PECAM-1 Is Down-Regulated in γδT Cells during Remission, but Up-Regulated in Relapse of Multiple Sclerosis
Abstract
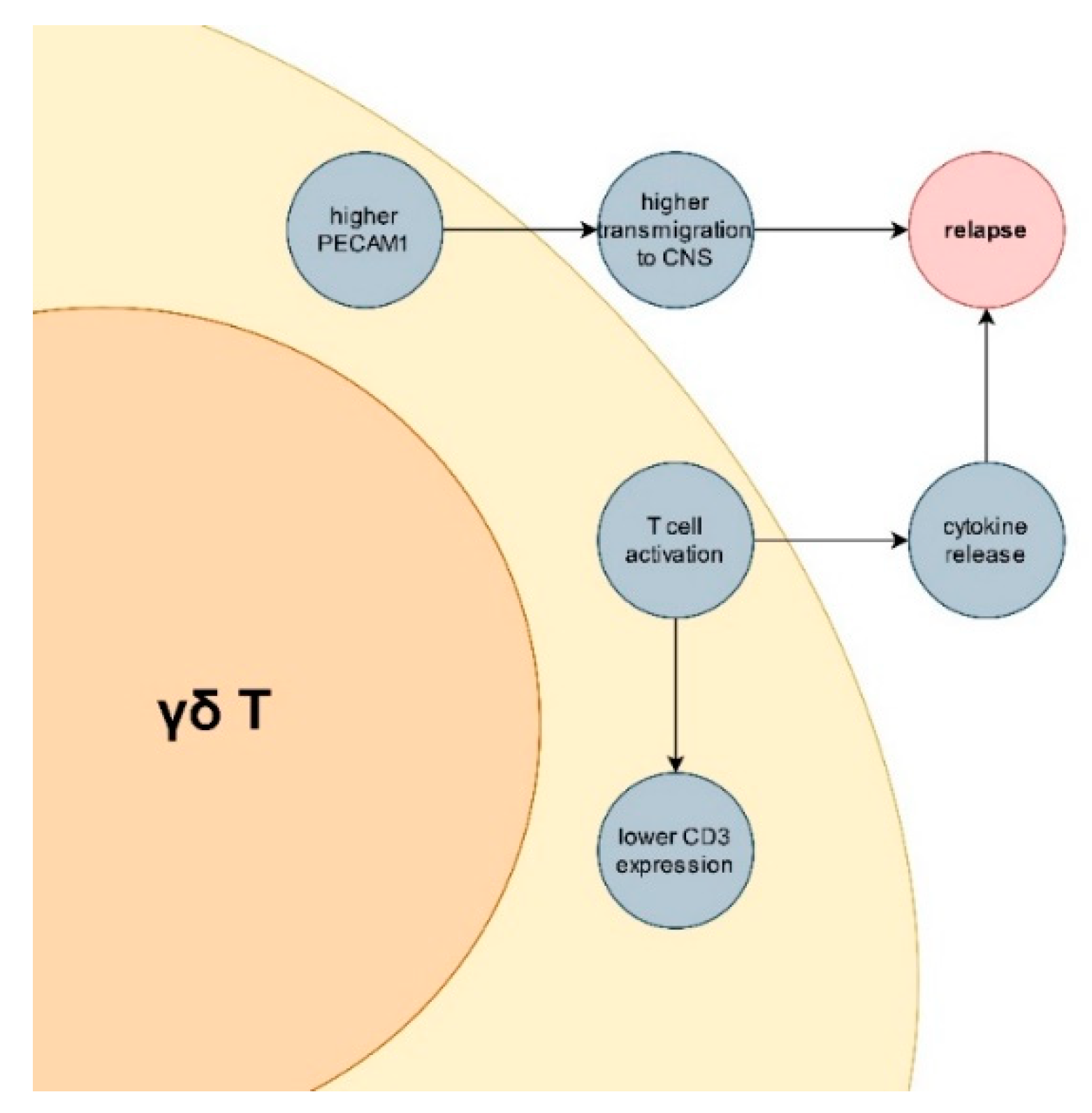
:1. Introduction
2. Materials and Methods
2.1. Study Group
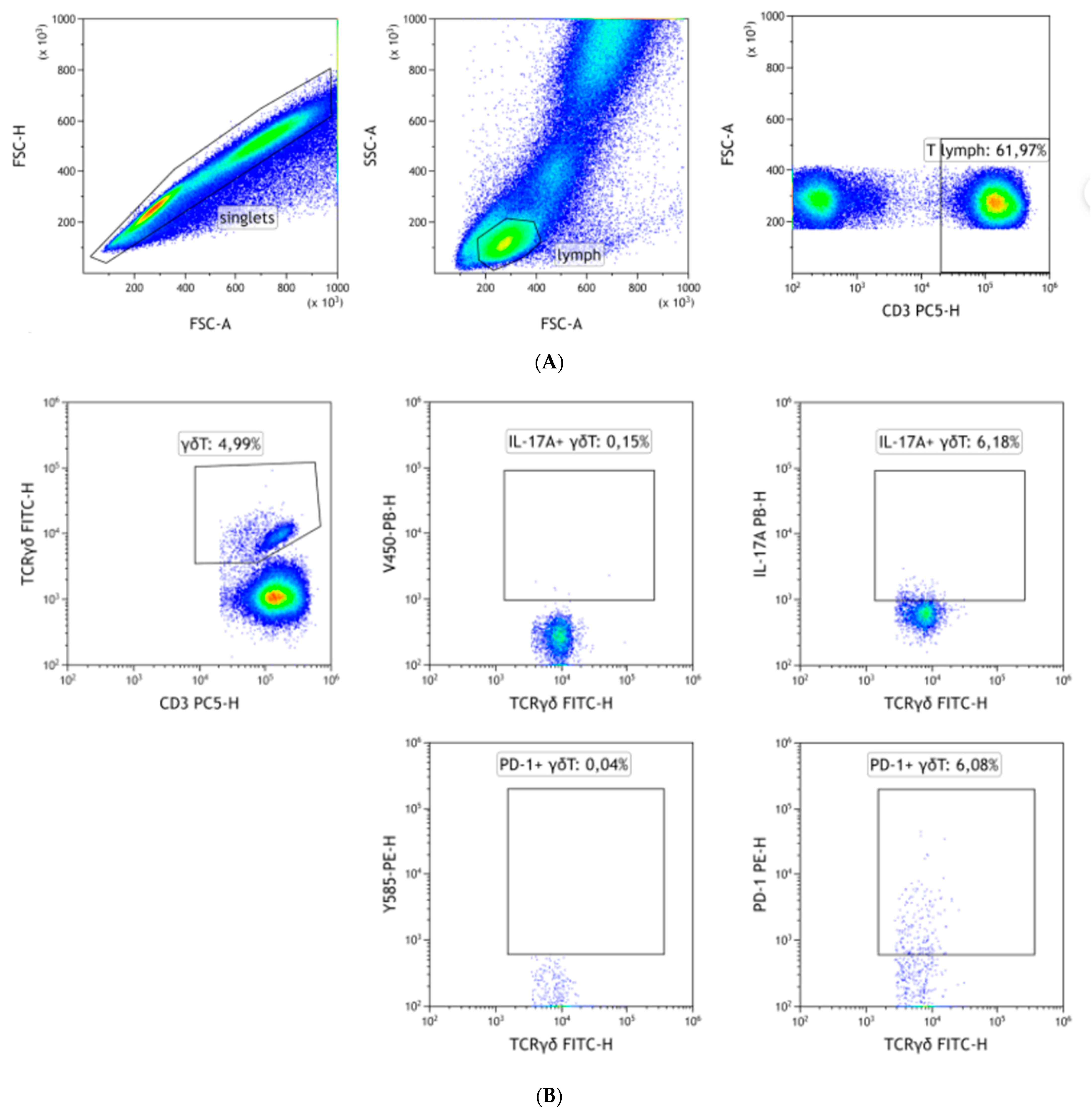
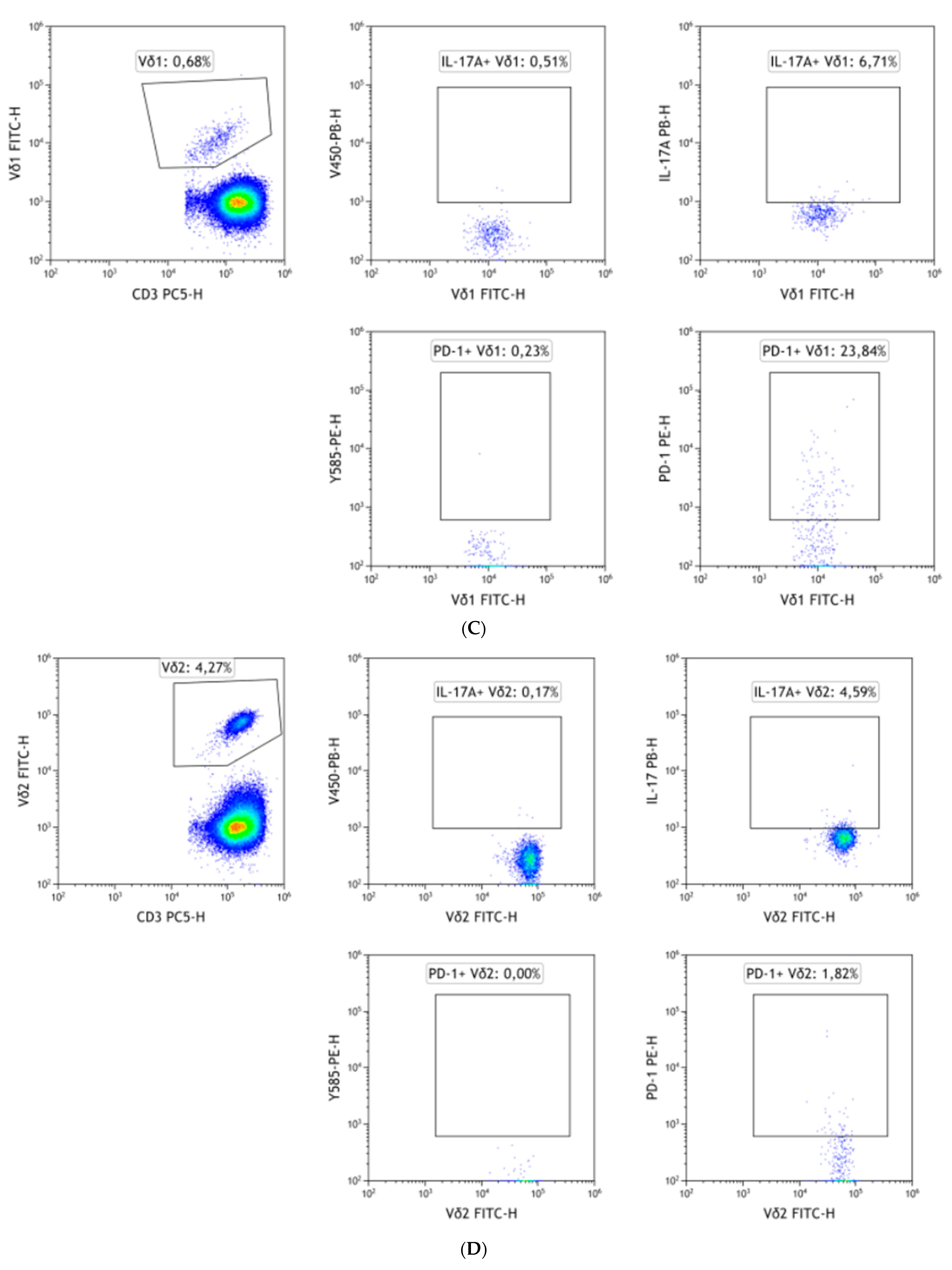
2.2. Flow Cytometry
2.3. Cell Sorting
2.4. RT-qPCR
2.5. Statistical Analysis
3. Results
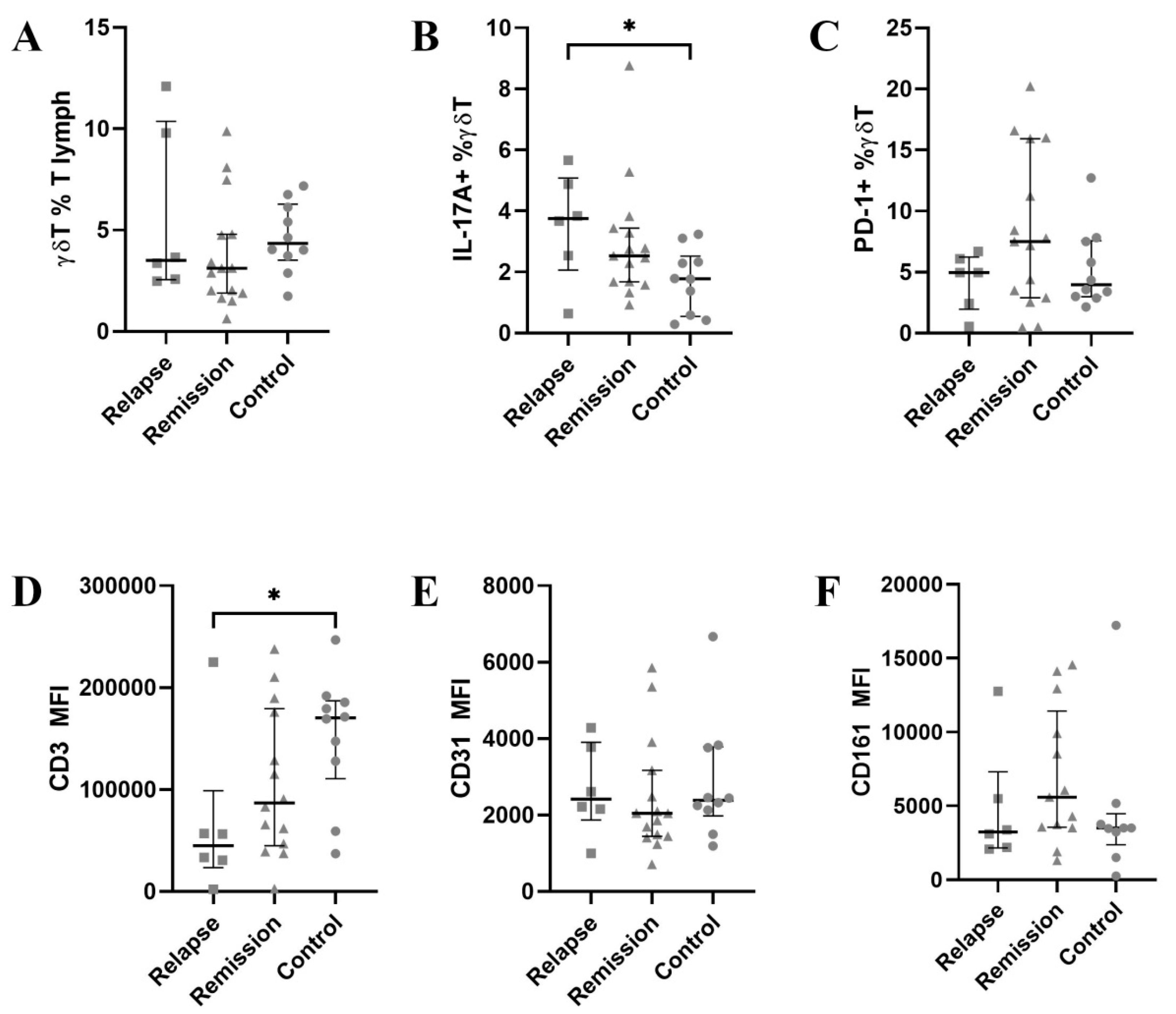
3.1. CD3 Is Significantly Down-Regulated on γδ T Cells
3.2. PECAM-1 Is Slightly Down-Regulated in Vδ1 during Remission
3.3. CD3 Is Further Down-Regulated in Vδ2 Cells
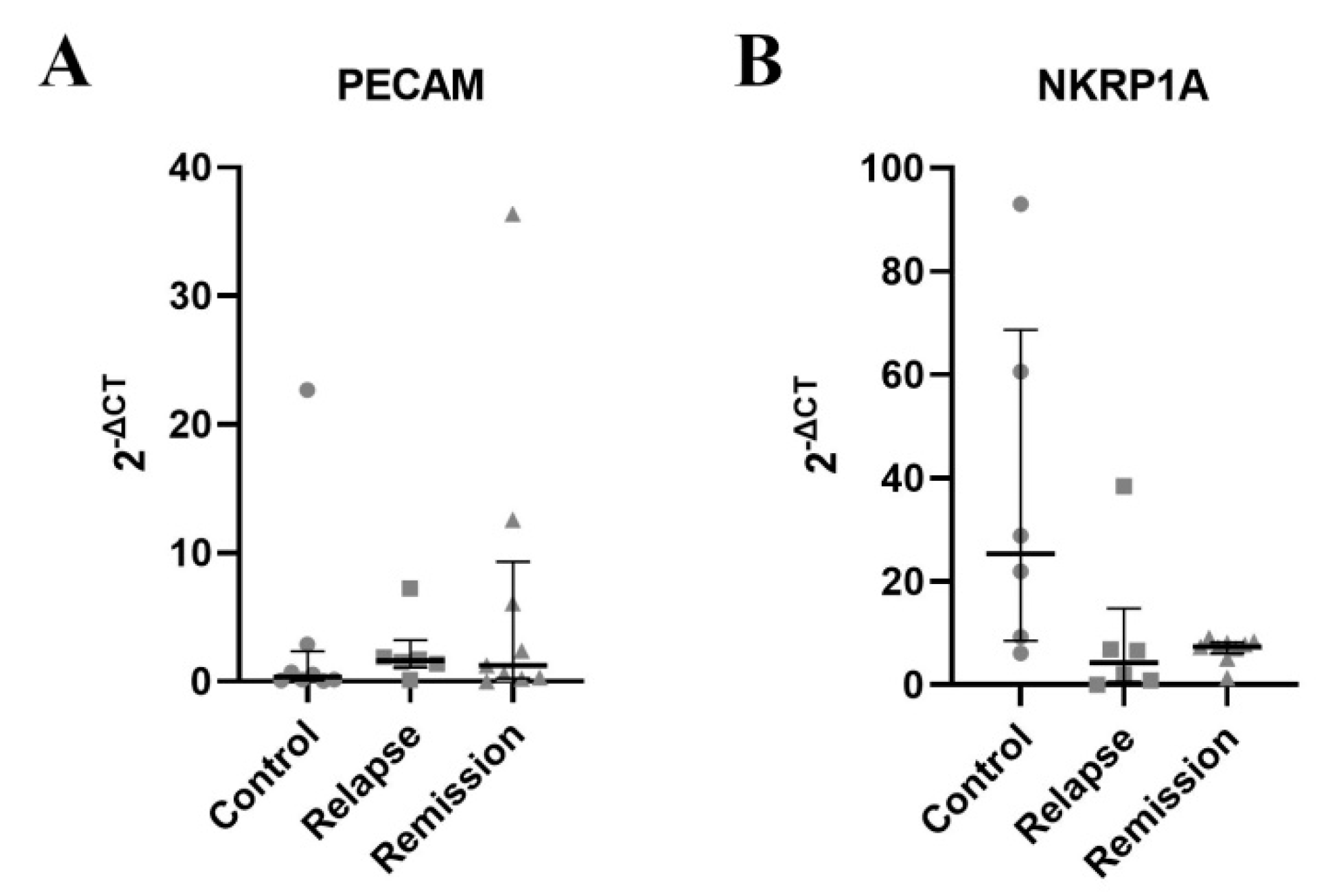
3.4. PECAM-1 mRNA Is Up-Regulated While NKRP1A mRNA Is Downregulated in γδ T during Relapse
3.5. Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sorensen, P.S.; Sellebjerg, F.; Hartung, H.-P.; Montalban, X.; Comi, G.; Tintoré, M. The Apparently Milder Course of Multiple Sclerosis: Changes in the Diagnostic Criteria, Therapy and Natural History. Brain 2020, 143, 2637–2652. [Google Scholar] [CrossRef]
- Koch-Henriksen, N.; Sørensen, P.S. The Changing Demographic Pattern of Multiple Sclerosis Epidemiology. Lancet Neurol. 2010, 9, 520–532. [Google Scholar] [CrossRef]
- Koch-Henriksen, N.; Thygesen, L.C.; Stenager, E.; Laursen, B.; Magyari, M. Incidence of MS Has Increased Markedly over Six Decades in Denmark Particularly with Late Onset and in Women. Neurology 2018, 90, e1954–e1963. [Google Scholar] [CrossRef] [PubMed]
- Wallin, M.T.; Culpepper, W.J.; Nichols, E.; Bhutta, Z.A.; Gebrehiwot, T.T.; Hay, S.I.; Khalil, I.A.; Krohn, K.J.; Liang, X.; Naghavi, M.; et al. Global, Regional, and National Burden of Multiple Sclerosis 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 269–285. [Google Scholar] [CrossRef] [Green Version]
- Davis, B.E.; Lakin, L.; Binns, C.C.; Currie, K.M.; Rensel, M.R. Patient and Provider Insights into the Impact of Multiple Sclerosis on Mental Health: A Narrative Review. Neurol. Ther. 2021, 10, 99–119. [Google Scholar] [CrossRef]
- Kavaliunas, A.; Danylaite Karrenbauer, V.; Hillert, J. Socioeconomic Consequences of Multiple Sclerosis—A Systematic Literature Review. Acta Neurol. Scand. 2021, 143, 587–601. [Google Scholar] [CrossRef]
- Hilt Pfleger, C.C.; Meulengracht Flachs, E.; Koch-Henriksen, N. Social Consequences of Multiple Sclerosis (1): Early Pension and Temporary Unemployment—A Historical Prospective Cohort Study. Mult. Scler. J. 2010, 16, 121–126. [Google Scholar] [CrossRef]
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef]
- Aust, J.G.; Gays, F.; Mickiewicz, K.M.; Buchanan, E.; Brooks, C.G. The Expression and Function of the NKRP1 Receptor Family in C57BL/6 Mice. J. Immunol. 2009, 183, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Rozbeský, D.; Ivanova, L.; Hernychová, L.; Grobárová, V.; Novák, P.; Černý, J. Nkrp1 Family, from Lectins to Protein Interacting Molecules. Molecules 2015, 20, 3463–3478. [Google Scholar] [CrossRef] [Green Version]
- Mesci, A.; Ljutic, B.; Makrigiannis, A.P.; Carlyle, J.R. NKR-P1 Biology: From Prototype to Missing Self. Immunol. Res. 2006, 35, 13–26. [Google Scholar] [CrossRef]
- Bialoszewska, A.; Malejczyk, J. Biological and Clinical Significance of Human NKRP1A/LLT1 Receptor/Ligand Interactions. Crit. Rev. Immunol. 2018, 38, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Balato, A.; Unutmaz, D.; Gaspari, A.A. Natural Killer T Cells: An Unconventional T-Cell Subset with Diverse Effector and Regulatory Functions. J. Investig. Dermatol. 2009, 129, 1628–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggi, L.; Santarlasci, V.; Capone, M.; Peired, A.; Frosali, F.; Crome, S.Q.; Querci, V.; Fambrini, M.; Liotta, F.; Levings, M.K.; et al. CD161 Is a Marker of All Human IL-17-Producing T-Cell Subsets and Is Induced by RORC. Eur. J. Immunol. 2010, 40, 2174–2181. [Google Scholar] [CrossRef]
- Woodfin, A.; Voisin, M.-B.; Nourshargh, S. PECAM-1: A Multi-Functional Molecule in Inflammation and Vascular Biology. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2514–2523. [Google Scholar] [CrossRef] [Green Version]
- Muller, W.A.; Weigl, S.A.; Deng, X.; Phillips, D.M. PECAM-1 Is Required for Transendothelial Migration of Leukocytes. J. Exp. Med. 1993, 178, 449–460. [Google Scholar] [CrossRef]
- Solowiej, A.; Biswas, P.; Graesser, D.; Madri, J.A. Lack of Platelet Endothelial Cell Adhesion Molecule-1 Attenuates Foreign Body Inflammation Because of Decreased Angiogenesis. Am. J. Pathol. 2003, 162, 953–962. [Google Scholar] [CrossRef] [Green Version]
- Newman, P.J.; Newman, D.K. Signal Transduction Pathways Mediated by PECAM-1. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 953–964. [Google Scholar] [CrossRef]
- Elovaara, I.; Ukkonen, M.; Leppäkynnäs, M.; Lehtimäki, T.; Luomala, M.; Peltola, J.; Dastidar, P. Adhesion Molecules in Multiple Sclerosis: Relation to Subtypes of Disease and Methylprednisolone Therapy. Arch. Neurol. 2000, 57, 546–551. [Google Scholar] [CrossRef] [Green Version]
- Rössler, K.; Neuchrist, C.; Kitz, K.; Scheiner, O.; Kraft, D.; Lassmann, H. Expression of Leucocyte Adhesion Molecules at the Human Blood-Brain Barrier (BBB). J. Neurosci. Res. 1992, 31, 365–374. [Google Scholar] [CrossRef]
- Cannella, B.; Raine, C.S. The Adhesion Molecule and Cytokine Profile of Multiple Sclerosis Lesions. Ann. Neurol. 1995, 37, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Minagar, A.; Jy, W.; Jimenez, J.J.; Sheremata, W.A.; Mauro, L.M.; Mao, W.W.; Horstman, L.L.; Ahn, Y.S. Elevated Plasma Endothelial Microparticles in Multiple Sclerosis. Neurology 2001, 56, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Kuenz, B.; Lutterotti, A.; Khalil, M.; Ehling, R.; Gneiss, C.; Deisenhammer, F.; Reindl, M.; Berger, T. Plasma Levels of Soluble Adhesion Molecules SPECAM-1, SP-Selectin and SE-Selectin Are Associated with Relapsing-Remitting Disease Course of Multiple Sclerosis. J. Neuroimmunol. 2005, 167, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Losy, J.; Niezgoda, A.; Wender, M. Increased Serum Levels of Soluble PECAM-1 in Multiple Sclerosis Patients with Brain Gadolinium-Enhancing Lesions. J. Neuroimmunol. 1999, 99, 169–172. [Google Scholar] [CrossRef]
- Wimmer, I.; Tietz, S.; Nishihara, H.; Deutsch, U.; Sallusto, F.; Gosselet, F.; Lyck, R.; Muller, W.A.; Lassmann, H.; Engelhardt, B. PECAM-1 Stabilizes Blood-Brain Barrier Integrity and Favors Paracellular T-Cell Diapedesis Across the Blood-Brain Barrier During Neuroinflammation. Front. Immunol. 2019, 10, 711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poggi, A.; Zocchi, M.R.; Costa, P.; Ferrero, E.; Borsellino, G.; Placido, R.; Galgani, S.; Salvetti, M.; Gasperini, C.; Ristori, G.; et al. IL-12-Mediated NKRP1A Up-Regulation and Consequent Enhancement of Endothelial Transmigration of Vδ2+ TCRγδ+ T Lymphocytes from Healthy Donors and Multiple Sclerosis Patients. J. Immunol. 1999, 162, 4349–4354. [Google Scholar] [PubMed]
- Zarobkiewicz, M.K.; Wawryk-Gawda, E.; Kowalska, W.; Janiszewska, M.; Bojarska-Junak, A. Γδ T Lymphocytes in Asthma: A Complicated Picture. Arch. Immunol. Ther. Exp. 2021, 69, 4. [Google Scholar] [CrossRef]
- Poggi, A.; Zocchi, M.R.; Carosio, R.; Ferrero, E.; Angelini, D.F.; Galgani, S.; Caramia, M.D.; Bernardi, G.; Borsellino, G.; Battistini, L. Transendothelial Migratory Pathways of V 1+TCR + and V 2+TCR + T Lymphocytes from Healthy Donors and Multiple Sclerosis Patients: Involvement of Phosphatidylinositol 3 Kinase and Calcium Calmodulin-Dependent Kinase II. J. Immunol. 2002, 168, 6071–6077. [Google Scholar] [CrossRef] [Green Version]
- Zarobkiewicz, M.K.; Kowalska, W.; Halczuk, P.; Woś, J.; Jodłowska-Jędrych, B.; Rejdak, K.; Roliński, J.; Bojarska-Junak, A.A. RORγT Is Overexpressed in INKT and Γδ T Cells during Relapse in Relapsing-Remitting Multiple Sclerosis. J. Neuroimmunol. 2019, 337, 577046. [Google Scholar] [CrossRef]
- Bekkema, R.; Tadema, A.; Daenen, S.M.G.J.; Kluin-Nelemans, H.C.; Mulder, A.B. An Improved Flow Cytometric Method Using FACS Lysing Solution for Measurement of ZAP-70 Expression in B-Cell Chronic Lymphocytic Leukemia. Cytometry B Clin. Cytom. 2008, 74, 40–44. [Google Scholar] [CrossRef]
- Joe, E.; Frey, T. Performance Comparison of Commercial Fixing and Permeabilizing Reagents. Blood 2008, 112, 4927. [Google Scholar] [CrossRef]
- Jason, J.; Inge, K.L. Mitogen-Induced Modulation of CD3, CD4, and CD8. Hum. Immunol. 2000, 61, 202–211. [Google Scholar] [CrossRef]
- Abuzakouk, M.; Kelleher, D.; Feighery, C.; O’Farrelly, C. Increased HLA-DR and Decreased CD3 on Human Intestinal Intraepithelial Lymphocytes: Evidence of Activation? Gut 1996, 39, 396–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado-Garcia, H.; Aguilar-Cazares, D.; Meneses-Flores, M.; Morales-Fuentes, J.; Lopez-Gonzalez, J.S. Lung Carcinomas Do Not Induce T-Cell Apoptosis via the Fas/Fas Ligand Pathway but Down-Regulate CD3 Epsilon Expression. Cancer Immunol. Immunother. 2008, 57, 325–336. [Google Scholar] [CrossRef]
- Valle, A.; Barbagiovanni, G.; Jofra, T.; Stabilini, A.; Perol, L.; Baeyens, A.; Anand, S.; Cagnard, N.; Gagliani, N.; Piaggio, E.; et al. Heterogeneous CD3 Expression Levels in Differing T Cell Subsets Correlate with the In Vivo Anti-CD3–Mediated T Cell Modulation. J. Immunol. 2015, 194, 2117–2127. [Google Scholar] [CrossRef] [Green Version]
- Ross, E.A.; Coughlan, R.E.; Flores-Langarica, A.; Bobat, S.; Marshall, J.L.; Hussain, K.; Charlesworth, J.; Abhyankar, N.; Hitchcock, J.; Gil, C.; et al. Cd31 Is Required on Cd4+ T Cells to Promote T Cell Survival during Salmonella Infection. J. Immunol. 2011, 187, 1553–1565. [Google Scholar] [CrossRef] [Green Version]
- Marelli-Berg, F.M.; Clement, M.; Mauro, C.; Caligiuri, G. An Immunologist’s Guide to CD31 Function in T-Cells. J. Cell Sci. 2013, 126, 2343–2352. [Google Scholar] [CrossRef] [Green Version]
- Ashman, L.K.; Aylett, G.W. Expression of CD31 Epitopes on Human Lymphocytes: CD31 Monoclonal Antibodies Differentiate between Naive (CD45RA+) and Memory (CD45RA−) CD4-positive T Cells. Tissue Antigens 1991, 38, 208–212. [Google Scholar] [CrossRef]
- Poggi, A.; Zancolli, M.; Catellani, S.; Borsellino, G.; Battistini, L.; Zocchi, M.R. Migratory Pathways of T Cells and Response to CXCR3 and CXCR4 Ligands: Adhesion Molecules Involved and Implications for Multiple Sclerosis Pathogenesis. Ann. N. Y. Acad. Sci. 2007, 1107, 68–78. [Google Scholar] [CrossRef]
- Schirmer, L.; Rothhammer, V.; Hemmer, B.; Korn, T. Enriched CD161high CCR6+ Γδ T Cells in the Cerebrospinal Fluid of Patients with Multiple Sclerosis. JAMA Neurol. 2013, 70, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Annibali, V.; Ristori, G.; Angelini, D.F.; Serafini, B.; Mechelli, R.; Cannoni, S.; Romano, S.; Paolillo, A.; Abderrahim, H.; Diamantini, A.; et al. CD161highCD8+T Cells Bear Pathogenetic Potential in Multiple Sclerosis. Brain 2011, 134, 542–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brundula, V.; Rewcastle, N.B.; Metz, L.M.; Bernard, C.C.; Yong, V.W. Targeting Leukocyte MMPs and Transmigration: Minocycline as a Potential Therapy for Multiple Sclerosis. Brain 2002, 125, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, T.G.; Romanescu, C.; Popescu, B.O. The Blood–Brain Barrier—A Key Player in Multiple Sclerosis Disease Mechanisms. Biomolecules 2022, 12, 538. [Google Scholar] [CrossRef] [PubMed]
- Rice, G.P.A.; Hartung, H.-P.; Calabresi, P.A. Anti-A4 Integrin Therapy for Multiple Sclerosis: Mechanisms and Rationale. Neurology 2005, 64, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Hawke, S.; Zinger, A.; Juillard, P.-G.; Holdaway, K.; Byrne, S.N.; Grau, G.E. Selective Modulation of Trans-Endothelial Migration of Lymphocyte Subsets in Multiple Sclerosis Patients under Fingolimod Treatment. J. Neuroimmunol. 2020, 349, 577392. [Google Scholar] [CrossRef] [PubMed]
- Zarobkiewicz, M.K.; Kowalska, W.; Roliński, J.; Bojarska-Junak, A.A. Γδ T Lymphocytes in the Pathogenesis of Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. J. Neuroimmunol. 2019, 330, 67–73. [Google Scholar] [CrossRef]
- Bühler, U.; Fleischer, V.; Luessi, F.; Rezk, A.; Belikan, P.; Graetz, C.; Gollan, R.; Wolf, C.; Lutz, J.; Bar-Or, A.; et al. Role of IL-17-Producing Lymphocytes in Severity of Multiple Sclerosis upon Natalizumab Treatment. Mult. Scler. J. 2017, 23, 567–576. [Google Scholar] [CrossRef]


| % Women | Age | EDSS | ARR | Years after Diagnosis | |
|---|---|---|---|---|---|
| MS patients | 76.47% | 43 (29.5–51) | 3.5 (3–5.5) | 0 (0–1) | 10 (7.5–14.75) |
| Healthy volunteers | 70.00% | 44 (26–58) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarobkiewicz, M.K.; Morawska, I.; Kowalska, W.; Halczuk, P.; Roliński, J.; Bojarska-Junak, A.A. PECAM-1 Is Down-Regulated in γδT Cells during Remission, but Up-Regulated in Relapse of Multiple Sclerosis. J. Clin. Med. 2022, 11, 3210. https://doi.org/10.3390/jcm11113210
Zarobkiewicz MK, Morawska I, Kowalska W, Halczuk P, Roliński J, Bojarska-Junak AA. PECAM-1 Is Down-Regulated in γδT Cells during Remission, but Up-Regulated in Relapse of Multiple Sclerosis. Journal of Clinical Medicine. 2022; 11(11):3210. https://doi.org/10.3390/jcm11113210
Chicago/Turabian StyleZarobkiewicz, Michał K., Izabela Morawska, Wioleta Kowalska, Paweł Halczuk, Jacek Roliński, and Agnieszka A. Bojarska-Junak. 2022. "PECAM-1 Is Down-Regulated in γδT Cells during Remission, but Up-Regulated in Relapse of Multiple Sclerosis" Journal of Clinical Medicine 11, no. 11: 3210. https://doi.org/10.3390/jcm11113210
APA StyleZarobkiewicz, M. K., Morawska, I., Kowalska, W., Halczuk, P., Roliński, J., & Bojarska-Junak, A. A. (2022). PECAM-1 Is Down-Regulated in γδT Cells during Remission, but Up-Regulated in Relapse of Multiple Sclerosis. Journal of Clinical Medicine, 11(11), 3210. https://doi.org/10.3390/jcm11113210









