Effects of Electromyographic Biofeedback on Functional Recovery of Patients Two Months after Total Knee Arthroplasty: A Randomized Controlled Trial
Abstract
:1. Introduction
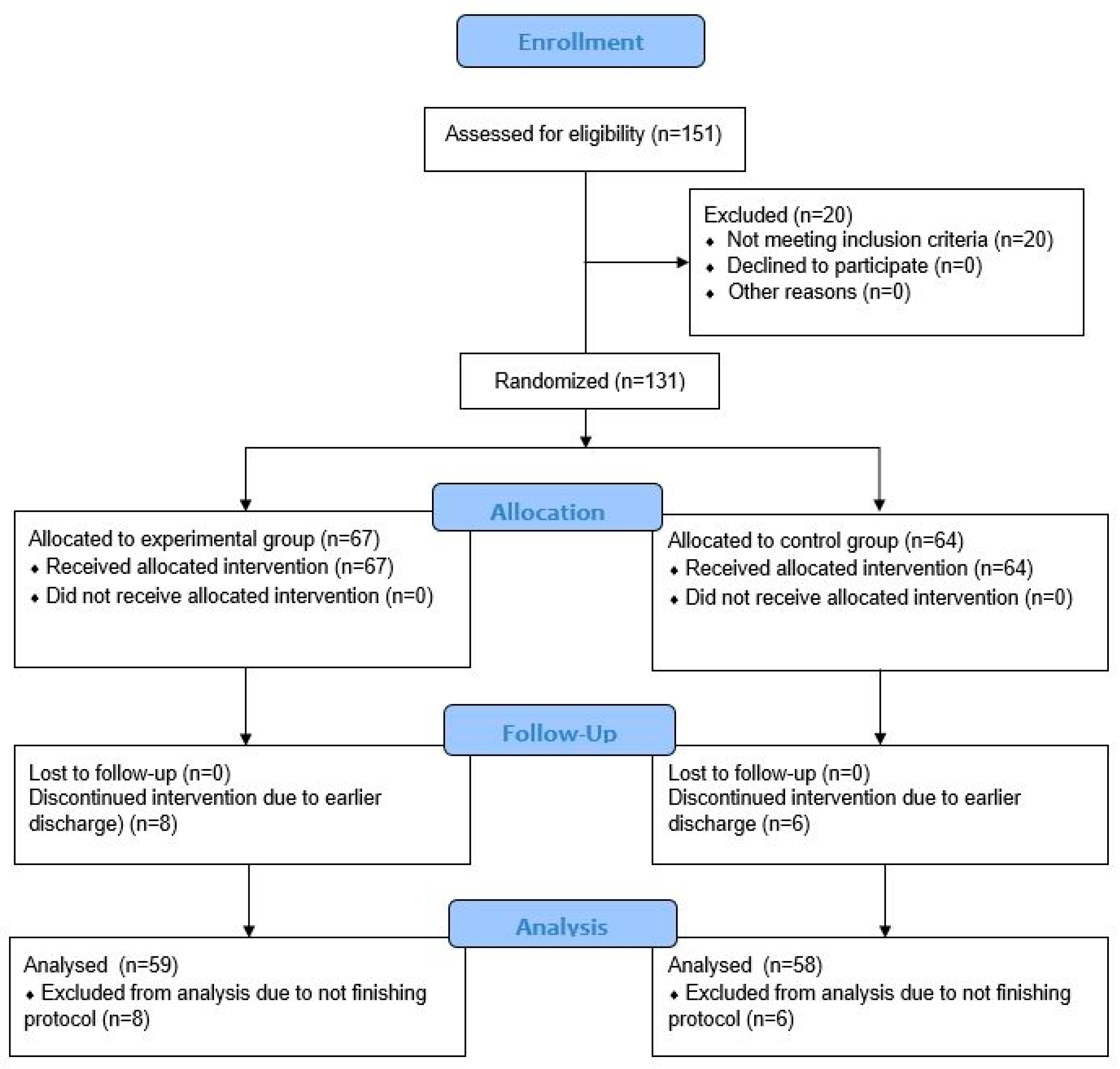
2. Materials and Methods
2.1. Study Design and Population
2.2. Randomization
2.3. Sample Size and Power Analysis
2.4. Rehabilitation Protocol
2.4.1. Experimental Biofeedback-Assisted Exercise Therapy
2.4.2. Conventional Rehabilitation Protocol
2.5. Outcome Measures
2.5.1. Knee Injury and Osteoarthritis Outcome Score
2.5.2. Numeric Rating Scale
2.5.3. Thirty-Second Chair Stand Test
2.5.4. Timed Up and Go Test
2.6. Statistical Analyses
3. Results
| Variable | EG (N = 59) | CG (N = 58) |
|---|---|---|
| Age (years; median (IQR)) | 70 (10) | 69 (9) |
| Body height (cm; median (IQR)) | 168 (13) | 165 (12) |
| Body mass (kg; median (IQR)) | 87 (20) | 84 (18) |
| Body mass index (kg/m2; median (IQR)) | 30.8 (9.3) | 30.3 (7.1) |
| Sex (N (%)) | ||
| Male | 17 (29) | 24 (41) |
| Female | 42 (71) | 34 (59) |
| Education (N (%)) | ||
| Secondary level | 49 (83) | 52 (90) |
| Tertiary level | 10 (17) | 6 (10) |
| Side of the operated knee (N (%)) | ||
| Left | 25 (42) | 32 (55) |
| Right | 34 (58) | 26 (45) |
| Place of the surgery (N (%)) | ||
| University hospital | 13 (22) | 15 (26) |
| General hospital | 46 (78) | 43 (74) |
| Postoperative day at the beginning of the inpatient rehabilitation (day; median (IQR)) | 64 (84) | 70.5 (84) |
| Use of walking aid upon admission (N (%)) | ||
| One crutch | 21 (36) | 17 (30) |
| Two crutches | 29 (49) | 32 (55) |
| Walker | 0 (0) | 0 (0) |
| No use of walking aid | 9 (15) | 9 (15) |
| KOOS score (0–100 scale; median (IQR)) | ||
| Pain | 25 (22) | 29.5 (30.8) |
| Symptoms | 32 (32) | 32 (33) |
| ADL function | 16 (17) | 21.5 (31) |
| Sport and recreation function | 5 (25) | 5 (26.3) |
| Quality of life | 44 (31) | 38 (34) |
| NRS (0–10 scale; median (IQR)) | 2 (5) | 3 (5) |
| 30 s chair stand test (no. of stands; median (IQR)) | 9 (2) | 9 (3) |
| TUG (seconds; median (IQR)) | 13.8 (5.5) | 15 (6.9) |
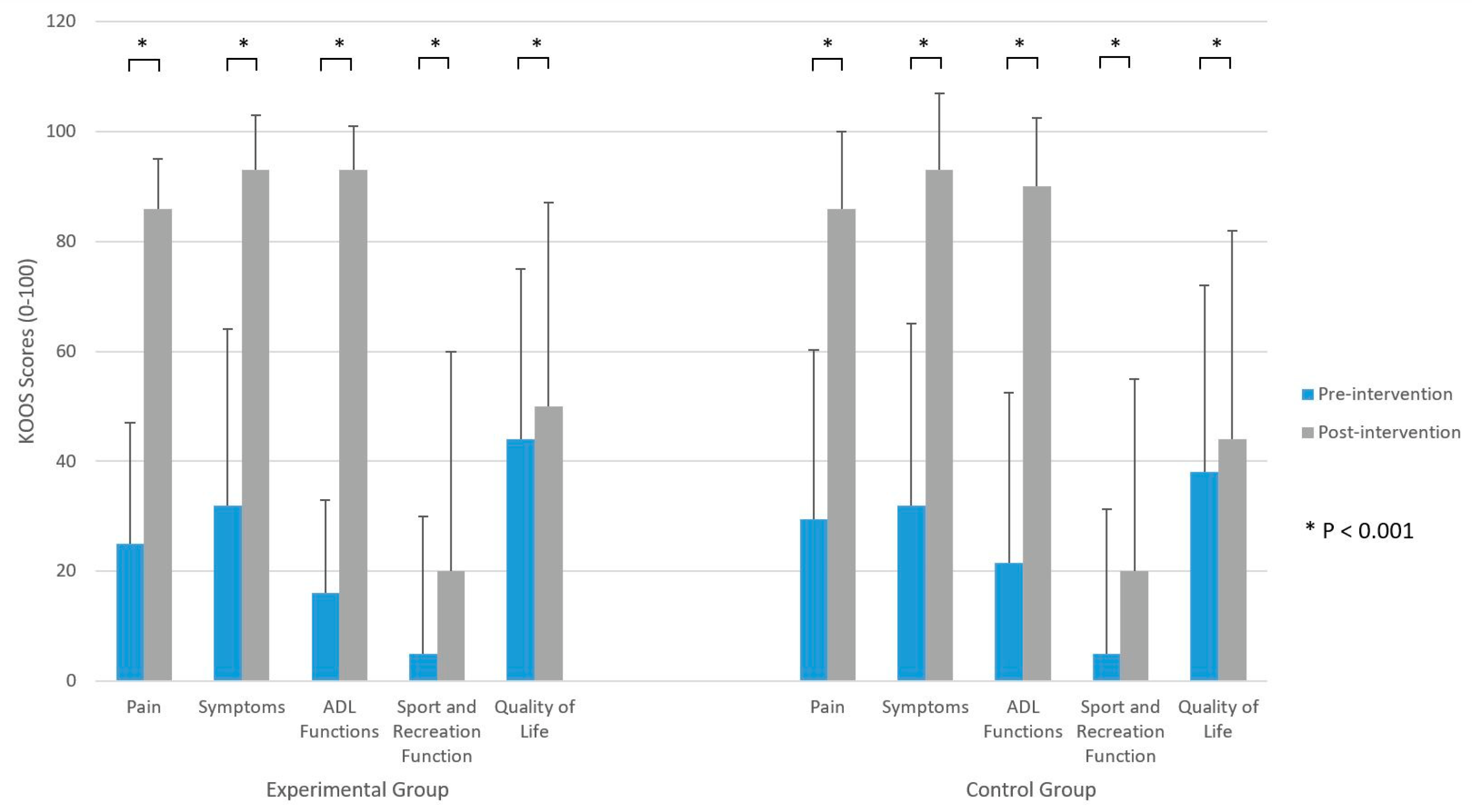
3.1. Within-Group Analyses
3.2. Between-Group Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vitaloni, M.; Botto-van Bemden, A.; Sciortino Contreras, R.M.; Scotton, D.; Bibas, M.; Quintero, M.; Monfort, J.; Carné, X.; de Abajo, F.; Oswald, E.; et al. Global Management of Patients with Knee Osteoarthritis Begins with Quality of Life Assessment: A Systematic Review. BMC Musculoskelet. Disord. 2019, 20, 493. [Google Scholar] [CrossRef] [PubMed]
- Litwic, A.; Edwards, M.H.; Dennison, E.M.; Cooper, C. Epidemiology and Burden of Osteoarthritis. Br. Med. Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, K.R.; Vincent, H.K. Resistance Exercise for Knee Osteoarthritis. PMR 2012, 4, S45–S52. [Google Scholar] [CrossRef] [PubMed]
- Cudejko, T.; van der Esch, M.; Schrijvers, J.; Richards, R.; van den Noort, J.C.; Wrigley, T.; van der Leeden, M.; Roorda, L.D.; Lems, W.; Harlaar, J.; et al. The Immediate Effect of a Soft Knee Brace on Dynamic Knee Instability in Persons with Knee Osteoarthritis. Rheumatology 2018, 57, 1735–1742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherian, J.J.; Kapadia, B.H.; Bhave, A.; McElroy, M.J.; Cherian, C.; Harwin, S.F.; Mont, M.A. Use of Transcutaneous Electrical Nerve Stimulation Device in Early Osteoarthritis of the Knee. J. Knee Surg. 2015, 28, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Steinmeyer, J.; Bock, F.; Stöve, J.; Jerosch, J.; Flechtenmacher, J. Pharmacological Treatment of Knee Osteoarthritis: Special Considerations of the New German Guideline. Orthop. Rev. 2018, 10, 7782. [Google Scholar] [CrossRef]
- Li, J.-W.; Ma, Y.-S.; Xiao, L.-K. Postoperative Pain Management in Total Knee Arthroplasty. Orthop. Surg. 2019, 11, 755–761. [Google Scholar] [CrossRef]
- Singh, J.A.; Vessely, M.B.; Harmsen, W.S.; Schleck, C.D.; Melton, L.J.; Kurland, R.L.; Berry, D.J. A Population-Based Study of Trends in the Use of Total Hip and Total Knee Arthroplasty, 1969–2008. Mayo Clin. Proc. 2010, 85, 898–904. [Google Scholar] [CrossRef]
- Wang, S.-Q.; Gao, Y.-Q.; Zhang, C.; Xie, Y.-J.; Wang, J.-X.; Xu, F.-Y. A Bibliometric Analysis Using CiteSpace of Publications from 1999 to 2018 on Patient Rehabilitation After Total Knee Arthroplasty. Med. Sci. Monit. 2020, 26, e920795. [Google Scholar] [CrossRef]
- Mizner, R.L.; Petterson, S.C.; Stevens, J.E.; Vandenborne, K.; Snyder-Mackler, L. Early Quadriceps Strength Loss after Total Knee Arthroplasty. The Contributions of Muscle Atrophy and Failure of Voluntary Muscle Activation. J. Bone Jt. Surg. 2005, 87, 1047–1053. [Google Scholar] [CrossRef]
- Fortier, L.M.; Rockov, Z.A.; Chen, A.F.; Rajaee, S.S. Activity Recommendations After Total Hip and Total Knee Arthroplasty. J. Bone Jt. Surg. 2021, 103, 446–455. [Google Scholar] [CrossRef]
- Dávila Castrodad, I.M.; Recai, T.M.; Abraham, M.M.; Etcheson, J.I.; Mohamed, N.S.; Edalatpour, A.; Delanois, R.E. Rehabilitation Protocols Following Total Knee Arthroplasty: A Review of Study Designs and Outcome Measures. Ann. Transl. Med. 2019, 7, S255. [Google Scholar] [CrossRef]
- Bade, M.J.; Kohrt, W.M.; Stevens-Lapsley, J.E. Outcomes before and after Total Knee Arthroplasty Compared to Healthy Adults. J. Orthop. Sports Phys. Ther. 2010, 40, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Mizner, R.L.; Snyder-Mackler, L. Altered Loading during Walking and Sit-to-Stand Is Affected by Quadriceps Weakness after Total Knee Arthroplasty. J. Orthop. Res. 2005, 23, 1083–1090. [Google Scholar] [CrossRef]
- Walsh, M.; Woodhouse, L.J.; Thomas, S.G.; Finch, E. Physical Impairments and Functional Limitations: A Comparison of Individuals 1 Year after Total Knee Arthroplasty with Control Subjects. Phys. Ther. 1998, 78, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Karaborklu Argut, S.; Celik, D.; Yasacı, Z. Effectiveness of Therapeutic Electromyographic Biofeedback after Orthopedic Knee Surgeries: A Systematic Review. Disabil. Rehabil. 2021, 1–9. [Google Scholar] [CrossRef]
- Giggins, O.M.; Persson, U.M.; Caulfield, B. Biofeedback in Rehabilitation. J. Neuroeng. Rehabil. 2013, 10, 60. [Google Scholar] [CrossRef] [Green Version]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Yuksel, E.; Kalkan, S.; Cekmece, S.; Unver, B.; Karatosun, V. Assessing Minimal Detectable Changes and Test-Retest Reliability of the Timed Up and Go Test and the 2-Minute Walk Test in Patients with Total Knee Arthroplasty. J. Arthroplast. 2017, 32, 426–430. [Google Scholar] [CrossRef]
- Mizner, R.L.; Petterson, S.C.; Snyder-Mackler, L. Quadriceps Strength and the Time Course of Functional Recovery after Total Knee Arthroplasty. J. Orthop. Sports Phys. Ther. 2005, 35, 424–436. [Google Scholar] [CrossRef] [Green Version]
- Roos, E.M.; Roos, H.P.; Lohmander, L.S.; Ekdahl, C.; Beynnon, B.D. Knee Injury and Osteoarthritis Outcome Score (KOOS)--Development of a Self-Administered Outcome Measure. J. Orthop. Sports Phys. Ther. 1998, 28, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Peer, M.A.; Lane, J. The Knee Injury and Osteoarthritis Outcome Score (KOOS): A Review of Its Psychometric Properties in People Undergoing Total Knee Arthroplasty. J. Orthop. Sports Phys. Ther. 2013, 43, 20–28. [Google Scholar] [CrossRef]
- Alviar, M.J.; Olver, J.; Brand, C.; Tropea, J.; Hale, T.; Pirpiris, M.; Khan, F. Do Patient-Reported Outcome Measures in Hip and Knee Arthroplasty Rehabilitation Have Robust Measurement Attributes? A Systematic Review. J. Rehabil. Med. 2011, 43, 572–583. [Google Scholar] [CrossRef] [Green Version]
- Downie, W.W.; Leatham, P.A.; Rhind, V.M.; Wright, V.; Branco, J.A.; Anderson, J.A. Studies with Pain Rating Scales. Ann. Rheum. Dis. 1978, 37, 378–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobson, F.; Hinman, R.S.; Roos, E.M.; Abbott, J.H.; Stratford, P.; Davis, A.M.; Buchbinder, R.; Snyder-Mackler, L.; Henrotin, Y.; Thumboo, J.; et al. OARSI Recommended Performance-Based Tests to Assess Physical Function in People Diagnosed with Hip or Knee Osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1042–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, C.J.; Rikli, R.E.; Beam, W.C. A 30-s Chair-Stand Test as a Measure of Lower Body Strength in Community-Residing Older Adults. Res. Q. Exerc. Sport 1999, 70, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Yeung, T.S.M.; Wessel, J.; Stratford, P.W.; MacDermid, J.C. The Timed up and Go Test for Use on an Inpatient Orthopaedic Rehabilitation Ward. J. Orthop. Sports Phys. Ther. 2008, 38, 410–417. [Google Scholar] [CrossRef]
- Bade, M.J.; Kittelson, J.M.; Kohrt, W.M.; Stevens-Lapsley, J.E. Predicting Functional Performance and Range of Motion Outcomes after Total Knee Arthroplasty. Am. J. Phys. Med. Rehabil. 2014, 93, 579–585. [Google Scholar] [CrossRef] [Green Version]
- Shanb, A.; Youssef, E. Effects of Adding Biofeedback Training to Active Exercises after Total Knee Arthroplasty. J. Musculoskelet. Res. 2014, 17, 1450001. [Google Scholar] [CrossRef]
- Wang, T.-J.; Chang, C.-F.; Lou, M.-F.; Ao, M.-K.; Liu, C.-C.; Liang, S.-Y.; Wu, S.-F.V.; Tung, H.-H. Biofeedback Relaxation for Pain Associated with Continuous Passive Motion in Taiwanese Patients after Total Knee Arthroplasty. Res. Nurs. Health 2015, 38, 39–50. [Google Scholar] [CrossRef]
- Draper, V.; Ballard, L. Electrical Stimulation versus Electromyographic Biofeedback in the Recovery of Quadriceps Femoris Muscle Function Following Anterior Cruciate Ligament Surgery. Phys. Ther. 1991, 71, 455–461; discussion 461–464. [Google Scholar] [CrossRef]
- Akkaya, N.; Ardic, F.; Ozgen, M.; Akkaya, S.; Sahin, F.; Kilic, A. Efficacy of Electromyographic Biofeedback and Electrical Stimulation Following Arthroscopic Partial Meniscectomy: A Randomized Controlled Trial. Clin. Rehabil. 2012, 26, 224–236. [Google Scholar] [CrossRef]
- Yilmaz, O.O.; Senocak, O.; Sahin, E.; Baydar, M.; Gulbahar, S.; Bircan, C.; Alper, S. Efficacy of EMG-Biofeedback in Knee Osteoarthritis. Rheumatol. Int. 2010, 30, 887–892. [Google Scholar] [CrossRef]
- Yip, S.L.M.; Ng, G.Y.F. Biofeedback Supplementation to Physiotherapy Exercise Programme for Rehabilitation of Patellofemoral Pain Syndrome: A Randomized Controlled Pilot Study. Clin. Rehabil. 2006, 20, 1050–1057. [Google Scholar] [CrossRef]
- Ng, G.Y.F.; Zhang, A.Q.; Li, C.K. Biofeedback Exercise Improved the EMG Activity Ratio of the Medial and Lateral Vasti Muscles in Subjects with Patellofemoral Pain Syndrome. J. Electromyogr. Kinesiol. 2008, 18, 128–133. [Google Scholar] [CrossRef]
- Dursun, N.; Dursun, E.; Kiliç, Z. Electromyographic Biofeedback-Controlled Exercise versus Conservative Care for Patellofemoral Pain Syndrome. Arch. Phys. Med. Rehabil. 2001, 82, 1692–1695. [Google Scholar] [CrossRef]
- Stevens, J.E.; Mizner, R.L.; Snyder-Mackler, L. Quadriceps Strength and Volitional Activation before and after Total Knee Arthroplasty for Osteoarthritis. J. Orthop. Res. 2003, 21, 775–779. [Google Scholar] [CrossRef]
- Ikezoe, T.; Asakawa, Y.; Tsutou, A. The Relationship between Quadriceps Strength and Balance to Fall of Elderly Admitted to a Nursing Home. J. Phys. Ther. Sci. 2003, 15, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Magan, A.A.; Ahmed, S.S.; Paton, B.; Konan, S.; Haddad, F.S. Does Multimodal Therapy Influence Functional Outcome After Total Knee Arthroplasty? Orthop. Clin. N. Am. 2020, 51, 453–459. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Ra, H.J. Patient Satisfaction after Total Knee Arthroplasty. Knee Surg. Relat. Res. 2016, 28, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeJong, G.; Hsieh, C.J.; Vita, M.T.; Zeymo, A.; Boucher, H.R.; Thakkar, S.C. Innovative Devices Did Not Provide Superior Total Knee Arthroplasty Outcomes in Post-Operative Rehabilitation: Results from a Four-Arm Randomized Clinical Trial. J. Arthroplast. 2020, 35, 2054–2065. [Google Scholar] [CrossRef] [PubMed]


| Variable | EG (N = 59) | CG (N = 58) | |
|---|---|---|---|
| Use of walking aid (N (%)) | |||
| One crutch | 26 (44) | 25 (43) | 0.557 a |
| Two crutches | 3 (5) | 6 (10) | |
| Walker | 0 (0) | 0 (0) | |
| No use of walking aid | 30 (51) | 27 (47) | |
| KOOS score (0–100 scale; median (IQR)) | |||
| Pain | 86 (9) | 86 (14) | 0.212 b |
| Symptoms | 93 (10) | 93 (14) | 0.488 b |
| ADL function | 93 (8) | 90 (12.5) | 0.073 b |
| Sport and recreation function | 20 (40) | 20 (35) | 0.660 b |
| Quality of life | 50 (37) | 44 (38) | 0.055 b |
| NRS (0–10 scale; median (IQR)) | 0 (2) | 1 (2.3) | 0.298 b |
| 30 s chair stand test (no. of stands; median (IQR)) | 12 (5) | 11 (5) | 0.129 b |
| TUG (seconds; median (IQR)) | 10 (4.4) | 11 (4.1) | 0.143 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sklempe Kokic, I.; Vuksanic, M.; Kokic, T.; Peric, I.; Duvnjak, I. Effects of Electromyographic Biofeedback on Functional Recovery of Patients Two Months after Total Knee Arthroplasty: A Randomized Controlled Trial. J. Clin. Med. 2022, 11, 3182. https://doi.org/10.3390/jcm11113182
Sklempe Kokic I, Vuksanic M, Kokic T, Peric I, Duvnjak I. Effects of Electromyographic Biofeedback on Functional Recovery of Patients Two Months after Total Knee Arthroplasty: A Randomized Controlled Trial. Journal of Clinical Medicine. 2022; 11(11):3182. https://doi.org/10.3390/jcm11113182
Chicago/Turabian StyleSklempe Kokic, Iva, Matko Vuksanic, Tomislav Kokic, Ivan Peric, and Ivana Duvnjak. 2022. "Effects of Electromyographic Biofeedback on Functional Recovery of Patients Two Months after Total Knee Arthroplasty: A Randomized Controlled Trial" Journal of Clinical Medicine 11, no. 11: 3182. https://doi.org/10.3390/jcm11113182








