (7.9%) | 0.473 | |
| >210 | 22/120 (18.3%) | 3/44 (6.8%) | 19/76 (25.0%) | 0.015 | |
| Endometriosis | 47/120 (39.2%) | 21/44 (47.7%) | 26/76 (34.2%) | 0.146 | |
| Myomas | 23/120 (19.2%) | 10/44 (22.7%) | 13/76 (17.1%) | 0.454 |
| Simple | Simple Regression | Multiple Regression | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Angle of Flexion > 210° | 4.47 | 1.23, 16.21 | 0.022 | 5.80 | 1.19, 28.3 | 0.029 |
| Uterus Volume (cm3) | 1.01 | 1.00,1.03 | 0.050 | 1.01 | 0.998, 1.03 | 0.0814 |
| Intermenstrual pain | 2.32 | 1.05, 5.16 | 0.038 | 2.17 | 0.91, 5.17 | 0.078 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xholli, A.; Scovazzi, U.; Londero, A.P.; Evangelisti, G.; Cavalli, E.; Schiaffino, M.G.; Vacca, I.; Oppedisano, F.; Ferraro, M.F.; Sirito, G.; et al. Angle of Uterine Flexion and Adenomyosis. J. Clin. Med. 2022, 11, 3214. https://doi.org/10.3390/jcm11113214
Xholli A, Scovazzi U, Londero AP, Evangelisti G, Cavalli E, Schiaffino MG, Vacca I, Oppedisano F, Ferraro MF, Sirito G, et al. Angle of Uterine Flexion and Adenomyosis. Journal of Clinical Medicine. 2022; 11(11):3214. https://doi.org/10.3390/jcm11113214
Chicago/Turabian StyleXholli, Anjeza, Umberto Scovazzi, Ambrogio Pietro Londero, Giulio Evangelisti, Elena Cavalli, Maria Giulia Schiaffino, Ilaria Vacca, Francesca Oppedisano, Mattia Francesco Ferraro, Giorgio Sirito, and et al. 2022. "Angle of Uterine Flexion and Adenomyosis" Journal of Clinical Medicine 11, no. 11: 3214. https://doi.org/10.3390/jcm11113214
APA StyleXholli, A., Scovazzi, U., Londero, A. P., Evangelisti, G., Cavalli, E., Schiaffino, M. G., Vacca, I., Oppedisano, F., Ferraro, M. F., Sirito, G., Molinari, F., & Cagnacci, A. (2022). Angle of Uterine Flexion and Adenomyosis. Journal of Clinical Medicine, 11(11), 3214. https://doi.org/10.3390/jcm11113214







