Age-Specific Characteristics of Adult and Pediatric Respiratory Viral Infections: A Retrospective Single-Center Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant Recruitment
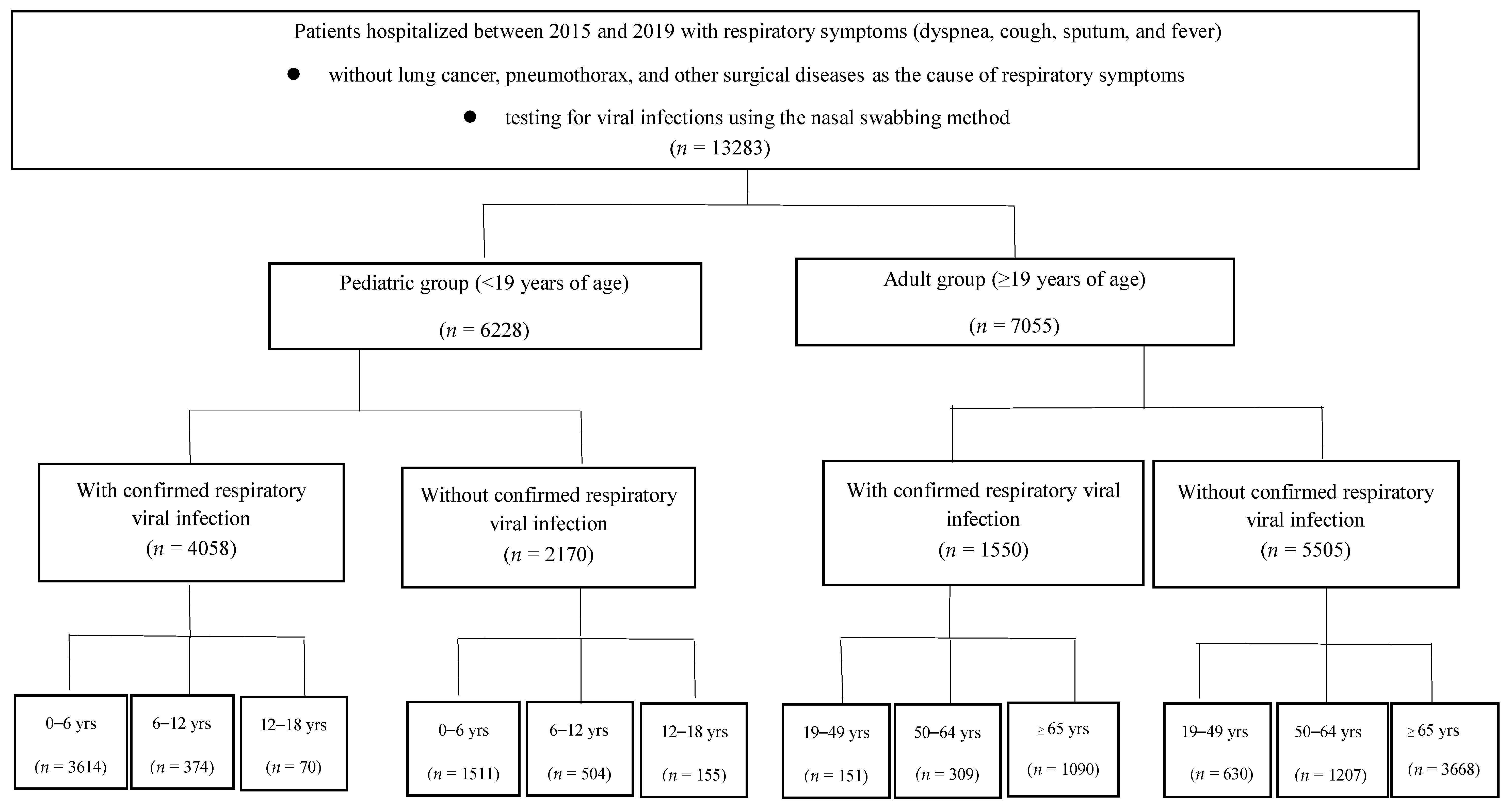
2.2. Inclusion and Exclusion Criteria
2.3. Viral Testing
2.4. Definitions
2.4.1. Viral Infection Rate
2.4.2. Co-Infection
2.5. Data Collection
2.6. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Types of Viruses Detected
3.3. Viral Infection Rates
3.4. Co-Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus Disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Marroquín, B.; Vine, V.; Morgan, R. Mental health during the COVID-19 pandemic: Effects of stay-at-home policies, social distancing behavior, and social resources. Psychiatry Res. 2020, 293, 113419. [Google Scholar] [CrossRef]
- Kim, M.R.; Lee, H.R.; Lee, G.M. Epidemiology of acute viral respiratory tract infections in Korean children. J. Infect. 2000, 41, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Guerrier, G.; Goyet, S.; Chheng, E.T.; Rammaert, B.; Borand, L.; Te, V.; Try, P.L.; Sareth, R.; Cavailler, P.; Mayaud, C.; et al. Acute viral lower respiratory tract infections in Cambodian children: Clinical and epidemiologic characteristics. Pediatr. Infect. Dis. J. 2013, 32, e8–e13. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [CrossRef] [Green Version]
- Yu, J.; Xie, Z.; Zhang, T.; Lu, Y.; Fan, H.; Yang, D.; Bénet, T.; Vanhems, P.; Shen, K.; Huang, F.; et al. Comparison of the prevalence of respiratory viruses in patients with acute respiratory infections at different hospital settings in North China, 2012–2015. BMC Infect. Dis. 2018, 18, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Chappell, J.D. Community-acquired pneumonia requiring hospitalization among US adults. N. Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ieven, M.; Coenen, S.; Loens, K.; Lammens, C.; Coenjaerts, F.; Vanderstraeten, A.; Henriques-Normark, B.; Crook, D.; Huygen, K.; Butler, C.; et al. Aetiology of lower respiratory tract infection in adults in primary care: A prospective study in 11 European countries. Clin. Microbiol. Infect. 2018, 24, 1158–1163. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.; Lui, C.Y.G.; Wong, K.T.; Li, T.C.M.; Tse, E.C.M.; Chan, J.Y.C.; Yu, J.; Wong, S.S.M.; Choi, K.W.; Wong, R.Y.K.; et al. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin. Infect. Dis. 2013, 57, 1069–1077. [Google Scholar] [CrossRef]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef]
- Katsurada, N.; Suzuki, M.; Aoshima, M.; Yaegashi, M.; Ishifuji, T.; Asoh, N.; Hamashige, N.; Abe, M.; Ariyoshi, K. The impact of virus infections on pneumonia mortality is complex in adults: A prospective multicentre observational study. BMC Infect. Dis. 2017, 17, 755. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.S.; Park, S.H.; Kim, M.-A.; Kim, H.J.; Park, J.S.; Lee, M.Y.; Lee, C.W.; Dauti, S.; Choi, W.-I. Risk of mortality associated with respiratory syncytial virus and influenza infection in adults. BMC Infect. Dis. 2017, 17, 785. [Google Scholar] [CrossRef]
- Simon, M.; Collins, M.S. The pediatric lung and aspiration. Perspect. Swallowing Swallowing Disord. 2013, 22, 142–154. [Google Scholar] [CrossRef]
- David, R.; Tohomas, M. Overview of innate lung immunity and inflammmation. In Lung Innate Immunity and Inflammation; Springer Humana: New York, NY, USA, 2018; Volume 1809, pp. 17–30. [Google Scholar] [CrossRef]
- Keith, M. The Role of Immunity and Inflammation in Lung Senescence and Susceptibility to Infection in the Elderly. Semin. Respir. Crit. Care Med. 2010, 31, 561–574. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, K.; Lee, Y.; Kim, K.; Oh, J. Analysis of the association among air pollutants, allergenic pollen, and respiratory virus infection of children in Guri, Korea during recent 5 years. Allergy Asthma Immunol. Res. 2022, 14, 289–299. [Google Scholar] [CrossRef]
- Annesi, I.; Maesano, C.N.; D’Amato, M.; D’Amato, G. Pros and cons for the role of air pollution on COVID-19 development. Allergy 2021, 76, 2647–2649. [Google Scholar] [CrossRef]
- Visseaux, B.; Collin, G.; Ichou, H.; Charpentier, C.; Bendhafer, S.; Dumitrescu, M.; Allal, L.; Cojocaru, B.; Desfrère, L.; Descamps, D.; et al. Usefulness of multiplex PCR methods and respiratory viruses’ distribution in children below 15 years old according to age, seasons and clinical units in France: A 3 years retrospective study. PLoS ONE 2017, 12, e0172809. [Google Scholar] [CrossRef] [Green Version]
- Stefanska, I.; Romanowska, M.; Donevski, S.; Gawryluk, D.; Brydak, L.B. Co-infections with influenza and other respiratory viruses. In Respiratory Regulation-The Molecular Approach; Springer: Dordrecht, The Netherlands, 2013; Volume 756, pp. 291–301. [Google Scholar] [CrossRef]
- Mandelia, Y.; Procop, G.; Richter, S.; Worley, S.; Liu, W.; Esper, F. Dynamics and predisposition of respiratory viral co-infections in children and adults. Clin. Microbiol. Infect. 2021, 27, 631.e1–631.e6. [Google Scholar] [CrossRef]
- Ching, N.S.; Kotsanas, D.; Easton, M.L.; Francis, M.J.; Korman, T.M.; Buttery, J.P. Respiratory virus detection and co-infection in children and adults in a large Australian hospital in 2009–2015. J. Paediatr. Child. Health 2018, 54, 1321–1328. [Google Scholar] [CrossRef]
- van den Bergh, M.R.; Biesbroek, G.; Rossen, J.W.A.; de Steenhuijsen Piters, W.A.A.; Bosch, A.A.T.M.; van Gils, E.J.M.; Wang, X.; Boonacker, C.W.; Veenhoven, R.H.; Bruin, J.P.; et al. Associations between pathogens in the upper respiratory tract of young children: Interplay between viruses and bacteria. PLoS ONE 2012, 7, e47711. [Google Scholar] [CrossRef]
- Glezen, W.P. Influenza surveillance in an urban area. Can. J. Infect. Dis. 1993, 4, 272–274. [Google Scholar] [CrossRef]


| N | Age, yrs | Sex (M:F) | |
|---|---|---|---|
| Total pediatric patients | 6228 | 3.6 ± 3.6 | 3479:2749 |
| 0–6 years old | 5125 | 2.2 ± 1.8 | 2869:2256 |
| 6–12 years old | 878 | 9.0 ± 1.6 | 500:378 |
| 12–18 years old | 225 | 14.6 ± 1.6 | 110:115 |
| Pediatric patients infected | 4058 | 3.0 ± 2.9 | 2265:1793 |
| 0–6 years old | 3614 | 2.2 ± 1.6 | 2010:1604 |
| 6–12 years old | 374 | 8.7 ± 1.6 | 217:157 |
| 12–18 years old | 70 | 14.2 ± 1.2 | 38:32 |
| Total adult patients | 7055 | 69.4 ± 15.4 | 4295:2760 |
| 19–49 years old | 781 | 38.0 ± 9.2 | 461:140 |
| 50–64 years old | 1516 | 57.9 ± 4.1 | 975:541 |
| ≥65 years old | 4758 | 78.1 ± 7.1 | 2859:1899 |
| Adult patients infected | 1550 | 70.2 ± 15.3 | 840:710 |
| 19–49 years old | 151 | 36.6 ± 9.7 | 84:67 |
| 50–64 years old | 309 | 57.8 ± 4.0 | 163:146 |
| ≥65 years old | 1090 | 78.3 ± 7.2 | 593:497 |
| Virus Infection Rate | ||||||
|---|---|---|---|---|---|---|
| 0–6 yrs | 7–12 yrs | 12–18 yrs | 19–49 yrs | 50–64 yrs | ≥65 yrs | |
| Influenza | 0.09 | 0.26 | 0.31 | 0.23 | 0.34 | 0.27 |
| ParaIinfluV | 0.14 | 0.08 | 0.07 | 0.05 | 0.08 | 0.10 |
| RSV | 0.19 | 0.03 | 0.03 | 0.09 | 0.08 | 0.14 |
| Corona | 0.06 | 0.05 | 0.02 | 0.09 | 0.10 | 0.13 |
| Adeno | 0.27 | 0.28 | 0.26 | 0.15 | 0.07 | 0.05 |
| Rhino | 0.37 | 0.35 | 0.39 | 0.35 | 0.29 | 0.26 |
| Hboca | 0.14 | 0.06 | 0.01 | 0.01 | 0.02 | 0.01 |
| MPV | 0.08 | 0.04 | 0.03 | 0.12 | 0.08 | 0.10 |
| Infection Rate | 1 Virus Infected | 2 Viruses Co-Infected | 3 Viruses Co-Infected | ≥4 Viruses Co-Infected | |
|---|---|---|---|---|---|
| Pediatric patients | 4058/6228 (65.2%) | 2829 (48.9%) | 1006 (17.4%) | 202 (3.5%) | 21 (0.3%) |
| 0–6 years | 3614/5125 (70.5%) | 2471 (68.4%) | 929 (25.7%) | 193 (5.3%) | 21 (0.6%) |
| 7–12 years | 374/878 (42.6%) | 300 (80.2%) | 65 (17.4%) | 9 (2.4%) | 0 |
| 13–18 years | 70/225 (31.1%) | 58 (82.9%) | 12 (17.1%) | 0 | 0 |
| Adult patients | 1550/7055 (22.0%) | 1304 (84.1%) | 237 (15.3%) | 19 (1.2%) | 0 (0%) |
| 19–49 years | 151/781 (19.3%) | 137 (90.7%) | 9 (6.0%) | 5 (3.3%) | 0 |
| 50–64 years | 309/1516 (20.4%) | 260 (84.1%) | 47 (15.2%) | 2 (0.6%) | 0 |
| ≥65 years | 1090/4758 (22.9%) | 907 (85.3%) | 181 (16.6%) | 12 (1.1%) | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.K.; Na, J.Y.; Kim, J.; Oh, J.-W.; Kim, Y.J.; Choi, Y.-J. Age-Specific Characteristics of Adult and Pediatric Respiratory Viral Infections: A Retrospective Single-Center Study. J. Clin. Med. 2022, 11, 3197. https://doi.org/10.3390/jcm11113197
Hwang JK, Na JY, Kim J, Oh J-W, Kim YJ, Choi Y-J. Age-Specific Characteristics of Adult and Pediatric Respiratory Viral Infections: A Retrospective Single-Center Study. Journal of Clinical Medicine. 2022; 11(11):3197. https://doi.org/10.3390/jcm11113197
Chicago/Turabian StyleHwang, Jae Kyoon, Jae Yoon Na, Jihye Kim, Jae-Won Oh, Yong Joo Kim, and Young-Jin Choi. 2022. "Age-Specific Characteristics of Adult and Pediatric Respiratory Viral Infections: A Retrospective Single-Center Study" Journal of Clinical Medicine 11, no. 11: 3197. https://doi.org/10.3390/jcm11113197







