Evaluation of Alpha-Synuclein Cerebrospinal Fluid Levels in Several Neurological Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CSF Analysis
2.3. Statistical Analysis
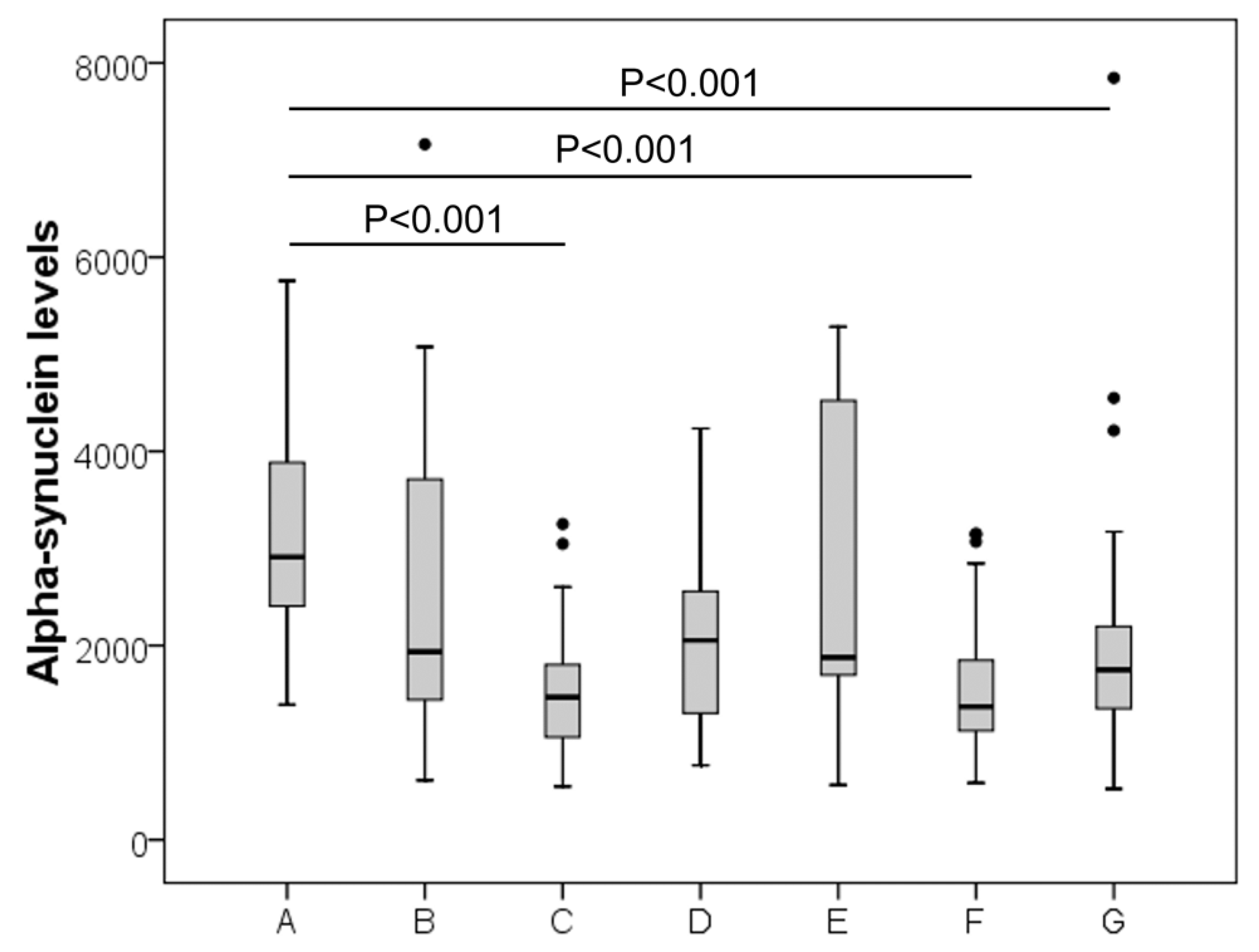
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clayton, D.F.; George, J.M. The synucleins: A family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998, 21, 249–254. [Google Scholar] [CrossRef]
- Del Tredici, K.; Braak, H. Review: Sporadic Parkinson’s disease: Development and distribution of α-synuclein pathology. Neuropathol. Appl. Neurobiol. 2016, 42, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Burré, J. The Synaptic Function of α-Synuclein. J. Park. Dis. 2015, 5, 699–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahul-Mellier, A.L.; Burtscher, J.; Maharjan, N.; Weerens, L.; Croisier, M.; Kuttler, F.; Leleu, M.; Knott, G.W.; Lashuel, H.A. The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc. Natl. Acad. Sci. USA 2020, 117, 4971–4982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paillusson, S.; Clairembault, T.; Biraud, M.; Neunlist, M.; Derkinderen, P. Activity-dependent secretion of alpha-synuclein by enteric neurons. J. Neurochem. 2013, 125, 512–517. [Google Scholar] [CrossRef]
- Emmanouilidou, E.; Elenis, D.; Papasilekas, T.; Stranjalis, G.; Gerozissis, K.; Ioannou, P.C.; Vekrellis, K. Assessment of α-synuclein secretion in mouse and human brain parenchyma. PLoS ONE 2011, 6, e22225. [Google Scholar] [CrossRef]
- Mollenhauer, B.; Locascio, J.J.; Schulz-Schaeffer, W.; Sixel-Döring, F.; Trenkwalder, C.; Schlossmacher, M.G. α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: A cohort study. Lancet Neurol. 2011, 10, 230–240. [Google Scholar] [CrossRef]
- El-Agnaf, O.M.; Salem, S.A.; Paleologou, K.E.; Cooper, L.J.; Fullwood, N.J.; Gibson, M.J.; Curran, M.D.; Court, J.A.; Mann, D.M.; Ikeda, S.; et al. Alpha-synuclein implicated in Parkinson’s disease is present in extracellular biological fluids, including human plasma. FASEB J. 2003, 17, 1945–1947. [Google Scholar] [CrossRef]
- Eusebi, P.; Giannandrea, D.; Biscetti, L.; Abraha, I.; Chiasserini, D.; Orso, M.; Calabresi, P.; Parnetti, L. Diagnostic utility of cerebrospinal fluid α-synuclein in Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2017, 32, 1389–1400. [Google Scholar] [CrossRef]
- Parnetti, L.; Gaetani, L.; Eusebi, P.; Paciotti, S.; Hansson, O.; El-Agnaf, O.; Mollenhauer, B.; Blennow, K.; Calabresi, P. CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol. 2019, 18, 573–586. [Google Scholar] [CrossRef]
- Constantinides, V.C.; Majbour, N.K.; Paraskevas, G.P.; Abdi, I.; Safieh-Garabedian, B.; Stefanis, L.; El-Agnaf, O.M.; Kapaki, E. Cerebrospinal Fluid α-Synuclein Species in Cognitive and Movements Disorders. Brain Sci. 2021, 11, 119. [Google Scholar] [CrossRef]
- Agnello, L.; Gambino, C.M.; Lo Sasso, B.; Bivona, G.; Milano, S.; Ciaccio, A.M.; Piccoli, T.; La Bella, V.; Ciaccio, M. Neurogranin as a Novel Biomarker in Alzheimer’s Disease. Lab. Med. 2021, 52, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Bradner, J.; Hancock, A.M.; Chung, K.A.; Quinn, J.F.; Peskind, E.R.; Galasko, D.; Jankovic, J.; Zabetian, C.P.; Kim, H.M.; et al. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann. Neurol. 2011, 69, 570–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Shi, M.; Chung, K.A.; Zabetian, C.P.; Leverenz, J.B.; Berg, D.; Srulijes, K.; Trojanowski, J.Q.; Lee, V.M.; Siderowf, A.D.; et al. Phosphorylated α-synuclein in Parkinson’s disease. Sci. Transl. Med. 2012, 4, 121ra20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compta, Y.; Revesz, T. Neuropathological and Biomarker Findings in Parkinson’s Disease and Alzheimer’s Disease: From Protein Aggregates to Synaptic Dysfunction. J. Park. Dis. 2021, 11, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.G.; Jeon, S.; Kang, S.W.; Park, M.; Baik, K.; Yoo, H.S.; Chung, S.J.; Jeong, S.H.; Jung, J.H.; Lee, P.H.; et al. Interaction of CSF α-synuclein and amyloid beta in cognition and cortical atrophy. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2021, 13, e12177. [Google Scholar] [CrossRef]
- Mackin, R.S.; Insel, P.; Zhang, J.; Mohlenhoff, B.; Galasko, D.; Weiner, M.; Mattsson, N. Cerebrospinal fluid α-synuclein and Lewy body-like symptoms in normal controls, mild cognitive impairment, and Alzheimer’s disease. J. Alzheimers Dis. 2015, 43, 1007–1016. [Google Scholar] [CrossRef] [Green Version]
- Visanji, N.P.; Lang, A.E.; Kovacs, G.G. Beyond the synucleinopathies: Alpha synuclein as a driving force in neurodegenerative comorbidities. Transl. Neurodegener. 2019, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Bae, J.R.; Kim, S.H. Synapses in neurodegenerative diseases. BMB Rep. 2017, 50, 237–246. [Google Scholar] [CrossRef] [Green Version]
- del Campo, M.; Mollenhauer, B.; Bertolotto, A.; Engelborghs, S.; Hampel, H.; Simonsen, A.H.; Kapaki, E.; Kruse, N.; Le Bastard, N.; Lehmann, S.; et al. Recommendations to standardize preanalytical confounding factors in Alzheimer’s and Parkinson’s disease cerebrospinal fluid biomarkers: An update. Biomark. Med. 2012, 6, 419–430. [Google Scholar] [CrossRef]
- Allegri, R.F. Moving from neurodegenerative dementias, to cognitive proteinopathies, replacing “where” by “what”…. Dement. Neuropsychol. 2020, 14, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Camporesi, E.; Nilsson, J.; Brinkmalm, A.; Becker, B.; Ashton, N.J.; Blennow, K.; Zetterberg, H. Fluid Biomarkers for Synaptic Dysfunction and Loss. Biomark. Insights 2020, 15, 1177271920950319. [Google Scholar] [CrossRef] [PubMed]
- Agnello, L.; Colletti, T.; Lo Sasso, B.; Vidali, M.; Spataro, R.; Gambino, C.M.; Giglio, R.V.; Piccoli, T.; Bivona, G.; La Bella, V.; et al. Tau protein as a diagnostic and prognostic biomarker in amyotrophic lateral sclerosis. Eur. J. Neurol. 2021, 28, 1868–1875. [Google Scholar] [CrossRef]
- Colletti, T.; Agnello, L.; Spataro, R.; Guccione, L.; Notaro, A.; Lo Sasso, B.; Blandino, V.; Graziano, F.; Gambino, C.M.; Giglio, R.V.; et al. Prognostic Role of CSF β-amyloid 1-42/1-40 Ratio in Patients Affected by Amyotrophic Lateral Sclerosis. Brain Sci. 2021, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H., Jr.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Agnello, L.; Piccoli, T.; Vidali, M.; Cuffaro, L.; Lo Sasso, B.; Iacolino, G.; Giglio, V.R.; Lupo, F.; Alongi, P.; Bivona, G.; et al. Diagnostic accuracy of cerebrospinal fluid biomarkers measured by chemiluminescent enzyme immunoassay for Alzheimer disease diagnosis. Scand. J. Clin. Lab. Investig. 2020, 80, 313–317. [Google Scholar] [CrossRef]
- McGrowder, D.A.; Miller, F.; Vaz, K.; Nwokocha, C.; Wilson-Clarke, C.; Anderson-Cross, M.; Brown, J.; Anderson-Jackson, L.; Williams, L.; Latore, L.; et al. Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease: Current Evidence and Future Perspectives. Brain Sci. 2021, 11, 215. [Google Scholar] [CrossRef]
- Twohig, D.; Nielsen, H.M. α-synuclein in the pathophysiology of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 23. [Google Scholar] [CrossRef] [Green Version]
- Sako, W.; Murakami, N.; Izumi, Y.; Kaji, R. Reduced alpha-synuclein in cerebrospinal fluid in synucleinopathies: Evidence from a meta-analysis. Mov. Disord. 2014, 29, 1599–1605. [Google Scholar] [CrossRef]
- Zhou, B.; Wen, M.; Yu, W.F.; Zhang, C.L.; Jiao, L. The Diagnostic and Differential Diagnosis Utility of Cerebrospinal Fluid α -Synuclein Levels in Parkinson’s Disease: A Meta-Analysis. Park. Dis. 2015, 2015, 567386. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Tang, H.; Nie, K.; Wang, L.; Zhao, J.; Gan, R.; Huang, J.; Zhu, R.; Feng, S.; Duan, Z.; et al. Cerebrospinal fluid alpha-synuclein as a biomarker for Parkinson’s disease diagnosis: A systematic review and meta-analysis. Int. J. Neurosci. 2015, 125, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Chalatsa, I.; Melachroinou, K.; Emmanouilidou, K.E.; Vekrellis, E.K. Assessment of cerebrospinal fluid α-synuclein as a potential biomarker in Parkinson’s disease and synucleinopathies. Neuroimmunol. Neuroinflamm. 2020, 7, 132–140. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Han, Z.M.; Liu, Q.F.; Tang, W.; Ye, K.; Yao, Y.Y. Use of CSF α-synuclein in the differential diagnosis between Alzheimer’s disease and other neurodegenerative disorders. Int. Psychogeriatr. 2015, 27, 1429–1438. [Google Scholar] [CrossRef]
- Chiasserini, D.; Biscetti, L.; Eusebi, P.; Salvadori, N.; Frattini, G.; Simoni, S.; De Roeck, N.; Tambasco, N.; Stoops, E.; Vanderstichele, H.; et al. Differential role of CSF fatty acid binding protein 3, α-synuclein, and Alzheimer’s disease core biomarkers in Lewy body disorders and Alzheimer’s dementia. Alzheimers Res. Ther. 2017, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Majbour, N.K.; Chiasserini, D.; Vaikath, N.N.; Eusebi, P.; Tokuda, T.; van de Berg, W.; Parnetti, L.; Calabresi, P.; El-Agnaf, O.M. Increased levels of CSF total but not oligomeric or phosphorylated forms of alpha-synuclein in patients diagnosed with probable Alzheimer’s disease. Sci. Rep. 2017, 7, 40263. [Google Scholar] [CrossRef]
- Vacchi, E.; Kaelin-Lang, A.; Melli, G. Tau and Alpha Synuclein Synergistic Effect in Neurodegenerative Diseases: When the Periphery Is the Core. Int. J. Mol. Sci. 2020, 21, 5030. [Google Scholar] [CrossRef]
- Jellinger, K.A. Interaction between α-synuclein and other proteins in neurodegenerative disorders. Sci. World J. 2011, 11, 1893–1907. [Google Scholar] [CrossRef] [Green Version]
- Larson, M.E.; Sherman, M.A.; Greimel, S.; Kuskowski, M.; Schneider, J.A.; Bennett, D.A.; Lesné, S.E. Soluble α-synuclein is a novel modulator of Alzheimer’s disease pathophysiology. J. Neurosci. 2012, 32, 10253–10266. [Google Scholar] [CrossRef] [Green Version]
- Clinton, L.K.; Blurton-Jones, M.; Myczek, K.; Trojanowski, J.Q.; LaFerla, F.M. Synergistic Interactions between Abeta, tau, and alpha-synuclein: Acceleration of neuropathology and cognitive decline. J. Neurosci. 2010, 30, 7281–7289. [Google Scholar] [CrossRef] [Green Version]
- Mollenhauer, B.; Bowman, F.D.; Drake, D.; Duong, J.; Blennow, K.; El-Agnaf, O.; Shaw, L.M.; Masucci, J.; Taylor, P.; Umek, R.M.; et al. Antibody-based methods for the measurement of α-synuclein concentration in human cerebrospinal fluid-method comparison and round robin study. J. Neurochem. 2019, 149, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Rong, H.; Jin, L.; Wei, W.; Wang, X.; Xi, Z. Alpha-synuclein is a potential biomarker in the serum and CSF of patients with intractable epilepsy. Seizure 2015, 27, 6–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Group | Sex, M% (Total nr) | Age (Median, IQR) | α-syn, pg/mL (Median, IQR) |
|---|---|---|---|
| AD | 56 (25) | 73 (67–76) | 2912 (2243–4036) |
| Cerebrovascular diseases | 67 (18) | 60 (53–70) | 1938 (1337–3719) |
| Controls | 54 (35) | 51 (37–66) | 1469 (1047–1827) |
| Inflammatory CNS diseases | 30 (10) | 64 (58–76) | 2053 (1284–2677) |
| Other neurological diseases | 43 (7) | 63 (48–72) | 1879 (1679–4698) |
| PD | 64 (22) | 65 (56–72) | 1372 (1103–2031) |
| Peripheral Neuropathy | 61 (41) | 60 (48–71) | 1751 (1333–2225) |
| α-syn | T-tau | P-tau | β42 | β40 | β42/40 Ratio | |
|---|---|---|---|---|---|---|
| α-synuclein | 0.630 p = 0.002 | 0.498 p = 0.018 | 0.485 p = 0.022 | 0.488 p = 0.021 | 0.222 p = 0.320 | |
| T-tau | 0.785 p < 0.001 | 0.291 p = 0.189 | 0.309 p = 0.162 | 0.040 p = 0.859 | ||
| P-tau | 0.233 p = 0.296 | 0.450 p = 0.036 | −0.142 p = 0.527 | |||
| β42 | 0.755 p < 0.001 | 0.622 p = 0.002 | ||||
| β40 | 0.028 p = 0.903 | |||||
| β42/40 ratio |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agnello, L.; Lo Sasso, B.; Vidali, M.; Scazzone, C.; Gambino, C.M.; Piccoli, T.; Bivona, G.; Ciaccio, A.M.; Giglio, R.V.; La Bella, V.; et al. Evaluation of Alpha-Synuclein Cerebrospinal Fluid Levels in Several Neurological Disorders. J. Clin. Med. 2022, 11, 3139. https://doi.org/10.3390/jcm11113139
Agnello L, Lo Sasso B, Vidali M, Scazzone C, Gambino CM, Piccoli T, Bivona G, Ciaccio AM, Giglio RV, La Bella V, et al. Evaluation of Alpha-Synuclein Cerebrospinal Fluid Levels in Several Neurological Disorders. Journal of Clinical Medicine. 2022; 11(11):3139. https://doi.org/10.3390/jcm11113139
Chicago/Turabian StyleAgnello, Luisa, Bruna Lo Sasso, Matteo Vidali, Concetta Scazzone, Caterina Maria Gambino, Tommaso Piccoli, Giulia Bivona, Anna Maria Ciaccio, Rosaria Vincenza Giglio, Vincenzo La Bella, and et al. 2022. "Evaluation of Alpha-Synuclein Cerebrospinal Fluid Levels in Several Neurological Disorders" Journal of Clinical Medicine 11, no. 11: 3139. https://doi.org/10.3390/jcm11113139
APA StyleAgnello, L., Lo Sasso, B., Vidali, M., Scazzone, C., Gambino, C. M., Piccoli, T., Bivona, G., Ciaccio, A. M., Giglio, R. V., La Bella, V., & Ciaccio, M. (2022). Evaluation of Alpha-Synuclein Cerebrospinal Fluid Levels in Several Neurological Disorders. Journal of Clinical Medicine, 11(11), 3139. https://doi.org/10.3390/jcm11113139












