Lymphatic Phenotype of Noonan Syndrome: Innovative Diagnosis and Possible Implications for Therapy
Abstract
:1. Introduction
2. Imaging the Lymphatic System
3. Clinical Manifestation Compared to Radiological Findings in Patients with NS
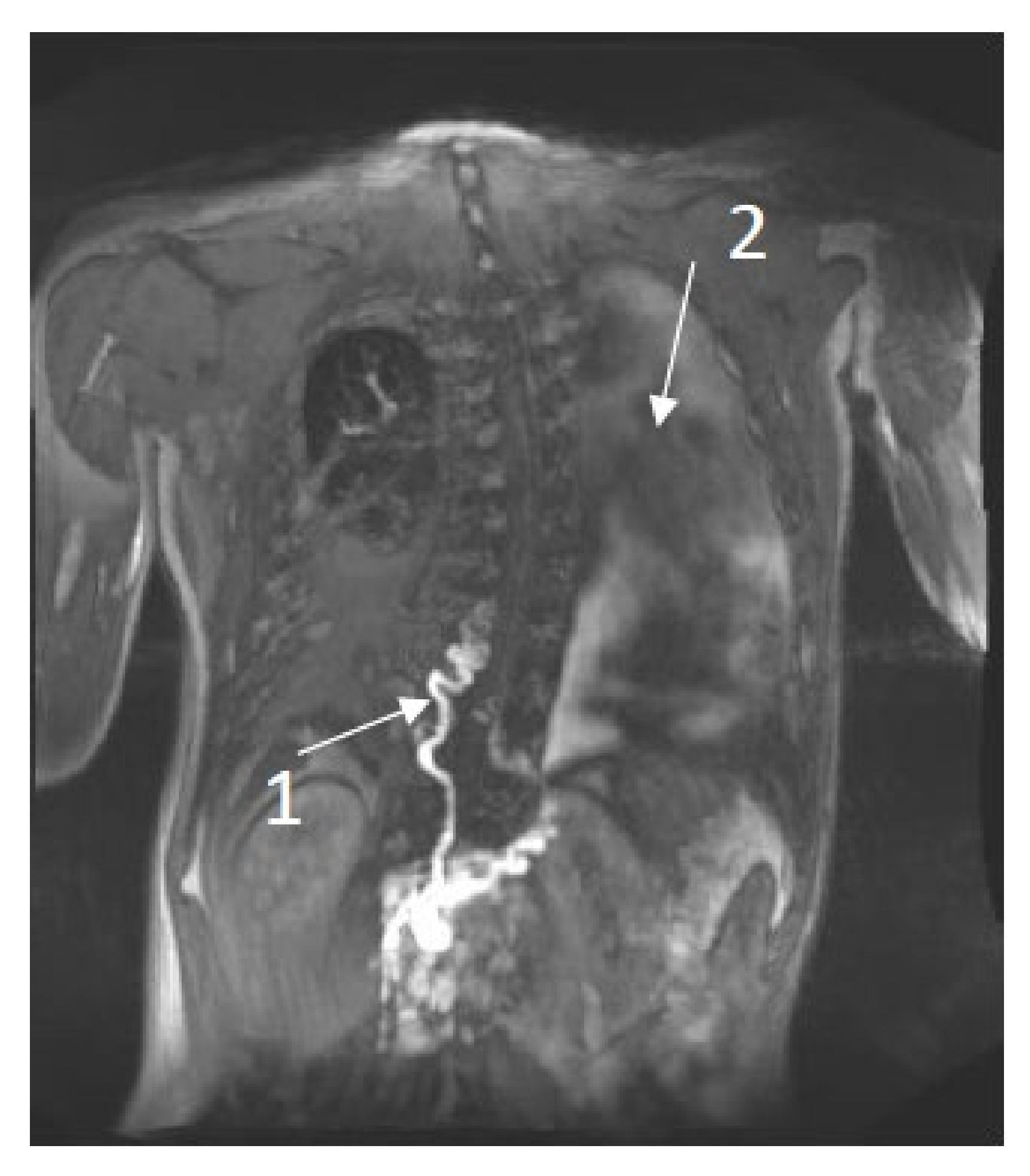
Literature Review
4. Possible Implications for Therapeutic Options
5. Future Research Opportunities
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biko, D.M.; Reisen, B.; Otero, H.J.; Ravishankar, C.; Victoria, T.; Glatz, A.C.; Rome, J.J.; Dori, Y. Imaging of central lymphatic abnormalities in Noonan syndrome. Pediatr. Radiol. 2019, 49, 586–592. [Google Scholar] [CrossRef]
- Kouz, K.; Lissewski, C.; Spranger, S.; Mitter, D.; Riess, A.; Lopez-Gonzalez, V.; Lüttgen, S.; Aydin, H.; Von Deimling, F.; Evers, C.; et al. Genotype and phenotype in patients with Noonan syndrome and a RIT1 mutation. Genet. Med. 2016, 18, 1226–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tidyman, W.E.; Rauen, K.A. Expansion of the RASopathies. Curr. Genet. Med. Rep. 2016, 4, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, A.R.; Cushman, B.J.; Cave, H.; Dillon, M.W.; Gelb, B.D.; Gripp, K.W.; Lee, J.A.; Mason-Suares, H.; Rauen, K.A.; Tartaglia, M.; et al. Assessing the gene-disease association of 19 genes with the RASopathies using the ClinGen gene curation framework. Hum. Mutat. 2018, 39, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.E.; Allanson, J.E.; Tartaglia, M.; Gelb, B.D. Noonan syndrome. Lancet 2013, 381, 333–342. [Google Scholar] [CrossRef] [Green Version]
- Tartaglia, M.; Gelb, B.D.; Zenker, M. Noonan syndrome and clinically related disorders. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 161–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Simons, M. Lymphatic fate determination: Playing RAF with ERK. Cell Cycle 2013, 12, 1157–1158. [Google Scholar] [CrossRef] [Green Version]
- Makinen, T.; Boon, L.M.; Vikkula, M.; Alitalo, K. Lymphatic Malformations: Genetics, Mechanisms and Therapeutic Strategies. Circ. Res. 2021, 129, 136–154. [Google Scholar] [CrossRef]
- Myers, A.; Bernstein, J.A.; Brennan, M.L.; Curry, C.; Esplin, E.D.; Fisher, J.; Homeyer, M.; Manning, M.A.; Muller, E.A.; Niemi, A.K.; et al. Perinatal features of the RASopathies: Noonan syndrome, cardiofaciocutaneous syndrome and Costello syndrome. Am. J. Med. Genet. A 2014, 164, 2814–2821. [Google Scholar] [CrossRef]
- Swarts, J.K.L.; Leenders, E.; Klein, W.M.; Draaisma, J.M.T. Lymphatic anomalies during lifetime in patients with Noonan Syndrome: Retrospective cohort study. Am. J. Hum. Genet. 2021; in press. [Google Scholar]
- Lissewski, C.; Chune, V.; Pantaleoni, F.; De Luca, A.; Capri, Y.; Brinkmann, J.; Lepri, F.; Daniele, P.; Leenders, E.; Mazzanti, L.; et al. Variants of SOS2 are a rare cause of Noonan syndrome with particular predisposition for lymphatic complications. Eur. J. Hum. Genet. 2021, 29, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Sleutjes, J.; Kleimeier, L.; Leenders, E.; Klein, W.; Draaisma, J. Lymphatic abnormalities in Noonan syndrome spectrum disorders: A systematic review. Mol. Syndromol. 2021, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dori, Y.; Smith, C.; Pinto, E.; Snyder, K.; March, M.E.; Hakonarson, H.; Belasco, J. Severe Lymphatic Disorder Resolved With MEK Inhibition in a Patient With Noonan Syndrome and SOS1 Mutation. Pediatrics 2020, 146, e20200167. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; March, M.E.; Gutierrez-Uzquiza, A.; Kao, C.; Seiler, C.; Pinto, E.; Matsuoka, L.S.; Battig, M.R.; Bhoj, E.J.; Wenger, T.L.; et al. ARAF recurrent mutation causes central conducting lymphatic anomaly treatable with a MEK inhibitor. Nat. Med. 2019, 25, 1116–1122. [Google Scholar] [CrossRef]
- Othman, S.; Azoury, S.C.; DiBardino, D.; Adams, D.M.; Itkin, M.; Kovach, S.J. Respiratory Failure in Noonan Syndrome Treated by Microsurgical Thoracic Duct-Venous Anastomosis. Ann. Thorac. Surg. 2021, 113, e219–e221. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kudo, T.; Endo, J.; Hashida, K.; Tachibana, N.; Murakoshi, T.; Hasebe, T. Transnodal lymphangiography and post-CT for protein-losing enteropathy in Noonan syndrome. Minim. Invasive Ther. Allied Technol. 2015, 24, 246–249. [Google Scholar] [CrossRef]
- Keberle, M.; Mork, H.; Jenett, M.; Hahn, D.; Scheurlen, M. Computed tomography after lymphangiography in the diagnosis of intestinal lymphangiectasia with protein-losing enteropathy in Noonan’s syndrome. Eur. Radiol. 2000, 10, 1591–1593. [Google Scholar] [CrossRef]
- Hilliard, R.I.; McKendry, J.B.; Phillips, M.J. Congenital abnormalities of the lymphatic system: A new clinical classification. Pediatrics 1990, 86, 988–994. [Google Scholar] [CrossRef]
- Wassef, M.; Blei, F.; Adams, D.; Alomari, A.; Baselga, E.; Berenstein, A.; Burrows, P.; Frieden, I.J.; Garzon, M.C.; Lopez-Gutierrez, J.C.; et al. Vascular Anomalies Classification: Recommendations From the International Society for the Study of Vascular Anomalies. Pediatrics 2015, 136, e203–e214. [Google Scholar] [CrossRef] [Green Version]
- Chavhan, G.B.; Amaral, J.G.; Temple, M.; Itkin, M. MR Lymphangiography in Children: Technique and Potential Applications. Radiographics 2017, 37, 1775–1790. [Google Scholar] [CrossRef] [Green Version]
- Ricci, K.W.; Iacobas, I. How we approach the diagnosis and management of complex lymphatic anomalies. Pediatr. Blood Cancer 2021, e28985. [Google Scholar] [CrossRef]
- Van Schaik, C.J.; Boer, L.L.; Draaisma, J.M.T.; van der Vleuten, C.J.M.; Janssen, J.J.; Fütterer, J.J.; Schultze Kool, L.J.; Klein, W.M. The lymphatic system throughout history: From hieroglyphic translations to state of the art radiological techniques. Clin. Anat. 2022. [Google Scholar] [CrossRef]
- De Haas, M. Lymphoscintigraphy of the Lower Extremities; Meander Medical Centre Amersfoort: Amersfoort, The Netherlands; Available online: richtlijnendatabase.nl (accessed on 17 December 2021).
- Itkin, M.; Chidekel, A.; Ryan, K.A.; Rabinowitz, D. Abnormal pulmonary lymphatic flow in patients with paediatric pulmonary lymphatic disorders: Diagnosis and treatment. Paediatr. Respir. Rev. 2020, 36, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Winters, H.; Tielemans, H.J.; Ulrich, D.J. Lymphovenous Anastomosis and Secondary Resection for Noonan Syndrome with Vulvar Lymphangiectasia. Plast. Reconstr. Surg. Glob. Open 2016, 4, e1007. [Google Scholar] [CrossRef] [PubMed]
- Damstra, R.J.; Halk, A.B.; Dutch Working Group on Lymphedema. The Dutch lymphedema guidelines based on the International Classification of Functioning, Disability, and Health and the chronic care model. J. Vasc. Surg. Venous Lymphat. Disord. 2017, 5, 756–765. [Google Scholar] [CrossRef]
- Strehl, J.; Schepke, M.; Wardelmann, E.; Caselmann, W.H.; Sauerbruch, T. Chronische Diarrhö bei einem 43-jährigen Patienten [Chronic diarrhea in a 43-year-old patient]. Internist 2003, 44, 626–630. [Google Scholar] [CrossRef]
- Prasad, D.; Srivastava, A.; Tambe, A.; Yachha, S.K.; Sarma, M.S.; Poddar, U. Clinical Profile, Response to Therapy, and Outcome of Children with Primary Intestinal Lymphangiectasia. Dig. Dis. 2019, 37, 458–466. [Google Scholar] [CrossRef]
- Downie, L.; Sasi, A.; Malhotra, A. Congenital chylothorax: Associations and neonatal outcomes. J. Paediatr. Child Health 2014, 50, 234–238. [Google Scholar] [CrossRef]
- Mitchell, K.; Weiner, A.; Ramsay, P.; Sahni, M. Use of Propranolol in the Treatment of Chylous Effusions in Infants. Pediatrics 2021, 148, e2020049699. [Google Scholar] [CrossRef]
- Nadolski, G.J.; Itkin, M. Thoracic duct embolization for nontraumatic chylous effusion: Experience in 34 patients. Chest 2013, 143, 158–163. [Google Scholar] [CrossRef]
- Othman, T.A.; Azenkot, T.; Moskoff, B.N.; Tenold, M.E.; Jonas, B.A. Venetoclax-based combinations for the treatment of newly diagnosed acute myeloid leukemia. Future Oncol. 2021, 17, 2989–3005. [Google Scholar] [CrossRef] [PubMed]
- Manevitz-Mendelson, E.; Leichner, G.S.; Barel, O.; Davidi-Avrahami, I.; Ziv-Strasser, L.; Eyal, E.; Pessach, I.; Rimon, U.; Barzilai, A.; Hirshberg, A.; et al. Somatic NRAS mutation in patient with generalized lymphatic anomaly. Angiogenesis 2018, 21, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Barclay, S.F.; Inman, K.W.; Luks, V.L.; McIntyre, J.B.; Al-Ibraheemi, A.; Church, A.J.; Perez-Atayde, A.R.; Mangray, S.; Jeng, M.; Kreimer, S.R.; et al. A somatic activating NRAS variant associated with kaposiform lymphangiomatosis. Genet. Med. 2019, 21, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. VEGFR and type-V RTK activation and signaling. Cold Spring Harb. Perspect. Biol. 2013, 5, a009092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Nr. | Age (y) (m/f) | Clinical Manifestation of Lymphatic Disease | Radiological Findings | Reference | ||
|---|---|---|---|---|---|---|
| TD | Flow abnormalities | Other findings | ||||
| 1 | 14 (f) | CT, PLE, MLA, RLA | ND | Retrograde mesenteric and pulmonary flow | Leak of contrast into duodenal lumen, abnormal CLS | Dori, Y [13] |
| 2 | 13 (m) | LE, PLE, HT | ND | Pleural fluids ascites | oedematous intestine | Keberle, M [17] |
| 3 | 17 (f) | PLE | absent | ND | abdominal collateral lymphatics and bilateral iliac lymphangiectasia | Matsumoto, T [16] |
| 4 | 61 (m) | LE, SLE | Occlusion at the neck | Increased pelvic and retroperitoneal flow | PLA, abdominal ascites | Othman, S [15] |
| 5 | 0.9 (f) | CT | Dilated | Bilateral perfusion | - | Biko [1] |
| 6 | 0.6 (m) | CT | Double duct, central TD not present | Bilateral perfusion | Body wall edema | Biko [1] |
| 7 | 0.1 (m) | CT | ND | Bilateral pleural effusions | Body wall edema, ascites | Biko [1] |
| 8 | 0.8 (m) | CT, ascites | absent | Bilateral perfusion | Body wall edema, ascites | Biko [1] |
| 9 | 7 (f) | CT | absent | Bilateral perfusion | Pericardial effusion, ascites | Biko [1] |
| 10 | 0.2 (m) | CT | absent | Bilateral perfusion | Body wall edema | Biko [1] |
| 11 | 0.1 (f) | CT | rudimentary | Bilateral perfusion | ascites | Biko [1] |
| 12 | 0.1 (f) | CT | Double duct | Perfusion right lung | - | Biko [1] |
| 13 | 0.1 (m) | CT, anasarca | Dilated | Bilateral perfusion | Network of lymphatic collaterals in left neck, body wall edema, ascites | Biko [1] |
| 14 | 5 (m) | ascites | absent | Peritoneum perfusion | Ascites | Biko [1] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleimeier, L.E.R.; van Schaik, C.; Leenders, E.; Itkin, M.; Klein, W.M.; Draaisma, J.M.T. Lymphatic Phenotype of Noonan Syndrome: Innovative Diagnosis and Possible Implications for Therapy. J. Clin. Med. 2022, 11, 3128. https://doi.org/10.3390/jcm11113128
Kleimeier LER, van Schaik C, Leenders E, Itkin M, Klein WM, Draaisma JMT. Lymphatic Phenotype of Noonan Syndrome: Innovative Diagnosis and Possible Implications for Therapy. Journal of Clinical Medicine. 2022; 11(11):3128. https://doi.org/10.3390/jcm11113128
Chicago/Turabian StyleKleimeier, Lotte E. R., Caroline van Schaik, Erika Leenders, Maxim Itkin, Willemijn M. Klein, and Jos M. T. Draaisma. 2022. "Lymphatic Phenotype of Noonan Syndrome: Innovative Diagnosis and Possible Implications for Therapy" Journal of Clinical Medicine 11, no. 11: 3128. https://doi.org/10.3390/jcm11113128






