Methylprednisolone Plus Low-Dose Methotrexate for Bullous Pemphigoid—A Single Center Retrospective Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bastuji-Garin, S.; Joly, P.; Lemordant, P.; Sparsa, A.; Bedane, C.; Delaporte, E.; Roujeau, J.; Bernard, P.; Guillaume, J.C.; Ingen-Housz-Oro, S.; et al. Risk Factors for Bullous Pemphigoid in the Elderly: A Prospective Case-Control Study. J. Investig. Dermatol. 2011, 131, 637–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feliciani, C.; Joly, P.; Jonkman, M.F.; Zambruno, G.; Zillikens, D.; Ioannides, D.; Kowalewski, C.; Jedlickova, H.; Kárpáti, S.; Marinovic, B.; et al. Management of Bullous Pemphigoid: The European Dermatology Forum Consensus in Collaboration with the European Academy of Dermatology and Venereology. Br. J. Dermatol. 2015, 172, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Santi, C.G.; Gripp, A.C.; Roselino, A.M.; Mello, D.S.; Gordilho, J.O.; de Marsillac, P.F.; Porro, A.M. Consensus on the Treatment of Autoimmune Bullous Dermatoses: Bullous Pemphigoid, Mucous Membrane Pemphigoid and Epidermolysis Bullosa Acquisita—Brazilian Society of Dermatology. An. Bras. Dermatol. 2019, 94, 33–47. [Google Scholar] [CrossRef]
- Ujiie, H.; Iwata, H.; Yamagami, J.; Nakama, T.; Aoyama, Y.; Ikeda, S.; Ishii, N.; Iwatsuki, K.; Kurosawa, M.; Sawamura, D.; et al. Japanese Guidelines for the Management of Pemphigoid (Including Epidermolysis Bullosa Acquisita). J. Dermatol. 2019, 46, 1102–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, E.; Sticherling, M.; Sárdy, M.; Eming, R.; Goebeler, M.; Hertl, M.; Hofmann, S.C.; Hunzelmann, N.; Kern, J.S.; Kramer, H.; et al. S2k Guidelines for the Treatment of Pemphigus Vulgaris/Foliaceus and Bullous Pemphigoid: 2019 Update. JDDG J. Ger. Soc. Dermatol. 2020, 18, 516–526. [Google Scholar] [CrossRef]
- Prince, M.J.; Wu, F.; Guo, Y.; Gutierrez Robledo, L.M.; O’Donnell, M.; Sullivan, R.; Yusuf, S. The Burden of Disease in Older People and Implications for Health Policy and Practice. Lancet 2015, 385, 549–562. [Google Scholar] [CrossRef]
- Zullo, A.R.; Gray, S.L.; Holmes, H.M.; Marcum, Z.A. Screening for Medication Appropriateness in Older Adults. Clin. Geriatr. Med. 2018, 34, 39–54. [Google Scholar] [CrossRef]
- Kjellman, P.; Eriksson, H.; Berg, P. A Retrospective Analysis of Patients with Bullous Pemphigoid Treated with Methotrexate. Arch. Dermatol. 2008, 144, 612–616. [Google Scholar] [CrossRef]
- Kalinska-Bienias, A.; Kowalczyk, E.; Jagielski, P.; Kowalewski, C.; Wozniak, K. Tetracycline, Nicotinamide, and Lesionally Administered Clobetasol as a Therapeutic Option to Prednisone in Patients with Bullous Pemphigoid: A Comparative, Retrospective Analysis of 106 Patients with Long-Term Follow-Up. Int. J. Dermatol. 2019, 58, 172–177. [Google Scholar] [CrossRef]
- Bedoui, Y.; Guillot, X.; Sélambarom, J.; Guiraud, P.; Giry, C.; Jaffar-Bandjee, M.C.; Ralandison, S.; Gasque, P. Methotrexate an Old Drug with New Tricks. Int. J. Mol. Sci. 2019, 20, 5023. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; O’Brien, T.; Yap, L.M.; Prince, H.M.; McCormack, C.J. The Use of Methotrexate in Dermatology: A Review. Australas. J. Dermatol. 2012, 53, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lever, W.F.; Hashimoto, K. The Etiology and Treatment of Pemphigus and Pemphigoid. J. Investig. Dermatol. 1969, 53, 373–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downham, T.F.; Chapel, T.A. Bullous Pemphigoid:Therapy in Patients with and without Diabetes Mellitus. Arch. Dermatol. 1978, 114, 1639–1642. [Google Scholar] [CrossRef] [PubMed]
- Heilborn, J.D.; Stahle-Backdahl, M.; Albertioni, F.; Vassilaki, I.; Peterson, C.; Stephansson, E. Low-Dose Oral Pulse Methotrexate as Monotherapy in Elderly Patients with Bullous Pemphigoid. J. Am. Acad. Dermatol. 1999, 40, 741–749. [Google Scholar] [CrossRef]
- Patton, T.; Korman, N. Role of Methotrexate in the Treatment of Bullous Pemphigoid in the Elderly. Drugs Aging 2008, 25, 623–629. [Google Scholar] [CrossRef]
- Paul, M.A.; Jorizzo, J.L.; Fleischer, A.B.; White, W.L. Low-Dose Methotrexate Treatment in Elderly Patients with Bullous Pemphigoid. J. Am. Acad. Dermatol. 1994, 31, 620–625. [Google Scholar] [CrossRef]
- Du-Thanh, A.; Merlet, S.; Maillard, H.; Bernard, P.; Joly, P.; Estève, E.; Richard, M.A.; Pauwels, C.; Ingen-Housz-Oro, S.; Guillot, B.; et al. Combined Treatment with Low-Dose Methotrexate and Initial Short-Term Superpotent Topical Steroids in Bullous Pemphigoid: An Open, Multicentre, Retrospective Study. Br. J. Dermatol. 2011, 165, 1337–1343. [Google Scholar] [CrossRef]
- Dereure, O.; Bessis, D.; Guillot, B.; Guilhou, J.J. Treatment of Bullous Pemphigoid by Low-Dose Methotrexate Associated with Short-Term Potent Topical Steroids: An Open Prospective Study of 18 Cases. Arch. Dermatol. 2002, 138, 1255–1256. [Google Scholar] [CrossRef]
- Bara, C. Methotrexate for Bullous Pemphigoid: Preliminary Study. Arch. Dermatol. 2003, 139, 1506–1507. [Google Scholar] [CrossRef]
- Kwatra, S.G.; Jorizzo, J.L. Bullous Pemphigoid: A Case Series with Emphasis on Long-Term Remission off Therapy. J. Dermatol. Treat. 2013, 24, 327–331. [Google Scholar] [CrossRef]
- Delaumenie, S.; Assikar, S.; Prudhomme, R.; Matei, I.; Souyri, N.; Dalmay, F.; Bedane, C. Methotrexate Is Safe and Efficient as Long-Term Treatment for Bullous Pemphigoid. Eur. J. Dermatol. 2019, 29, 217–218. [Google Scholar] [CrossRef] [PubMed]
- Gürcan, H.M.; Razzaque Ahmed, A. Analysis of Current Data on the Use of Methotrexate in the Treatment of Pemphigus and Pemphigoid. Br. J. Dermatol. 2009, 161, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Gravani, A.; Gaitanis, G.; Tsironi, T.; Tigas, S.; Bassukas, I.D. Changing Prevalence of Diabetes Mellitus in Bullous Pemphigoid: It Is the Dipeptidyl Peptidase-4 Inhibitors. J. Eur. Acad. Dermatol. Venereol. 2018, 32, e438–e439. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, K.; Spirova, M.; Gaitanis, G.; Tsartsarakis, A.; Bassukas, I.D.D. Drug-Induced Bullous Pemphigoid in Diabetes Mellitus Patients Receiving Dipeptidyl Peptidase-IV Inhibitors plus Metformin. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Kuwata, H.; Nishioka, Y.; Noda, T.; Kubo, S.; Myojin, T.; Higashino, T.; Takahashi, Y.; Ishii, H.; Imamura, T. Association between Dipeptidyl Peptidase-4 Inhibitors and Increased Risk for Bullous Pemphigoid within 3 Months from First Use: A 5-Year Population-Based Cohort Study Using the Japanese National Database. J. Diabetes Investig. 2022, 13, 460–467. [Google Scholar] [CrossRef]
- Fisch, A.; Morin, L.; Talme, T.; Johnell, K.; Gallais Sérézal, I. Low-Dose Methotrexate Use and Safety for Older Patients with Bullous Pemphigoid and Impaired Renal Function: A Cohort Study. J. Am. Acad. Dermatol. 2020, 82, 1532–1534. [Google Scholar] [CrossRef]
- Smaje, A.; Weston-Clark, M.; Raj, R.; Orlu, M.; Davis, D.; Rawle, M. Factors Associated with Medication Adherence in Older Patients: A Systematic Review. Aging Med. Milt. 2018, 1, 254–266. [Google Scholar] [CrossRef] [Green Version]
- Uchmanowicz, B.; Chudiak, A.; Uchmanowicz, I.; Rosińczuk, J.; Froelicher, E.S. Factors Influencing Adherence to Treatment in Older Adults with Hypertension. Clin. Interv. Aging 2018, 13, 2425–2441. [Google Scholar] [CrossRef] [Green Version]
- Yap, A.F.; Thirumoorthy, T.; Kwan, Y.H. Systematic Review of the Barriers Affecting Medication Adherence in Older Adults. Geriatr. Gerontol. Int. 2016, 16, 1093–1101. [Google Scholar] [CrossRef]
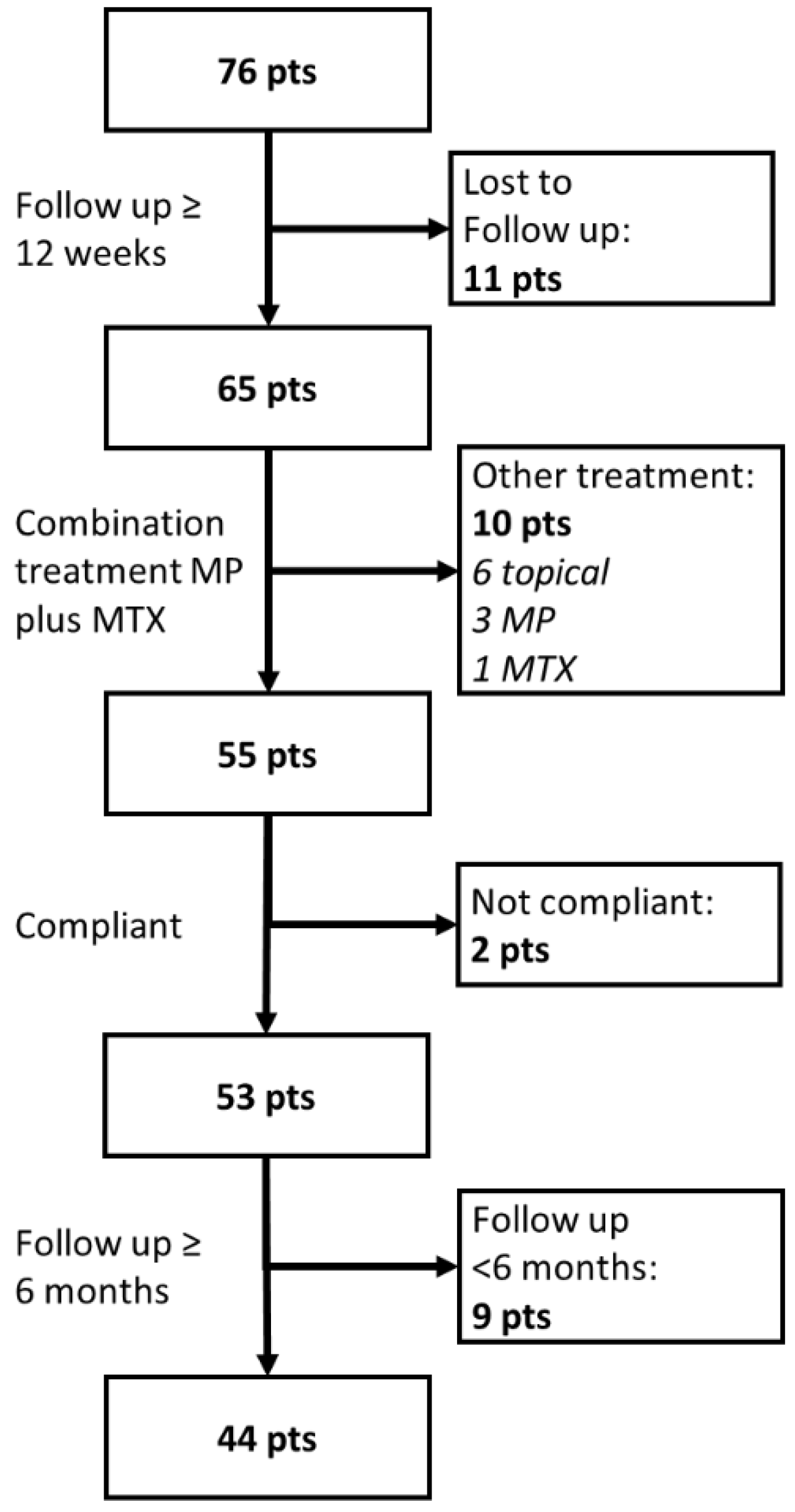
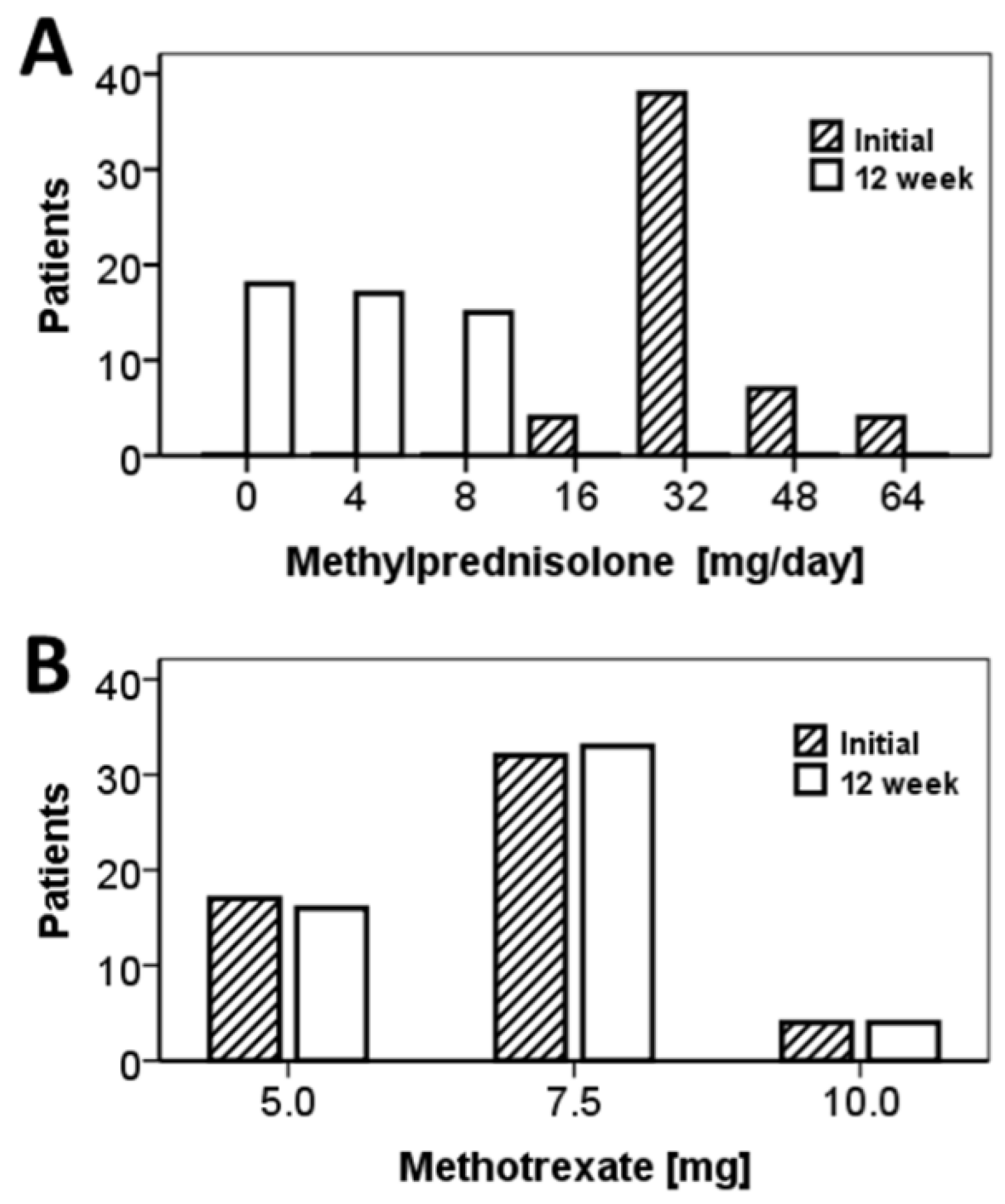

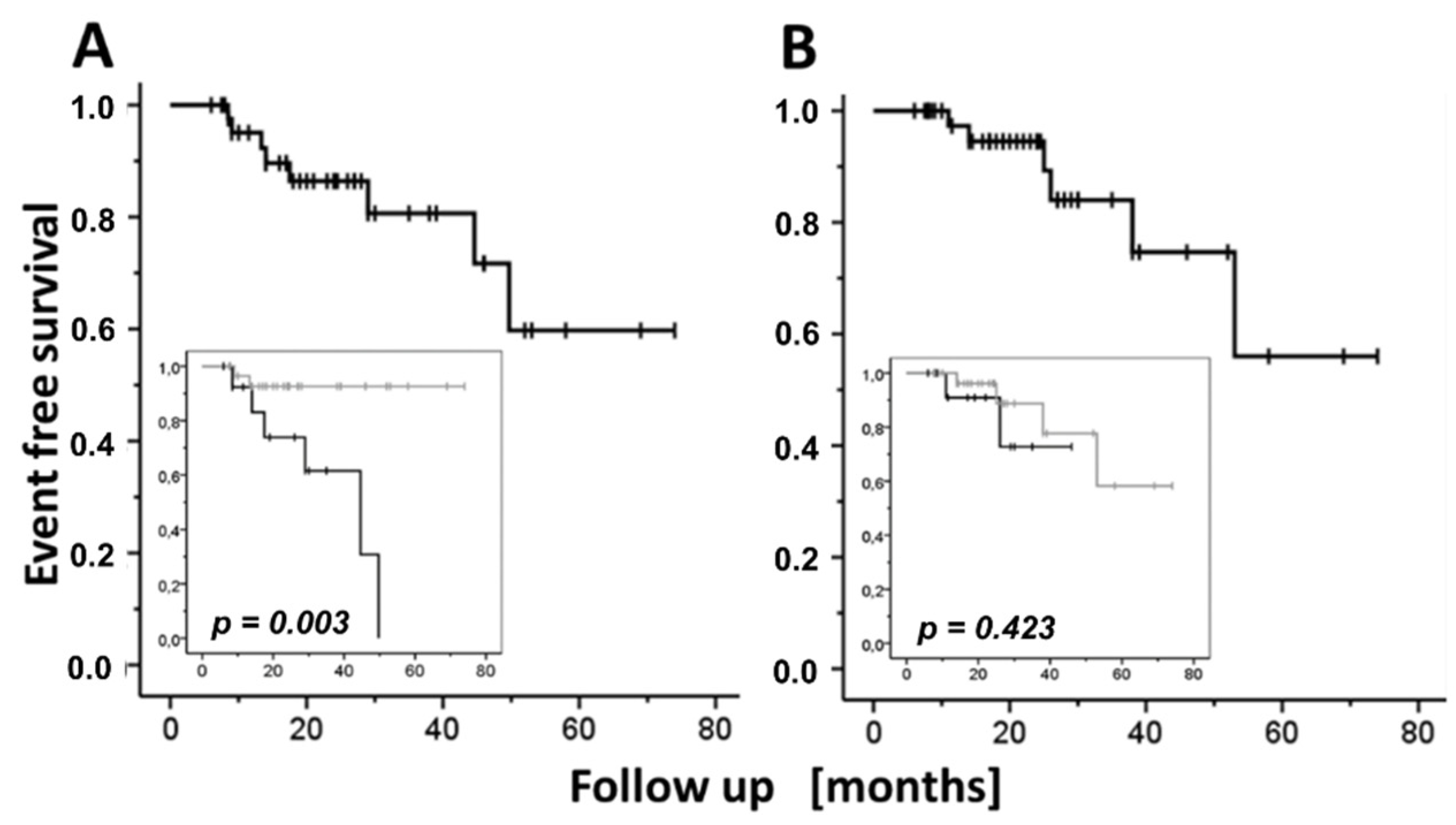

| Parameter | DPP4i a | n | Mean | S.E. b | 95% CI c of Mean | p * | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Age [years] | No | 29 | 80.31 | 1.47 | 77.30 | 83.32 | 0.234 |
| Yes | 24 | 77.46 | 1.88 | 73.57 | 81.35 | ||
| Total | 53 | 79.02 | 1.18 | 76.66 | 81.38 | ||
| Initial daily MP d dose [mg/day] | No | 29 | 35.31 | 2.30 | 30.60 | 40.02 | 0.770 |
| Yes | 24 | 35.33 | 1.92 | 31.36 | 39.31 | ||
| Total | 53 | 35.32 | 1.52 | 32.28 | 38.36 | ||
| 12-week daily MP dose [mg/day] | No | 29 | 4.00 | 0.77 | 2.43 | 5.57 | 0.828 |
| Yes | 24 | 3.92 | 0.60 | 2.68 | 5.15 | ||
| Total | 53 | 3.96 | 0.50 | 2.97 | 4.96 | ||
| Cumulative MP dose to TDC e [mg] | No | 29 | 679 | 78 | 517 | 840 | 0.791 |
| Yes | 24 | 677 | 57 | 557 | 797 | ||
| Total | 53 | 678 | 49 | 578 | 777 | ||
| 12-week cumulative MP dose [mg] | No | 29 | 1037 | 79 | 875 | 1199 | 0.805 |
| Yes | 24 | 971 | 66 | 833 | 1110 | ||
| Total | 53 | 1009 | 53 | 902 | 1115 | ||
| TDC [days] | No | 29 | 30.29 | 3.50 | 23.11 | 37.46 | 0.894 |
| Yes | 24 | 28.57 | 2.83 | 22.69 | 34.44 | ||
| Total | 53 | 29.51 | 2.29 | 24.92 | 34.10 | ||
| MP dose reduction rate [mg/week] f | No | 29 | 8.33 | 1.24 | 5.80 | 10.87 | 0.394 |
| Yes | 24 | 8.18 | 0.80 | 6.51 | 9.84 | ||
| Total | 53 | 8.26 | 0.76 | 6.73 | 9.80 | ||
| Initial MTX g dose [mg/week] | No | 29 | 6.55 | 0.26 | 6.02 | 7.09 | 0.070 |
| Yes | 24 | 7.29 | 0.30 | 6.68 | 7.91 | ||
| Total | 53 | 6.89 | 0.20 | 6.48 | 7.29 | ||
| 12-week MTX dose [mg/week] | No | 29 | 6.72 | 0.45 | 5.80 | 7.64 | 0.258 |
| Yes | 24 | 6.98 | 0.40 | 6.16 | 7.80 | ||
| Total | 53 | 6.84 | 0.30 | 6.23 | 7.45 | ||
| Parameter | Age | Mean | S.E. a | 95% CI of Mean b | p * | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| 12-week cumulative MP c dose [mg] | <75 | 1112 | 124 | 841 | 1383 | 0.259 |
| ≥75 | 974 | 57 | 858 | 1090 | ||
| TDC d [days] | <75 | 37.93 | 5.87 | 25.24 | 50.62 | 0.022 |
| ≥75 | 26.32 | 2.07 | 22.13 | 30.52 | ||
| Follow up [months] | <75 | 28.18 | 5.38 | 16.55 | 39.81 | 0.275 |
| ≥75 | 21.97 | 2.78 | 16.33 | 27.62 | ||
| MP dose reduction rate [mg/week] e | <75 | 7.22 | 1.28 | 4.45 | 9.99 | 0.407 |
| ≥75 | 8.66 | 0.93 | 6.76 | 10.55 | ||
| % Event-Free Patients [SE] b | |||
|---|---|---|---|
| Relapse OR SAE a | 12 Months (n = 34) c | 24 Months (n = 18) | 36 Months (n = 10) |
| Either event [n = 44] d | 92.5 [4.2] | 77.9 [7.0] | 73.4 [7.9] |
| Relapse [n = 44] | 95.1 [3.4] | 86.4 [5.7] | 80.7 [7.7] |
| SAE [n = 44] | 97.3 [2.7] | 94.5 [3.8] | 84.0 [7.8] |
| SAE [n = 53] | 95.2 [3.3] | 92.5 [4.2] | 82.2 [7.8] |
| Gender, Age | DMT2/History of DPP4i a Use | Comorbidities | Severe Adverse Event | Time from Diagnosis to SAE b [Months] | Clinical Course-Outcome |
|---|---|---|---|---|---|
| Female, 79 | No/No | Hypertension, COPD c, hyperlipidemia, epilepsy | Respiratory tract infection | 53 | Deceased |
| Female, 81 | No/No | Hypertension, hypothyroidism | Hyponatremia | 5 | Stopped MP d, treatment continued with topical steroids. Patient stopped MTX e by herself. Without new lesions at last follow-up visit (58 months since diagnosis). |
| Female, 87 | Yes/Yes | Hypertension, hypothyroidism | Stroke | 38 | Stopped MP intake and reduction of MTX dose. |
| Male, 89 | Yes/Yes | Hyperlipidemia, depression | Hip fracture | 14 | Stopped MP intake. Patient continued MTX (overall treatment duration: 12 months). |
| Female, 69 | No/No | Hypertension, atrial fibrillation, rheumatoid arthritis, hypothyroidism, heterozygous beta thalassemia, GERD f | Respiratory tract infection | 25 | Stopped MP and MTX. |
| Female, 90 | Yes/Yes | Psoriasis, colitis ulcerosa, cataract, glaucoma | Stroke | 26 | Stopped MTX after the stroke. Without new lesions at last follow-up (28 months since diagnosis; 2 months after the SAE) |
| Female, 84 | Yes/Yes | Hypertension, hyperlipidemia, GERD, osteoporosis | Respiratory tract infection | 15 | Deceased |
| Parameter | SAE a | Mean | S.E. b | 95% CI c for Mean | p d | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age [years] | No | 78.1 | 1.25 | 75.6 | 80.6 | 0.177 |
| Yes | 82.7 | 2.75 | 76.0 | 89.4 | ||
| 12-week cumulative MP e [mg] | No | 979 | 53 | 871 | 1087 | 0.157 |
| Yes | 1201 | 204 | 703 | 1699 | ||
| 12-week daily MP dose [mg] | No | 3.92 | 0.34 | 3.26 | 4.61 | 0.051 |
| Yes | 5.82 | 1.06 | 3.21 | 8.41 | ||
| Predictor | p a | OR b | 95.0% CI c for OR | |
|---|---|---|---|---|
| Lower | Upper | |||
| Gender (male vs. Female) | 0.193 | 3.167 | 0.559 | 17.941 |
| Age (<75 vs. ≥75 years) | 0.057 | 0.121 | 0.014 | 1.061 |
| Gliptin (intake at BP diagnosis: yes vs. no) d | 0.188 | 2.608 | 0.626 | 10.858 |
| MP e (initial daily dose [mg]) | 0.139 | 1.036 | 0.989 | 1.086 |
| MP (12-week daily dose: 0 mg vs. >0 mg) f | 0.013 | 8.726 | 1.572 | 48.439 |
| Methotrexate (initial weekly dose [mg]) | 0.132 | 1.513 | 0.883 | 2.593 |
| Adverse Event | 12-Week MP a Dose [mg/Day] | Event-Free Follow-Up Time [Months] | p d | |||
|---|---|---|---|---|---|---|
| Mean | S.E. b | 95% CI c | ||||
| Lower | Upper | |||||
| Relapse OR SAE e | 0 | 29.12 | 4.78 | 19.76 | 38.48 | 0.002 |
| >0 f | 56.60 | 5.44 | 45.93 | 67.27 | ||
| Relapse | 0 | 36.14 | 5.06 | 26.23 | 46.06 | 0.003 |
| >0 | 69.34 | 3.17 | 63.12 | 75.56 | ||
| SAE5 | 0 | 39.18 | 4.22 | 30.91 | 47.45 | 0.423 |
| >0 | 60.00 | 5.83 | 48.58 | 71.42 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gravani, A.; Gaitanis, G.; Spyridonos, P.; Alexis, I.; Tigas, S.; Bassukas, I.D. Methylprednisolone Plus Low-Dose Methotrexate for Bullous Pemphigoid—A Single Center Retrospective Analysis. J. Clin. Med. 2022, 11, 3193. https://doi.org/10.3390/jcm11113193
Gravani A, Gaitanis G, Spyridonos P, Alexis I, Tigas S, Bassukas ID. Methylprednisolone Plus Low-Dose Methotrexate for Bullous Pemphigoid—A Single Center Retrospective Analysis. Journal of Clinical Medicine. 2022; 11(11):3193. https://doi.org/10.3390/jcm11113193
Chicago/Turabian StyleGravani, Agoritsa, Georgios Gaitanis, Panagiota Spyridonos, Ioannis Alexis, Stelios Tigas, and Ioannis D. Bassukas. 2022. "Methylprednisolone Plus Low-Dose Methotrexate for Bullous Pemphigoid—A Single Center Retrospective Analysis" Journal of Clinical Medicine 11, no. 11: 3193. https://doi.org/10.3390/jcm11113193
APA StyleGravani, A., Gaitanis, G., Spyridonos, P., Alexis, I., Tigas, S., & Bassukas, I. D. (2022). Methylprednisolone Plus Low-Dose Methotrexate for Bullous Pemphigoid—A Single Center Retrospective Analysis. Journal of Clinical Medicine, 11(11), 3193. https://doi.org/10.3390/jcm11113193







