Sepsis Related Mortality Associated with an Inflammatory Burst in Patients Admitting to the Department of Internal Medicine with Apparently Normal C-Reactive Protein Concentration
Abstract
:1. Introduction
2. Patients, Controls and Methods
2.1. The Patients
2.2. The Method to Determine the CRP Cutoff
2.3. The MDClone System
2.4. Laboratory Methods
2.5. Review of Death Causes
2.6. Statistical Methods
2.7. Ethics Committee Approval
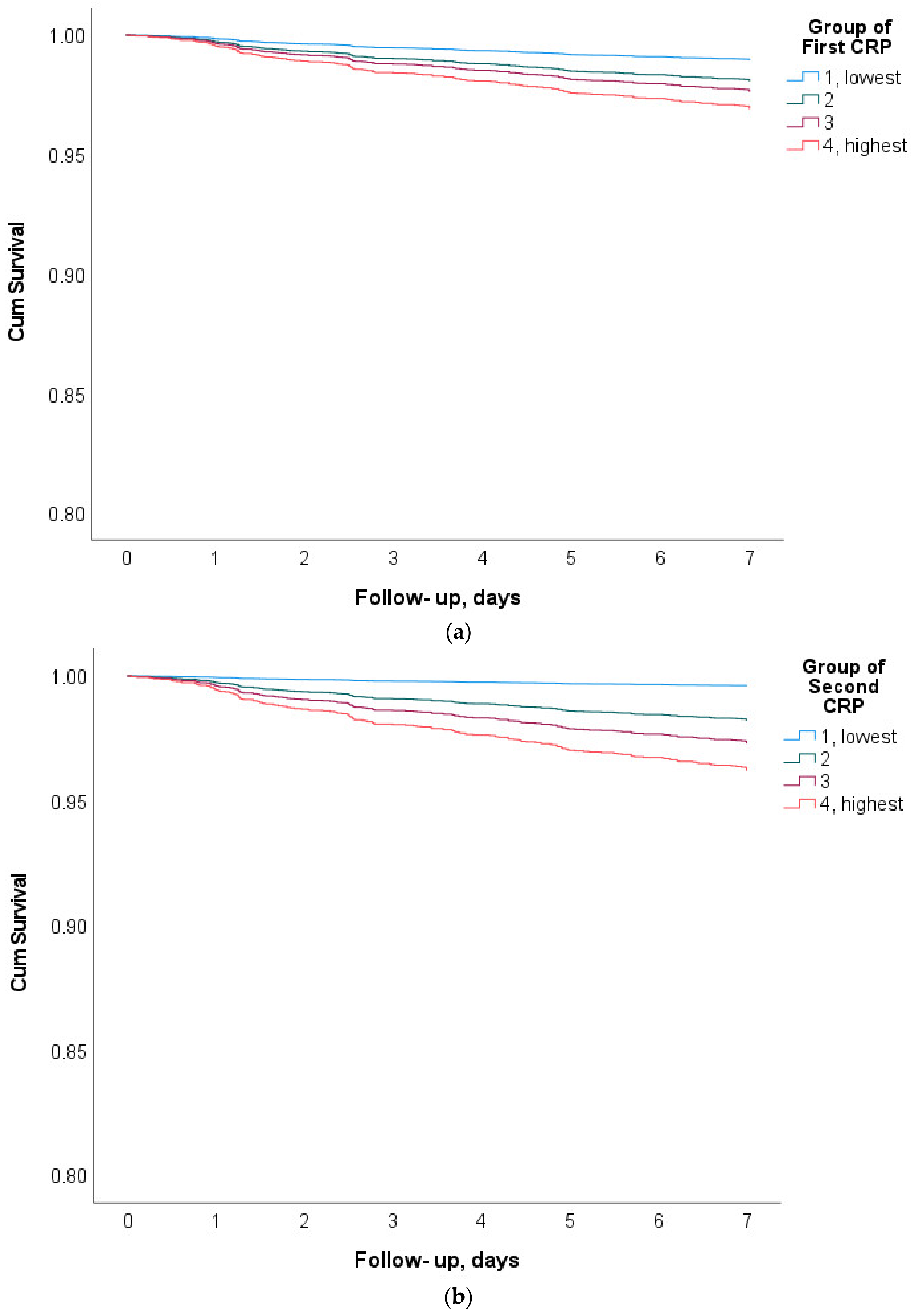
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board System
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.U. An Overview of Inflammation: Mechanism and Consequences. Front. Biol. China 2011, 6, 274. [Google Scholar] [CrossRef]
- Feigin, E.; Levinson, T.; Berliner, S.; Zeltser, D.; Itzhak, S.; Shenhar-Tsarfaty, S.; Egoz, E.; Meilik, A.; Goldiner, I.; Rogowski, O.; et al. Patients Who Are Admitted to the Department of Internal Medicine with a Very Low C-Reactive Protein Concentration. Eur. J. Inflamm. 2021, 19, 1–7. [Google Scholar] [CrossRef]
- Goldberg, I.; Shalmon, D.; Shteinvil, R.; Berliner, S.; Paran, Y.; Zeltser, D.; Shapira, I.; Shenhar-Tsarfaty, S.; Meilik, A.; Wasserman, A.; et al. A Second C-Reactive Protein (CRP) Test to Detect Inflammatory Burst in Patients with Acute Bacterial Infections Presenting with a First Relatively Low CRP. Medicine 2020, 99, e22551. [Google Scholar] [CrossRef]
- Bower, J.K.; Lazo, M.; Juraschek, S.P.; Selvin, E. Within-Person Variability in High-Sensitivity C-Reactive Protein. Arch. Intern. Med. 2012, 172, 1519–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocco, A.; Ringleb, P.A.; Grittner, U.; Nolte, C.H.; Schneider, A.; Nagel, S. Follow-up C-Reactive Protein Level Is More Strongly Associated with Outcome in Stroke Patients than Admission Levels. Neurol. Sci. 2015, 36, 2235–2241. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, Y.H.; Park, Y.H.; Kim, Y.H.; Hong, C.K.; Cho, K.W.; Hwang, S.Y. The Difference in C-Reactive Protein Value between Initial and 24 Hours Follow-up (D-CRP) Data as a Predictor of Mortality in Organophosphate Poisoned Patients. Clin. Toxicol. 2013, 51, 29–34. [Google Scholar] [CrossRef]
- Gill, D.; Sivakumaran, P.; Wilding, P.; Love, M.; Veltkamp, R.; Kar, A. Trends in C-Reactive Protein Levels Are Associated with Neurological Change Twenty-Four Hours after Thrombolysis for Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2016, 25, 1966–1969. [Google Scholar] [CrossRef]
- Levinson, T.; Tamir, N.; Shenhar-Tsarfaty, S.; Paran, Y.; Zeltzer, D.; Shapira, I.; Halpern, P.; Meilik, A.; Raykhshtat, E.; Goldiner, I.; et al. The Potential Benefit of a Second C-Reactive Protein Measurement in Patients with Gram-Negative Bacteraemia Presenting to the Emergency Medicine Department. Biomarkers 2020, 25, 533–538. [Google Scholar] [CrossRef]
- Wasserman, A.; Karov, R.; Shenhar-Tsarfaty, S.; Paran, Y.; Zeltzer, D.; Shapira, I.; Trotzky, D.; Halpern, P.; Meilik, A.; Raykhshtat, E.; et al. Septic Patients Presenting with Apparently Normal C-Reactive Protein: A Point of Caution for the ER Physician. Medicine 2019, 98, e13989. [Google Scholar] [CrossRef]
- Rogowski, O.; Toker, S.; Shapira, I.; Melamed, S.; Shirom, A.; Zeltser, D.; Berliner, S. Values of High-Sensitivity C-Reactive Protein in Each Month of the Year in Apparently Healthy Individuals. Am. J. Cardiol. 2005, 95, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Rogowski, O.; Shapira, I.; Peretz, H.; Berliner, S. Glycohaemoglobin as a Determinant of Increased Fibrinogen Concentrations and Low-Grade Inflammation in Apparently Healthy Nondiabetic Individuals. Clin. Endocrinol. 2008, 68, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Rogowski, O.; Shapira, I.; Bassat, O.K.-B.; Chundadze, T.; Finn, T.; Berliner, S.; Steinvil, A. Waist Circumference as the Predominant Contributor to the Micro-Inflammatory Response in the Metabolic Syndrome: A Cross Sectional Study. J. Inflamm. 2010, 7, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrenwald, M.; Wasserman, A.; Shenhar-Tsarfaty, S.; Zeltser, D.; Friedensohn, L.; Shapira, I.; Berliner, S.; Rogowski, O. Exercise Capacity and Body Mass Index-Important Predictors of Change in Resting Heart Rate. BMC Cardiovasc. Disord. 2019, 19, 307–308. [Google Scholar] [CrossRef]
- Rogowski, O.; Vered, Y.; Shapira, I.; Hirsh, M.; Zakut, V.; Berliner, S. Introducing the Wide Range C-Reactive Protein (Wr-CRP) into Clinical Use for the Detection of Microinflammation. Clin. Chim. Acta 2005, 358, 151–158. [Google Scholar] [CrossRef]
- Holzknecht, M.; Tiller, C.; Reindl, M.; Lechner, I.; Fink, P.; Lunger, P.; Mayr, A.; Henninger, B.; Brenner, C.; Klug, G.; et al. Association of c-reactive protein velocity with early left ventricular dysfunction in patients with first st-elevation myocardial infarction. J. Clin. Med. 2021, 10, 5494. [Google Scholar] [CrossRef]
- Banai, A.; Levit, D.; Morgan, S.; Loewenstein, I.; Merdler, I.; Hochstadt, A.; Szekely, Y.; Topilsky, Y.; Banai, S.; Shacham, Y. Association between C-Reactive Protein Velocity and Left Ventricular Function in Patients with ST-Elevated Myocardial Infarction. J. Clin. Med. 2022, 11, 401. [Google Scholar] [CrossRef]
- Ries, W.; Torzewski, J.; Heigl, F.; Pfluecke, C.; Kelle, S.; Darius, H.; Ince, H.; Mitzner, S.; Nordbeck, P.; Butter, C.; et al. C-Reactive Protein Apheresis as Anti-inflammatory Therapy in Acute Myocardial Infarction: Results of the CAMI-1 Study. Front. Cardiovasc. Med. 2021, 8, 591714. [Google Scholar] [CrossRef]
- Paces, J.; Strizova, Z.; Smrz, D.; Cerny, J. COVID-19 and the Immune System. Physiol. Res. 2020, 69, 379–388. [Google Scholar] [CrossRef]
- Lazzaroni, M.G.; Piantoni, S.; Masneri, S.; Garrafa, E.; Martini, G.; Tincani, A.; Andreoli, L.; Franceschini, F. Coagulation Dysfunction in COVID-19: The Interplay between Inflammation, Viral Infection and the Coagulation System. Blood Rev. 2021, 46, 100745. [Google Scholar] [CrossRef]
- Choudhary, S.; Sharma, K.; Silakari, O. The Interplay between Inflammatory Pathways and COVID-19: A Critical Review on Pathogenesis and Therapeutic Options. Microb. Pathog. 2021, 150, 104673. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-Phase Proteins and Other Systemic Responses to Inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Tripepi, G.; Mallamaci, F.; Zoccali, C. Inflammation Markers, Adhesion Molecules, and All-Cause and Cardiovascular Mortality in Patients with ESRD: Searching for the Best Risk Marker by Multivariate Modeling. J. Am. Soc. Nephrol. 2005, 16, S83–S88. [Google Scholar] [CrossRef] [Green Version]
- Faix, J.D. Biomarkers of Sepsis. Crit. Rev. Clin. Lab. Sci. 2013, 50, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Germolec, D.R.; Shipkowski, K.A.; Frawley, R.P.; Evans, E. Markers of Inflammation. Methods Mol. Biol. 2018, 1803, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Grondman, I.; Pirvu, A.; Riza, A.; Ioana, M.; Netea, M.G. Biomarkers of Inflammation and the Etiology of Sepsis. Biochem. Soc. Trans. 2020, 48, 1–14. [Google Scholar] [CrossRef]
- Luyendyk, J.P.; Schoenecker, J.G.; Flick, M.J. The Multifaceted Role of Fibrinogen in Tissue Injury and Inflammation. Blood 2019, 133, 511–520. [Google Scholar] [CrossRef]
- Lapić, I.; Padoan, A.; Bozzato, D.; Plebani, M. Erythrocyte Sedimentation Rate and C-Reactive Protein in Acute Inflammation. Am. J. Clin. Pathol. 2020, 153, 14–29. [Google Scholar] [CrossRef]
- Lien, F.; Wang, H.Y.; Lu, J.J.; Wen, Y.H.; Chiueh, T.S. Predicting 2-Day Mortality of Thrombocytopenic Patients Based on Clinical Laboratory Data Using Machine Learning. Med. Care 2021, 59, 245–250. [Google Scholar] [CrossRef]
- Moor, M.; Rieck, B.; Horn, M.; Jutzeler, C.R.; Borgwardt, K. Early Prediction of Sepsis in the ICU Using Machine Learning: A Systematic Review. Front. Med. 2021, 8, 607952. [Google Scholar] [CrossRef]
- Tseng, Y.J.; Wang, H.Y.; Lin, T.W.; Lu, J.J.; Hsieh, C.H.; Liao, C.T. Development of a Machine Learning Model for Survival Risk Stratification of Patients with Advanced Oral Cancer. JAMA Netw. Open 2020, 3, e2011768. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O.; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of Inflammation and Cardiovascular Disease. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Lenz, A.; Franklin, G.A.; Cheadle, W.G. Systemic Inflammation after Trauma. Injury 2007, 38, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Hietbrink, F.; Koenderman, L.; Rijkers, G.T.; Leenen, L.P.H. Trauma: The Role of the Innate Immune System. World J. Emerg. Surg. 2006, 1, 15. [Google Scholar] [CrossRef] [Green Version]
- Helmy, A.; de Simoni, M.G.; Guilfoyle, M.R.; Carpenter, K.L.H.; Hutchinson, P.J. Cytokines and Innate Inflammation in the Pathogenesis of Human Traumatic Brain Injury. Prog. Neurobiol. 2011, 95, 352–372. [Google Scholar] [CrossRef]
- Huber-Lang, M.; Lambris, J.D.; Ward, P.A. Innate Immune Responses to Trauma. Nat. Immunol. 2018, 19, 327–341. [Google Scholar] [CrossRef]
| Total Population | N = 3504 |
|---|---|
| Gender (% of males) | 1.7 |
| Age (Years: mean ± SD) | 64.3 ± 18.5 |
| Hypertension, % | 26.7 |
| Diabetes, % | 14.4 |
| Ischemic heart disease, % | 13.1 |
| Dyslipidemia, % | 11.3 |
| CVA % | 2.9 |
| Factor | First CRP (Median, IQR) | Second CRP (Median, IQR) | ||||
|---|---|---|---|---|---|---|
| With | Without | p Value | With | Without | p Value | |
| Gender, (Female) | 6.24 (1.6–14.0) | 5.37 (1.4–13.8) | <0.001 | 8.45 (2.3–19.5) | 7.74 (2.0–19.3) | <0.001 |
| Diabetes Mellitus | 7.54 (2.4–15.7) | 5.50 (1.3–13.8) | <0.001 | 9.48 (3.2–20.2) | 7.8 (2.0–19.3) | <0.001 |
| Hypertension | 6.91 (2.-15.0) | 5.38 (1.3–13.8) | <0.001 | 9.09 (2.9–19.9) | 7.7 (1.9–7.7) | <0.001 |
| Dyslipidemia | 5.57 (1.7–13.2) | 5.81 (1.4–14.3) | 0.684 | 7.47 (2.3–17.6) | 8.15 (2.1–19.7) | <0.001 |
| Ischemic heart disease | 6.65 (1.9–14.9) | 5.66 (1.4–14.0) | <0.001 | 8.42 (2.4–19.3) | 8.02 (2.1–19.4) | 0.008 |
| First CRP Measurement | Second CRP Measurement | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile | n | CRP, mg/L | Deaths within 7 Days n, (%) | Sepsis Related Deaths, n, (%) | Quartile | n | CRP, mg/L | Deaths within 7 Days n, (%) | Sepsis Related Deaths, n, (%) |
| 1 | 878 | 0.6 (0.5) | 15, (1.7%) | 1, (6.7%) | 1 | 879 | 0.85 (0.6) | 4, (0.5%) | 0, (0%) |
| 2 | 874 | 3.4 (1.2) | 33, (3.8%) | 6, (18.2%) | 2 | 873 | 4.4 (1.6) | 27, (3.1%) | 6, (22.2%) |
| 3 | 876 | 9.5 (2.4) | 47, (5.4%) | 19, (40.4%) | 3 | 876 | 12.2 (3.1) | 49, (5.6%) | 18, (36.7%) |
| 4 | 876 | 21.5 (5.1) | 68, (7.8%) | 37, (54.4%) | 4 | 876 | 40.9 (29.3) | 83, (9.5%) | 39, (47.0%) |
| Sum | 3504 | 8.8 (8.5) | 163, (4.65%) | 63, (38.7%) | Sum | 3504 | 14.6 (21.6) | 163, (4.65%) | 63, (38.7%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meilik, R.; Ben-Assayag, H.; Meilik, A.; Berliner, S.; Zeltser, D.; Shapira, I.; Rogowski, O.; Goldiner, I.; Shenhar-Tsarfaty, S.; Wasserman, A. Sepsis Related Mortality Associated with an Inflammatory Burst in Patients Admitting to the Department of Internal Medicine with Apparently Normal C-Reactive Protein Concentration. J. Clin. Med. 2022, 11, 3151. https://doi.org/10.3390/jcm11113151
Meilik R, Ben-Assayag H, Meilik A, Berliner S, Zeltser D, Shapira I, Rogowski O, Goldiner I, Shenhar-Tsarfaty S, Wasserman A. Sepsis Related Mortality Associated with an Inflammatory Burst in Patients Admitting to the Department of Internal Medicine with Apparently Normal C-Reactive Protein Concentration. Journal of Clinical Medicine. 2022; 11(11):3151. https://doi.org/10.3390/jcm11113151
Chicago/Turabian StyleMeilik, Ronnie, Hadas Ben-Assayag, Ahuva Meilik, Shlomo Berliner, David Zeltser, Itzhak Shapira, Ori Rogowski, Ilana Goldiner, Shani Shenhar-Tsarfaty, and Asaf Wasserman. 2022. "Sepsis Related Mortality Associated with an Inflammatory Burst in Patients Admitting to the Department of Internal Medicine with Apparently Normal C-Reactive Protein Concentration" Journal of Clinical Medicine 11, no. 11: 3151. https://doi.org/10.3390/jcm11113151
APA StyleMeilik, R., Ben-Assayag, H., Meilik, A., Berliner, S., Zeltser, D., Shapira, I., Rogowski, O., Goldiner, I., Shenhar-Tsarfaty, S., & Wasserman, A. (2022). Sepsis Related Mortality Associated with an Inflammatory Burst in Patients Admitting to the Department of Internal Medicine with Apparently Normal C-Reactive Protein Concentration. Journal of Clinical Medicine, 11(11), 3151. https://doi.org/10.3390/jcm11113151





