The Prognostic Role of ST2L and sST2 in Patients Who Underwent Carotid Plaque Endarterectomy: A Five-Year Follow-Up Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Tissue Samples
2.3. Immunohistochemistry
2.4. ELISA Test
2.5. Statistics and Data Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aimo, A.; Vergaro, G.; Passino, C.; Ripoli, A.; Ky, B.; Miller, W.L.; Bayes-Genis, A.; Anand, I.; Januzzi, J.L.; Emdin, M. Prognostic Value of Soluble Suppression of Tumorigenicity-2 in Chronic Heart Failure: A Meta-Analysis. JACC Heart Fail. 2017, 5, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Vergaro, G.; Ripoli, A.; Bayes-Genis, A.; Pascual Figal, D.A.; de Boer, R.A.; Lassus, J.; Mebazaa, A.; Gayat, E.; Breidthardt, T.; et al. Meta-analysis of soluble suppression of tumorigenicity-2 and prognosis in acute heart failure. JACC Heart Fail. 2017, 5, 287–296. [Google Scholar] [CrossRef]
- Aimo, A.; Januzzi, J.L., Jr.; Vergaro, G.; Richards, A.M.; Lam, C.S.P.; Latini, R.; Anand, I.S.; Cohn, J.N.; Ueland, T.; Gullestad, L.; et al. Circulating levels and prognostic value of soluble ST2 in heart failure are less influenced by age than N-terminal pro-B-type natriuretic peptide and high-sensitivity troponin T. Eur. J. Heart Fail. 2020, 22, 2078–2088. [Google Scholar] [CrossRef]
- Hou, Z.W.; Yu, H.B.; Liang, Y.C.; Gao, Y.; Xu, G.Q.; Wu, M.; Mei, Z.; Wang, Z.L.; Li, Z.G.; Li, Y.Y.; et al. Circulating Soluble ST2 Predicts All-Cause Mortality in Severe Heart Failure Patients with an Implantable Cardioverter Defibrillator. Cardiol. Res. Pract. 2020, 2020, 4375651. [Google Scholar] [CrossRef]
- Aurora, L.; Peterson, E.; Gui, H.; Zeld, N.; McCord, J.; Pinto, Y.; Cook, B.; Sabbah, H.N.; Keoki Williams, L.; Snider, J.; et al. Suppression tumorigenicity 2 (ST2) turbidimetric immunoassay compared to enzyme-linked immunosorbent assay in predicting survival in heart failure patients with reduced ejection fraction. Clin. Chim. Acta. 2020, 510, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M.; Xu, D.; Asquith, D.L.; Denby, L.; Li, Y.; Sattar, N.; Baker, A.H.; McInnes, I.B.; Liew, F.Y. IL-33 reduces the development of atherosclerosis. J. Exp. Med. 2008, 205, 339–346. [Google Scholar] [CrossRef]
- Stojkovic, S.; Demyanets, S.; Kopp, C.W.; Hengstenberg, C.; Wojta, J.; Eichelberger, B.; Panzer, S.; Gremmel, T. Association of Soluble Suppression of Tumorigenesis 2 (sST2) With Platelet Activation, Monocyte Tissue Factor and Ischemic Outcomes Following Angioplasty and Stenting. Front. Cardiovasc. Med. 2020, 7, 605669. [Google Scholar] [CrossRef]
- Stankovic, M.; Ljujic, B.; Babic, S.; Maravic-Stojkovic, V.; Mitrovic, S.; Arsenijevic, N.; Radak, D.; Pejnovic, N.; Lukic, M.L. IL-33/IL-33R in various types of carotid artery atherosclerotic lesions. Cytokine 2019, 120, 242–250. [Google Scholar] [CrossRef]
- Willems, S.; Quax, P.H.; de Borst, G.J.; de Vries, J.P.; Moll, F.L.; de Kleijn, D.P.; Hoefer, I.E.; Pasterkamp, G. Soluble ST2 levels are not associated with secondary cardiovascular events and vulnerable plaque phenotype in patients with carotid artery stenosis. Atherosclerosis 2013, 231, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Pfetsch, V.; Sanin, V.; Jaensch, A.; Dallmeier, D.; Mons, U.; Brenner, H.; Koenig, W.; Rothenbacher, D. Increased Plasma Concentrations of Soluble ST2 Independently Predict Mortality but not Cardiovascular Events in Stable Coronary Heart Disease Patients: 13-Year Follow-up of the KAROLA Study. Cardiovasc. Drugs Ther. 2017, 31, 167–177. [Google Scholar] [CrossRef]
- Liu, N.; Hang, T.; Gao, X.; Yang, W.; Kong, W.; Lou, Q.; Yang, J. The association between soluble suppression of tumorigenicity-2 and long-term prognosis in patients with coronary artery disease: A meta-analysis. PLoS ONE 2020, 15, e0238775. [Google Scholar] [CrossRef] [PubMed]
- Naylor, A.R.; Ricco, J.B.; de Borst, G.J.; Debus, S.; de Haro, J.; Halliday, A.; Hamilton, G.; Kakisis, J.; Kakkos, S.; Lepidi, S.; et al. Editor’s Choice—Management of Atherosclerotic Carotid and Vertebral Artery Disease: 2017 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 3–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. Editor’s Choice—2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 305–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Ciccone, M.M.; Cortese, F.; Gesualdo, M.; Riccardi, R.; Di Nunzio, D.; Moncelli, M.; Iacoviello, M.; Scicchitano, P. A Novel Cardiac Bio- Marker: ST2: A Review. Molecules 2013, 18, 15314–15328. [Google Scholar] [CrossRef]
- Marzullo, A.; Ambrosi, F.; Inchingolo, M.; Manca, F.; Devito, F.; Angiletta, D.; Zito, A.; Scicchitano, P.; Ciccone, M.M. ST2L Transmembrane Receptor Expression: An Immunochemical Study on Endarterectomy Samples. PLoS ONE 2016, 11, e0156315. [Google Scholar] [CrossRef]
- Cardellini, M.; Rizza, S.; Casagrande, V.; Cardolini, I.; Ballanti, M.; Davato, F.; Porzio, O.; Canale, M.P.; Legramante, J.M.; Mavilio, M.; et al. Soluble ST2 is a biomarker for cardiovascular mortality related to abnormal glucose metabolism in high-risk subjects. Acta Diabetol. 2019, 56, 273–280. [Google Scholar] [CrossRef]
- Filali, Y.; Kesäniemi, Y.A.; Ukkola, O. Soluble ST2, a biomarker of fibrosis, is associated with multiple risk factors, chronic diseases and total mortality in the OPERA study. Scand. J. Clin. Lab. Investig. 2021, 81, 324–331. [Google Scholar] [CrossRef]
- Kim, H.L.; Lee, J.P.; Wong, N.; Lim, W.H.; Seo, J.B.; Zo, J.H.; Kim, M.A.; Kim, S.H. Prognostic value of serum soluble ST2 in stable coronary artery disease: A prospective observational study. Sci. Rep. 2021, 11, 15203. [Google Scholar] [CrossRef]
- Li, M.; Duan, L.; Cai, Y.; Hao, B.; Chen, J.; Li, H.; Liu, H. Prognostic value of soluble suppression of tumorigenesis-2 (sST2) for cardiovascular events in coronary artery disease patients with and without diabetes mellitus. Cardiovasc. Diabetol. 2021, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Dieplinger, B.; Egger, M.; Haltmayer, M.; Kleber, M.E.; Scharnagl, H.; Silbernagel, G.; de Boer, R.A.; Maerz, W.; Mueller, T. Increased soluble ST2 predicts long-term mortality in patients with stable coronary artery disease: Results from the Ludwigshafen risk and cardiovascular health study. Clin. Chem. 2014, 60, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Guo, Y.; Wang, X.; Pei, L.; Wang, X.; Wu, J.; Sun, S.; Li, Y.; Ning, M.; Buonanno, F.S.; et al. Serum soluble ST2 is a potential long-term prognostic biomarker for transient ischaemic attack and ischaemic stroke. Eur. J. Neurol. 2020, 27, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.; Sastre, C.; Wolcott, Z.; Bevers, M.B.; Kimberly, W.T. Time-dependent, dynamic prediction of fatty acid-binding protein 4, Galectin-3, and soluble ST2 measurement with poor outcome after acute stroke. Int. J. Stroke 2021, 16, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Preis, S.R.; Beiser, A.; DeCarli, C.; Wollert, K.C.; Wang, T.J.; Januzzi, J.L., Jr.; Vasan, R.S.; Seshadri, S. Associations of Circulating Growth Differentiation Factor-15 and ST2 Concentrations With Subclinical Vascular Brain Injury and Incident Stroke. Stroke 2015, 46, 2568–2575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Lin, A.; Yu, Y.; Zhang, L.; Yang, G.; Hu, H.; Luo, Y. Serum Soluble ST2 as a Novel Inflammatory Marker in Acute Ischemic Stroke. Clin. Lab. 2018, 64, 1349–1356. [Google Scholar] [CrossRef]
- Sastre, C.; Bevers, M.B.; Kimberly, W.T. Role of Interleukin-1 Receptor-Like 1 (ST2) in Cerebrovascular Disease. Neurocrit. Care 2021, 35, 887–893. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, Y.; Xiao, W.; Liang, Z.; Dai, J.; Weng, X.; Wu, X. Interleukin-33 ameliorates ischemic brain injury in experimental stroke through promoting Th2 response and suppressing Th17 response. Brain Res. 2015, 1597, 86–94. [Google Scholar] [CrossRef]
- Korhonen, P.; Kanninen, K.M.; Lehtonen, Š.; Lemarchant, S.; Puttonen, K.A.; Oksanen, M.; Dhungana, H.; Loppi, S.; Pollari, E.; Wojciechowski, S.; et al. Immunomodulation by interleukin-33 is protective in stroke through modulation of inflammation. Brain Behav. Immun. 2015, 49, 322–336. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, H.; Zhang, H.; Ye, Q.; Wang, J.; Yang, B.; Mao, L.; Zhu, W.; Leak, R.K.; Xiao, B.; et al. ST2/IL-33-Dependent Microglial Response Limits Acute Ischemic Brain Injury. J. Neurosci. 2017, 37, 4692–4704. [Google Scholar] [CrossRef]
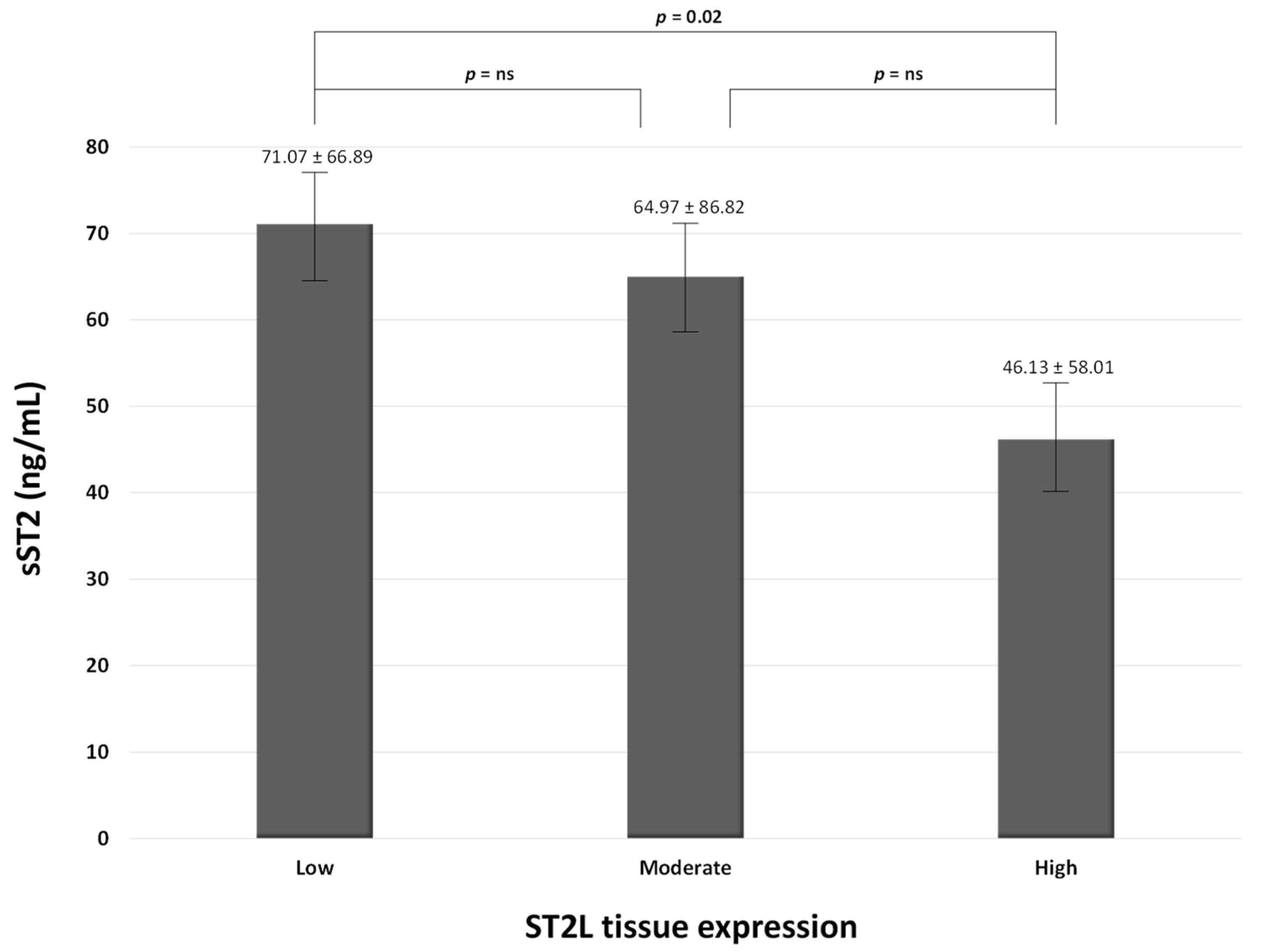

| Characteristics | Survivors (n = 58) | Non-Survivors (n = 24) | p-Values |
|---|---|---|---|
| Age (years) | 71.3 ± 7.9 | 73.1 ± 8.6 | 0.36 |
| Female (n/%) | 38 (65.5) | 20 (83.3) | 0.12 |
| Weight (kg) | 78.4 ± 11.9 | 76.6 ± 12.9 | 0.55 |
| Height (cm) | 167.4 ± 7.9 | 166.2 ± 9.0 | 0.53 |
| BMI (kg/m2) | 28.0 ± 3.6 | 27.8 ± 4.4 | 0.84 |
| Systolic arterial pressure (mmHg) | 130.3 ± 14.4 | 130.6 ± 10.5 | 0.91 |
| Diastolic arterial pressure (mmHg) | 76.3 ± 8.0 | 75.8 ± 7.5 | 0.81 |
| Heart rate (bpm) | 63.6 ± 9.2 | 67.8 ± 12.6 | 0.09 |
| Symptomatic (n/%) | 23 (39.6) | 14 (58.3) | 0.12 |
| Diabetes (n/%) | 18 (31.0) | 8 (33.3) | 0.84 |
| Hypertension (n/%) | 53 (91.4) | 22 (91.7) | 0.97 |
| Smokers (n/%) | 8 (13.8) | 6 (25) | 0.22 |
| Ex-smokers (n/%) | 22 (37.9) | 7 (29.2) | 0.46 |
| C-reactive protein (mg/L) | 4.2 ± 3.9 | 5.2 ± 3.5 | 0.90 |
| Leucocytes (admission) (×103/mm3) | 7.3 ± 2.0 | 8.4 ± 2.4 | 0.049 |
| Leucocytes (peak) (×103/mm3) | 11.9 ± 4.3 | 11.5 ± 3.3 | 0.74 |
| Total Cholesterol (mg/dL) | 158.6 ± 35.3 | 159.4 ± 51.7 | 0.93 |
| LDL-C (mg/dL) | 85.9 ± 33.1 | 85.8 ± 42.5 | 0.99 |
| HDL-C (mg/dL) | 51.9 ± 19.7 | 45.5 ± 11.3 | 0.14 |
| Triglycerides (mg/dL) | 104.0 ± 45.6 | 140.5 ± 66.4 | 0.005 |
| Troponin I (ng/mL) | 0.023 ± 0.035 | 0.027 ± 0.028 | 0.61 |
| Plaque instability features in asymptomatic pts | |||
| - Stenosis progression > 30% (n/%) | 9 (25.7) | 3 (30) | 0.79 |
| - Large plaque (n/%) | 10 (28.6) | 6 (25) | 0.07 |
| - Necrotic core (n/%) | 7 (20) | 4 (16.7) | 0.20 |
| - Echolucent plaque (n/%) | 7 (20) | 4 (16.7) | 0.20 |
| - Hypoechoic areas (n/%) | 6 (17.1) | 4 (16.7) | 0.13 |
| - Intraplaque hemorrhages (n/%) | 7 (20) | 5 (20.8) | 0.06 |
| - Surface irregularity (n/%) | 17 (48.6) | 8 (33.3) | 0.08 |
| - Silent infarction on CT/MRI (n/%) | 10 (28.6) | 6 (25) | 0.07 |
| sST2 (ng/mL) | 38.0 ± 30.0 | 117.0 ± 103.9 | <0.001 |
| Pharmacological treatments | |||
| ACEi/sartans (n/%) | 51 (87.9) | 20 (83.3) | 0.58 |
| Beta-blockers (n/%) | 42 (72.4) | 17 (70.8) | 0.88 |
| Diuretics (n/%) | 20 (34.5) | 9 (37.5) | 0.80 |
| MRA (n/%) | 11 (19.0) | 5 (20.8) | 0.84 |
| Statins (n/%) | 47 (81.0) | 21 (87.5) | 0.48 |
| Ezetimibe (n/%) | 10 (17.2) | 5 (20.8) | 0.71 |
| PCSK9i (n/%) | 4 (6.9) | 2 (8.3) | 0.82 |
| Characteristic | Survivors (n = 58) | Non-Survivors (n = 24) | Cut-Off | Sensibility | Specificity | AUC | p |
| sST2, ng/mL | 38.0 ± 30.0 | 117.0 ± 103.9 | >98.44 | 54.2 | 98.3 | 0.791 | <0.0001 |
| Tryglicerides, mg/dL | 104.0 ± 45.6 | 140.5 ± 66.4 | >105 | 70.8 | 67.2 | 0.685 | =0.0043 |
| Characteristic | Asymptomatic (n = 37) | Symptomatic (n = 45) | Cut-Off | Sensibility | Specificity | AUC | p |
| Total Cholesterol, mg/dL | 146.6 ± 31.6 | 173.7 ± 45.3 | >175.6 | 56.8 | 88.9 | 0.722 | =0.0002 |
| HDL-C, mg/dL | 54.2 ± 21.3 | 44.9 ± 10.5 | ≤55 | 89.2 | 37.8 | 0.628 | =0.0363 |
| LDL-C, mg/dL | 70.9 ± 26.1 | 104.1 ± 37.9 | >95 | 70.3 | 86.7 | 0.775 | <0.0001 |
| Tryglicerides, mg/dL | 107.5 ± 54.2 | 123.5 ± 54.8 | >94 | 75.7 | 51.1 | 0.623 | =0.0466 |
| sST2, ng/mL | 45.4 ± 41.4 | 80.3 ± 92.1 | >95.43 | 32.4 | 91.1 | 0.609 | 0.0855 |
| Univariate Cox Regression Analysis | Adjusted Cox Regression Analysis | ||||
|---|---|---|---|---|---|
| Survival Analysis | |||||
| Parameter | HR (95% CI) | p-Value | HR (95% CI) | Wald | p-Value |
| sST2, ng/mL | 1.012 (1.008–1.016) | <0.0001 | 1.012 (1.008–1.016) | 34.6856 | <0.0001 |
| Triglycerides, mg/dL | 1.008 (1.002–1.015) | 0.0058 | 1.008 (1.002–1.015) | 6.0960 | 0.0135 |
| Symptomatic Cerebrovascular Diseases | |||||
| Parameter | HR (95% CI) | p-Value | HR (95% CI) | Wald | p-Value |
| Smoking habit | 2.327 (1.083–4.998) | 0.0304 | |||
| Hemoglobin, g/dL | 0.841 (0.716–0.988) | 0.0347 | 0.744 (0.613–0.903) | 8.9739 | 0.0027 |
| HDL-C, mg/dL | 0.972 (0.946–0–999) | 0.0440 | |||
| LDL-C, mg/dL | 1.010 (1.001–1.020) | 0.0282 | |||
| Triglycerides, mg/dL | 1.007 (1.001–1.013) | 0.0162 | |||
| Ezetimibe | 0.151 (0.021–1.104) | 0.0102 | |||
| sST2, ng/mL | 1.013 (1.008–1.017) | <0.0001 | 1.013 (1.008–1.018) | 21.998 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scicchitano, P.; Marzullo, A.; Santoro, A.; Zito, A.; Cortese, F.; Galeandro, C.; Ciccone, A.S.; Angiletta, D.; Manca, F.; Pulli, R.; et al. The Prognostic Role of ST2L and sST2 in Patients Who Underwent Carotid Plaque Endarterectomy: A Five-Year Follow-Up Study. J. Clin. Med. 2022, 11, 3142. https://doi.org/10.3390/jcm11113142
Scicchitano P, Marzullo A, Santoro A, Zito A, Cortese F, Galeandro C, Ciccone AS, Angiletta D, Manca F, Pulli R, et al. The Prognostic Role of ST2L and sST2 in Patients Who Underwent Carotid Plaque Endarterectomy: A Five-Year Follow-Up Study. Journal of Clinical Medicine. 2022; 11(11):3142. https://doi.org/10.3390/jcm11113142
Chicago/Turabian StyleScicchitano, Pietro, Andrea Marzullo, Annarita Santoro, Annapaola Zito, Francesca Cortese, Cristina Galeandro, Andrea Sebastiano Ciccone, Domenico Angiletta, Fabio Manca, Raffaele Pulli, and et al. 2022. "The Prognostic Role of ST2L and sST2 in Patients Who Underwent Carotid Plaque Endarterectomy: A Five-Year Follow-Up Study" Journal of Clinical Medicine 11, no. 11: 3142. https://doi.org/10.3390/jcm11113142
APA StyleScicchitano, P., Marzullo, A., Santoro, A., Zito, A., Cortese, F., Galeandro, C., Ciccone, A. S., Angiletta, D., Manca, F., Pulli, R., Navarese, E. P., Gurbel, P. A., & Ciccone, M. M. (2022). The Prognostic Role of ST2L and sST2 in Patients Who Underwent Carotid Plaque Endarterectomy: A Five-Year Follow-Up Study. Journal of Clinical Medicine, 11(11), 3142. https://doi.org/10.3390/jcm11113142







