Magnetic-Resonance-Imaging-Based Left Atrial Strain and Left Atrial Strain Rate as Diagnostic Parameters in Cardiac Amyloidosis
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Hypertrophic Cardiomyopathy Population
2.3. Healthy Control Group
2.4. Cardiac MRI
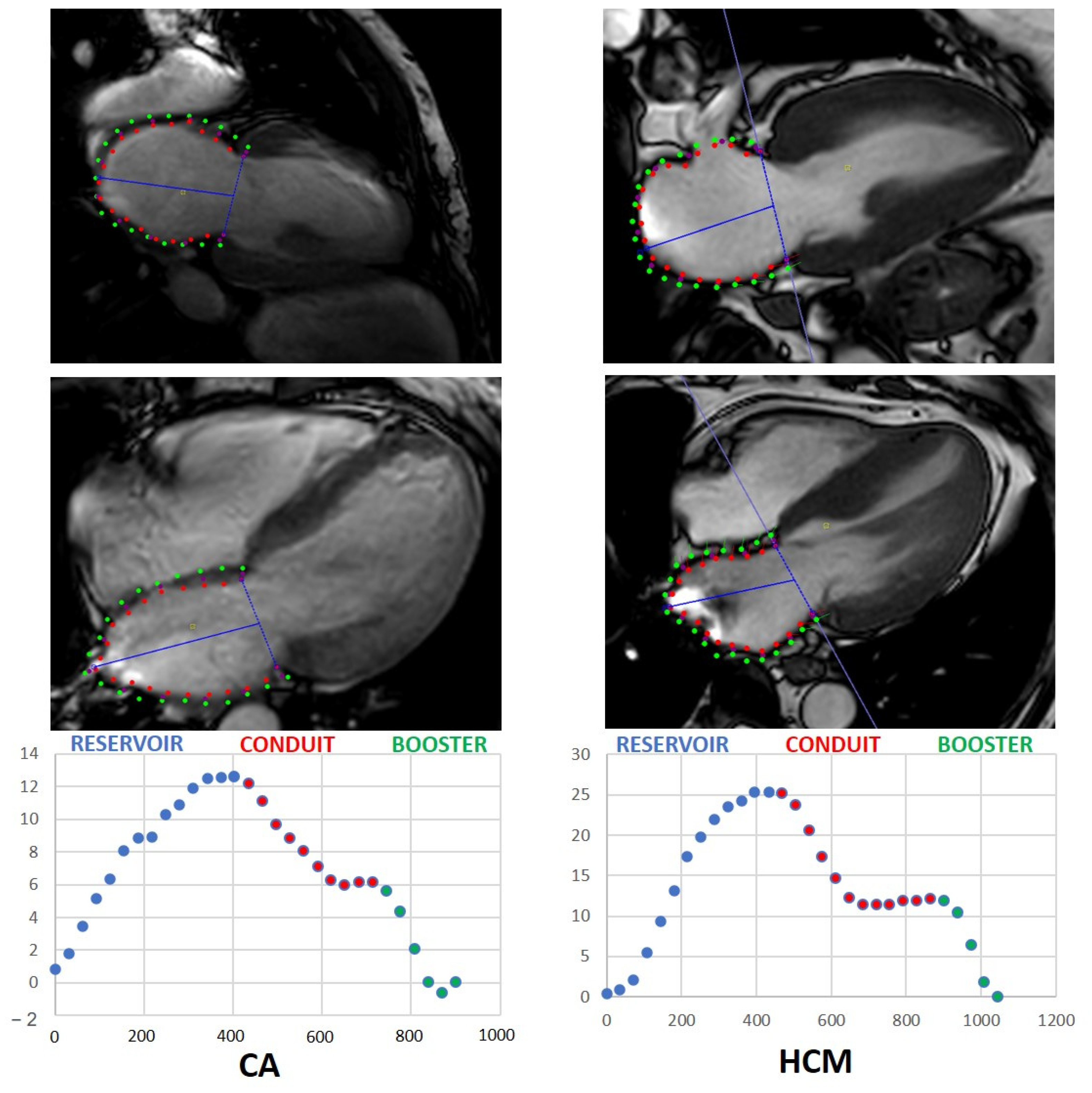
2.5. Strain Analysis
2.6. Statistics
3. Results
3.1. Patient Baseline Characteristics
3.2. Control Group Baseline Characteristics
3.3. Amyloidosis-Specific Characteristics
3.4. Left Atrial Strain and Strain Rate in Patients with ATTR and AL
3.5. Functional LA Parameters in Patients with CA and HCM and Healthy Subjects
3.6. Correlation of LAS in CA with Established Parameters
4. Discussion
- MRI-assessed LAS as well as LASR are significantly impaired in patients with CA compared to healthy subjects.
- MRI-assessed LAS and LASR significantly differ in patients with CA and HCM with a significantly stronger decrease in patients with CA.
- Reservoir LAS and conduit LAS correlate with LA-EF and LV strain, respectively. Booster LASR correlates with LA-EF and LA end diastolic area.
4.1. Diagnostic Value of MRI-Based Left Atrial Strain Analysis in Cardiac Amyloidosis
4.2. Differentiation between Cardiac Amyloidosis and Hypertrophic Cardiomyopathy
4.3. Clinical Outlook
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fontana, M.; Ćorović, A.; Scully, P.; Moon, J.C. Myocardial Amyloidosis: The Exemplar Interstitial Disease. JACC Cardiovasc. Imag. 2019, 12, 2345–2356. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.P.; Vendruscolo, M.; Dobson, C.M. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 2014, 15, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Ruberg, F.L.; Berk, J.L. Transthyretin (TTR) cardiac amyloidosis. Circulation 2012, 126, 1286–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapezzi, C.; Merlini, G.; Quarta, C.C.; Riva, L.; Longhi, S.; Leone, O.; Salvi, F.; Ciliberti, P.; Pastorelli, F.; Biagini, E.; et al. Systemic cardiac amyloidoses: Disease profiles and clinical courses of the 3 main types. Circulation 2009, 120, 1203–1212. [Google Scholar] [CrossRef] [Green Version]
- Falk, R.H.; Kruger, J.; Quarta, C.C. Senile systemic amyloidosis is a markedly underdiagnosed cardiomyopathy: Experience of a cardiac amyloidosis program. J. Am. Coll. Cardiol. 2013, 61 (Suppl. 10), E1241. [Google Scholar] [CrossRef] [Green Version]
- Maurer, M.S.; Bokhari, S.; Damy, T.; Dorbala, S.; Drachman, B.M.; Fontana, M.; Grogan, M.; Kristen, A.V.; Lousada, I.; Nativi-Nicolau, J.; et al. Consensus Recommendations for the Suspicion and Diagnosis of Transthyretin Cardiac Amyloidosis. Circ. Heart Fail. 2019, 12, e006075. [Google Scholar] [CrossRef]
- Oerlemans, M.; Rutten, K.; Minnema, M.C.; Raymakers, R.; Asselbergs, F.W.; de Jonge, N. Cardiac amyloidosis: The need for early diagnosis.Cardiac amyloidosis: The need for early diagnosis. Neth. Heart J. 2019, 27, 525–536. [Google Scholar] [CrossRef] [Green Version]
- Quarta, C.C.; Solomon, S.D.; Uraizee, I.; Kruger, J.; Longhi, S.; Ferlito, M.; Gagliardi, C.; Milandri, A.; Rapezzi, C.; Falk, R.H. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation 2014, 129, 1840–1849. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Naharro, A.; Treibel, T.A.; Abdel-Gadir, A.; Bulluck, H.; Zumbo, G.; Knight, D.S.; Kotecha, T.; Francis, R.; Hutt, D.F.; Rezk, T.; et al. Magnetic Resonance in Transthyretin Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2017, 70, 466–477. [Google Scholar] [CrossRef]
- Duffy, G.; Cheng, P.P.; Yuan, N.; He, B.; Kwan, A.C.; Shun-Shin, M.J.; Alexander, K.M.; Ebinger, J.; Lungren, M.P.; Rader, F.; et al. High-Throughput Precision Phenotyping of Left Ventricular Hypertrophy With Cardiovascular Deep Learning. JAMA Cardiol. 2022, 7, 386–395. [Google Scholar] [CrossRef]
- Bisbal, F.; Baranchuk, A.; Braunwald, E.; Bayés, de Luna, A.; Bayés-Genís, A. Atrial Failure as a Clinical Entity: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 75, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Modin, D.; Biering-Sørensen, S.R.; Møgelvang, R.; Alhakak, A.S.; Jensen, J.S.; Biering-Sørensen, T. Prognostic value of left atrial strain in predicting cardiovascular morbidity and mortality in the general population. Eur. Heart J. Cardiovasc. Imaging. 2019, 20, 804–815. [Google Scholar] [CrossRef]
- Zhang, Q.; Yip, G.W.; Yu, C.M. Approaching regional left atrial function by tissue Doppler velocity and strain imaging. Europace 2008, 10 (Suppl. 3), iii62–iii69. [Google Scholar] [CrossRef]
- Kwong, R.Y.; Heydari, B.; Abbasi, S.; Steel, K.; Al-Mallah, M.; Wu, H.; Falk, R.H. Characterization of Cardiac Amyloidosis by Atrial Late Gadolinium Enhancement Using Contrast-Enhanced Cardiac Magnetic Resonance Imaging and Correlation With Left Atrial Conduit and Contractile Function. Am. J. Cardiol. 2015, 116, 622–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohty, D.; Boulogne, C.; Magne, J.; Varroud-Vial, N.; Martin, S.; Ettaif, H.; Fadel, B.M.; Bridoux, F.; Aboyans, V.; Damy, T.; et al. Prognostic value of left atrial function in systemic light-chain amyloidosis: A cardiac magnetic resonance study. Eur. Heart J. Cardiovasc. Imaging. 2016, 17, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Bandera, F.; Martone, R.; Chacko, L.; Ganesananthan, S.; Gilbertson, J.A.; Ponticos, M.; Lane, T.; Martinez-Naharro, A.; Whelan, C.; Quarta, C.; et al. Clinical Importance of Left Atrial Infiltration in Cardiac Transthyretin Amyloidosis. JACC Cardiovasc. Imag. 2022, 15, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Rausch, K.; Scalia, G.M.; Sato, K.; Edwards, N.; Lam, A.K.Y.; Platts, D.G.; Chan, J. Left atrial strain imaging differentiates cardiac amyloidosis and hypertensive heart disease. Int. J. Cardiovasc. Imag. 2021, 37, 81–90. [Google Scholar] [CrossRef]
- Buss, S.J.; Emami, M.; Mereles, D.; Korosoglou, G.; Kristen, A.V.; Voss, A.; Schellberg, D.; Zugck, C.; Galuschky, C.; Giannitsis, E.; et al. Longitudinal left ventricular function for prediction of survival in systemic light-chain amyloidosis: Incremental value compared with clinical and biochemical markers. J. Am. Coll Cardiol. 2012, 60, 1067–1076. [Google Scholar] [CrossRef] [Green Version]
- Mohty, D.; Pibarot, P.; Dumesnil, J.G.; Darodes, N.; Lavergne, D.; Echahidi, N.; Virot, P.; Bordessoule, D.; Jaccard, A. Left atrial size is an independent predictor of overall survival in patients with primary systemic amyloidosis. Arch. Cardiovasc. Dis. 2011, 104, 611–618. [Google Scholar] [CrossRef]
- Yilmaz, A.; Sechtem, U. Diagnostic approach and differential diagnosis in patients with hypertrophied left ventricles. Heart 2014, 100, 662–671. [Google Scholar] [CrossRef]
- Giusca, S.; Steen, H.; Montenbruck, M.; Patel, A.R.; Pieske, B.; Erley, J.; Kelle, S.; Korosoglou, G. Multi-parametric assessment of left ventricular hypertrophy using late gadolinium enhancement, T1 mapping and strain-encoded cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2021, 23, 92. [Google Scholar] [CrossRef] [PubMed]
- Sohns, C.; Sossalla, S.; Schmitto, J.D.; Jacobshagen, C.; Raab, B.W.; Obenauer, S.; Maier, L.S. Visualization of transcoronary ablation of septal hypertrophy in patients with hypertrophic obstructive cardiomyopathy: A comparison between cardiac MRI, invasive measurements and echocardiography. Clin. Res. Cardiol. 2010, 99, 359–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staab, W.; Bergau, L.; Schuster, A.; Hinojar, R.; Dorenkamp, M.; Obenauer, S.; Lotz, J.; Sohns, C. Detection of intracardiac masses in patients with coronary artery disease using cardiac magnetic resonance imaging: A comparison with transthoracic echocardiography. Int. J. Cardiovasc. Imag. 2014, 30, 647–657. [Google Scholar] [CrossRef] [PubMed]




| Male, n (%) | 33 (75) |
| Female, n (%) | 11 (25) |
| Age [years] | 73.7 ± 8.9 |
| BMI [kg/m2] | 25.2 ± 3.5 |
| Body surface [m2] | 1.9± 0.2 |
| Arterial hypertension, n (%) | 34 (77.3) |
| Systolic BP [mmHg] | 119.6 ± 20.9 |
| Diastolic BP [mmHg] | 74.3 ± 14.1 |
| Heart rate [min] | 72.5 ± 10.7 |
| Left ventricular EF [%] | 46.4 ± 9.6 |
| NYHA level | |
| NYHA I, n (%) | 0 (0) |
| NYHA II, n (%) | 6 (13.6) |
| NYHA III, n (%) | 36 (81.1) |
| NYHA IV, n (%) | 2 (4.5) |
| NTproBNP [pg/mL] | 3310 ± 897.3 |
| Coronary artery disease, n (%) | 14 (31.8) |
| Atrial fibrillation, n (%) | 17 (38.6) |
| CHA2DS2-VASC Score | 4 {3;5} |
| COPD, n (%) | 3 (6.8) |
| Diabetes mellitus, n (%) | 1 (2.3) |
| Stroke, n (%) | 5 (11.4) |
| Male, n (%) | 10 (47.6) |
| Female, n (%) | 11 (52.4) |
| Age [years] | 63.9 ± 7.4 |
| BMI [kg/m2] | 28.3 ± 3.9 |
| Body surface [m2] | 1.9 ± 0.2 |
| Arterial hypertension, n (%) | 10 (47.6) |
| Systolic BP [mmHg] | 129.7 ± 13.6 |
| Diastolic BP [mmHg] | 73.1 ± 9.5 |
| Heart rate [min] | 66.1 ± 7.9 |
| Left ventricular EF [%] | 53.5 ± 5.6 |
| Maximum IVSd | 20.3 ± 3.3 |
| NYHA level | |
| NYHA I, n (%) | 1 (4.8) |
| NYHA II, n (%) | 8 (38.1) |
| NYHA III, n (%) | 12 (57.1) |
| NYHA IV, n (%) | 0 |
| Coronary artery disease, n (%) | 2 (9.5) |
| Atrial fibrillation, n (%) | 1 (4.8) |
| CHA2DS2-VASC Score | 2 {1;3} |
| COPD, n (%) | 1 (4.8) |
| Diabetes mellitus, n (%) | 3 (14.3) |
| Male, n (%) | 25 (54.3) |
| Female, n (%) | 21 (45.7) |
| Age [years] | 57.3 ± 5.6 |
| BMI [kg/m2] | 74.8 ± 12.8 |
| Body surface [m2] | 1.9 ± 0.2 |
| Left ventricular EF [%] | 55 ± 1.2 |
| Histologically Proven Cardiac Amyloidosis, n (%) | 44 (100) |
| ATTR, n (%) | 22 (50) |
| WT, n (%) | 22 (100) |
| MT, n (%) | 0 (0) |
| AL, n (%) | 22 (50) |
| Lambda, n (%) | 20 (90.9) |
| Kappa, n (%) | 2 (9.1) |
| Histological quantification | |
| High grade, n (%) | 38 (86.4) |
| Intermediate, n (%) | 6 (13.6) |
| Low grade, n (%) | 0 (0) |
| ATTR | AL | p-Value | |
|---|---|---|---|
| LA global longitudinal strain | |||
| Reservoir [%] | 7.9 ± 5.9 | 8.7 ± 4.6 | 0.637 |
| Conduit [%] | 5.5 ± 2.9 | 5.5 ± 4.6 | 0.988 |
| Booster [%] | 2.5 ± 4.9 | 3.2 ± 4.1 | 0.585 |
| LA global strain rate | |||
| Reservoir [%] | 0.5 ± 0.5 | 0.5 ± 0.3 | 0.697 |
| Conduit [%] | −0.3 ± 0.3 | −0.5 ± 0.2 | 0.051 |
| Booster [%] | −0.4 ± 0.4 | −0.6 ± 0.4 | 0.104 |
| LA function biplanar | |||
| LA volume at LVED | 86.6 ± 37 | 79.8 ± 32.0 | 0.233 |
| LA volume at LVES | 104.8 ± 39.9 | 102.0 ± 35.8 | 0.354 |
| LA minimum volume | 83.9 ± 35.3 | 43.6 ± 17.7 | 0.597 |
| LA maximum volume | 106.3 ± 40.1 | 103.7 ± 36.4 | 0.856 |
| LA-EF | 20.1 ± 10.9 | 26.2 ± 13.4 | 0.421 |
| LA area at LVED (4CH) | 23.7 ± 6.2 | 22.9 ± 6.1 | 0.139 |
| LA area at LVES (4 CH) | 27.7 ± 6.2 | 27.8 ± 6.3 | 0.163 |
| Pearson’s Correlation | p-Value | |
|---|---|---|
| Global LA reservoir strain | ||
| LV strain | 0.445 | 0.002 |
| LA area (LVED) | 0.374 | 0.012 |
| LA-EF | 0.518 | <0.001 |
| LV-EF | 0.365 | 0.015 |
| Global LA conduit strain | ||
| Global longitudinal LV strain | 0.500 | <0.001 |
| LA-EF | 0.483 | <0.001 |
| Global LA reservoir strain rate | ||
| LV strain | 0.318 | 0.036 |
| LA-EF | 0.335 | 0.026 |
| LV-EF | 0.319 | 0.035 |
| Global LA conduit strain rate | ||
| LV strain | 0.318 | 0.035 |
| Global LA booster strain rate | ||
| LV-EF | 0.311 | 0.04 |
| LA-EF | 0.576 | <0.001 |
| LA area (LVED) | 0.531 | <0.001 |
| LA area (LVES) | 0.39 | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciacca, V.; Eckstein, J.; Körperich, H.; Fink, T.; Bergau, L.; El Hamriti, M.; Imnadze, G.; Guckel, D.; Fox, H.; Gerçek, M.; et al. Magnetic-Resonance-Imaging-Based Left Atrial Strain and Left Atrial Strain Rate as Diagnostic Parameters in Cardiac Amyloidosis. J. Clin. Med. 2022, 11, 3150. https://doi.org/10.3390/jcm11113150
Sciacca V, Eckstein J, Körperich H, Fink T, Bergau L, El Hamriti M, Imnadze G, Guckel D, Fox H, Gerçek M, et al. Magnetic-Resonance-Imaging-Based Left Atrial Strain and Left Atrial Strain Rate as Diagnostic Parameters in Cardiac Amyloidosis. Journal of Clinical Medicine. 2022; 11(11):3150. https://doi.org/10.3390/jcm11113150
Chicago/Turabian StyleSciacca, Vanessa, Jan Eckstein, Hermann Körperich, Thomas Fink, Leonard Bergau, Mustapha El Hamriti, Guram Imnadze, Denise Guckel, Henrik Fox, Muhammed Gerçek, and et al. 2022. "Magnetic-Resonance-Imaging-Based Left Atrial Strain and Left Atrial Strain Rate as Diagnostic Parameters in Cardiac Amyloidosis" Journal of Clinical Medicine 11, no. 11: 3150. https://doi.org/10.3390/jcm11113150
APA StyleSciacca, V., Eckstein, J., Körperich, H., Fink, T., Bergau, L., El Hamriti, M., Imnadze, G., Guckel, D., Fox, H., Gerçek, M., Farr, M., Burchert, W., Sommer, P., Sohns, C., & Piran, M. (2022). Magnetic-Resonance-Imaging-Based Left Atrial Strain and Left Atrial Strain Rate as Diagnostic Parameters in Cardiac Amyloidosis. Journal of Clinical Medicine, 11(11), 3150. https://doi.org/10.3390/jcm11113150






