Vascular Occlusion in Kidney Biopsy Is Characteristic of Clinically Manifesting Thrombotic Microangiopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Laboratory Analyses
2.3. Pathological Analyses
2.4. Statistical Analyses
3. Results
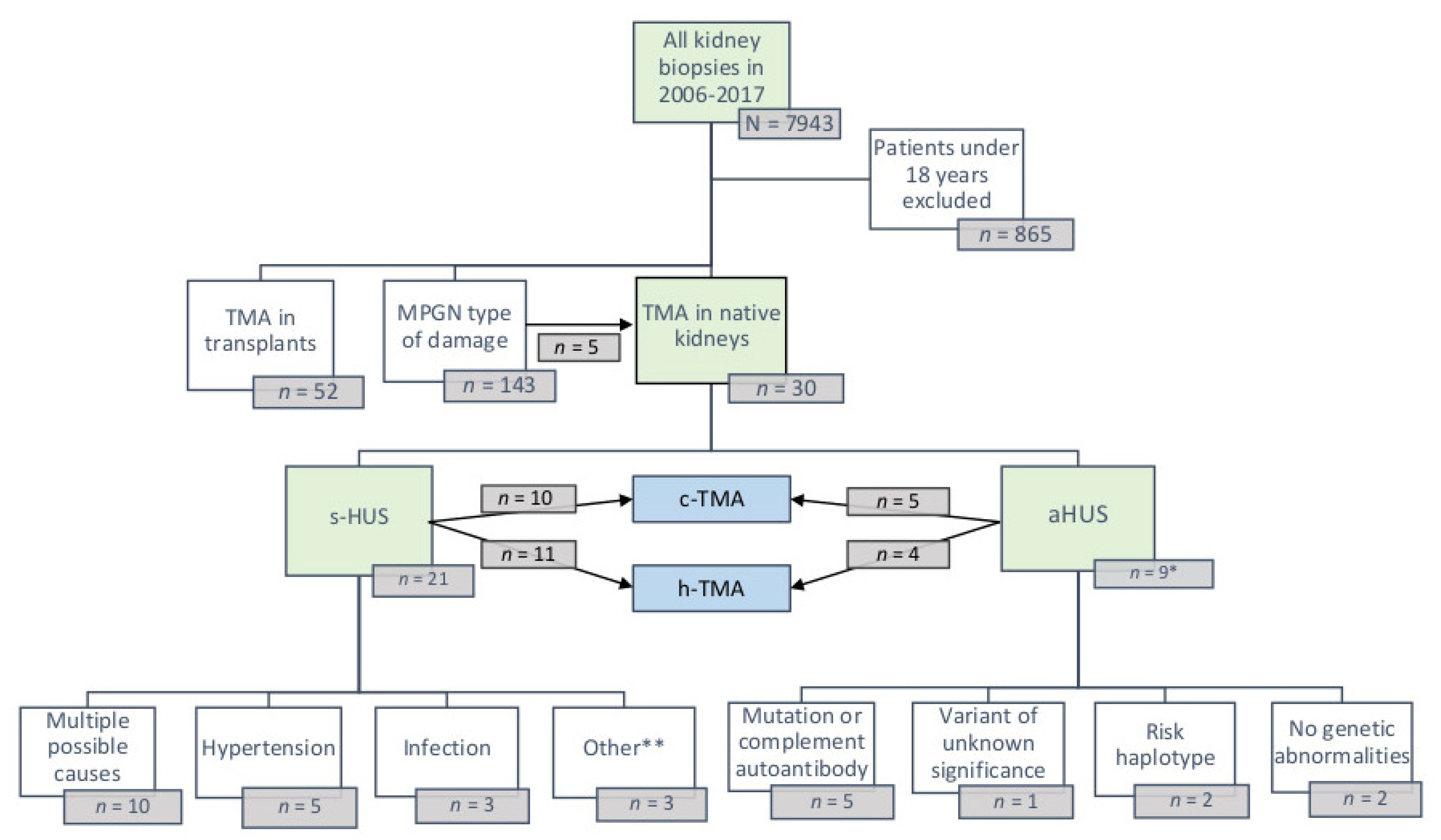
3.1. Patient Population and Clinical Findings
3.2. Predisposing and Causative Factors of Atypical and Secondary HUS
3.3. Laboratory Variables and Paraprotein Findings
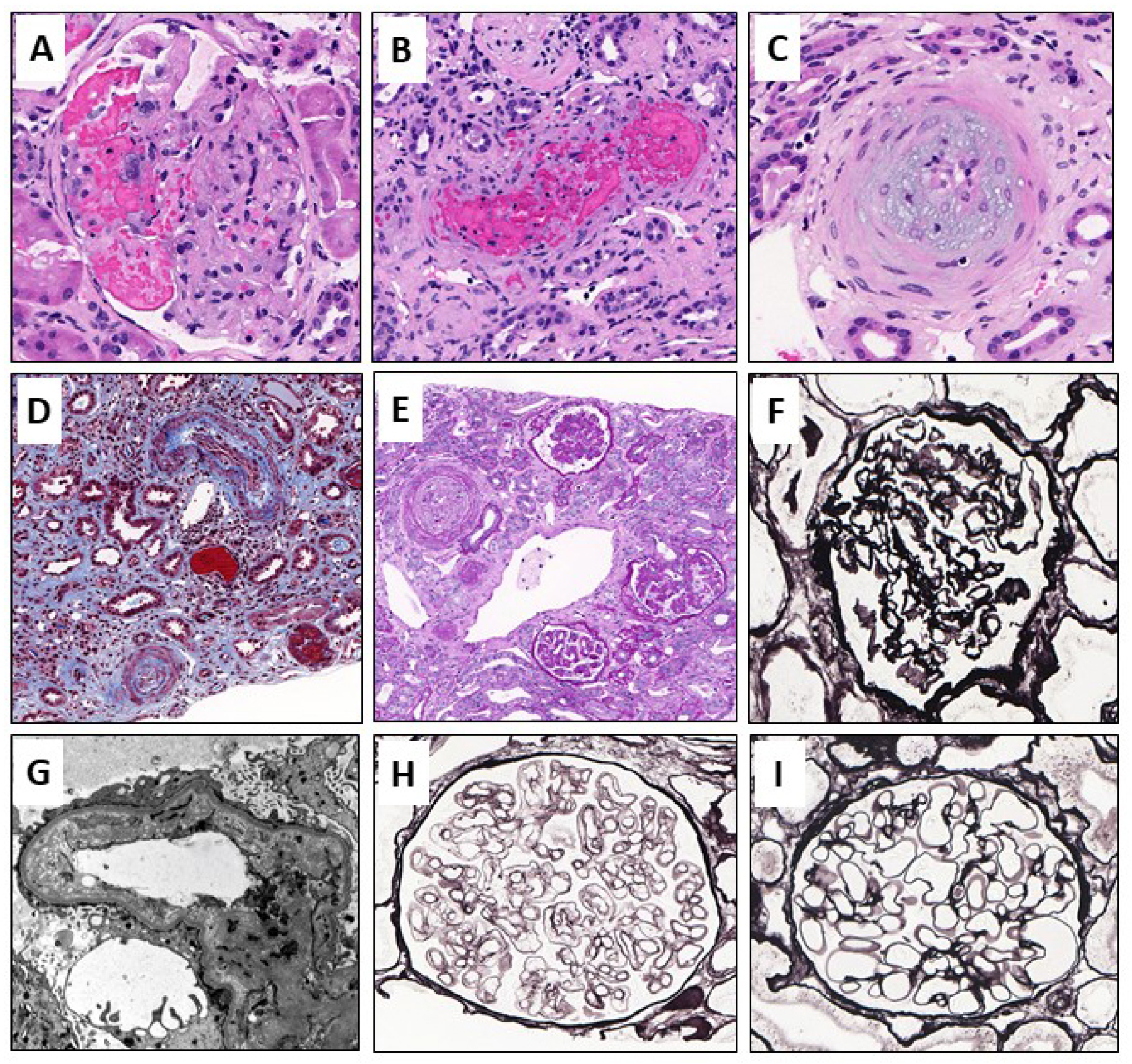
3.4. Histological Findings
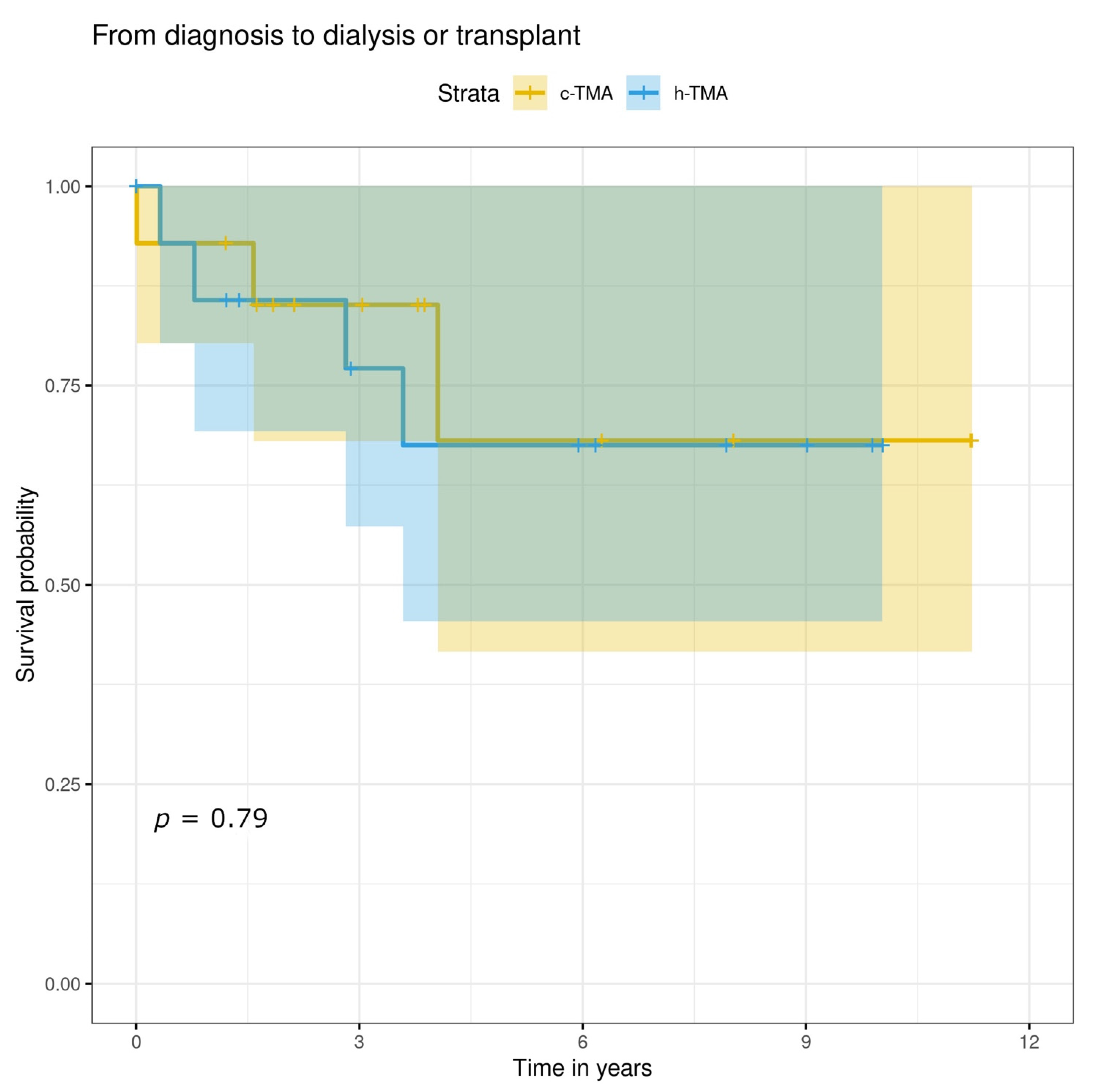
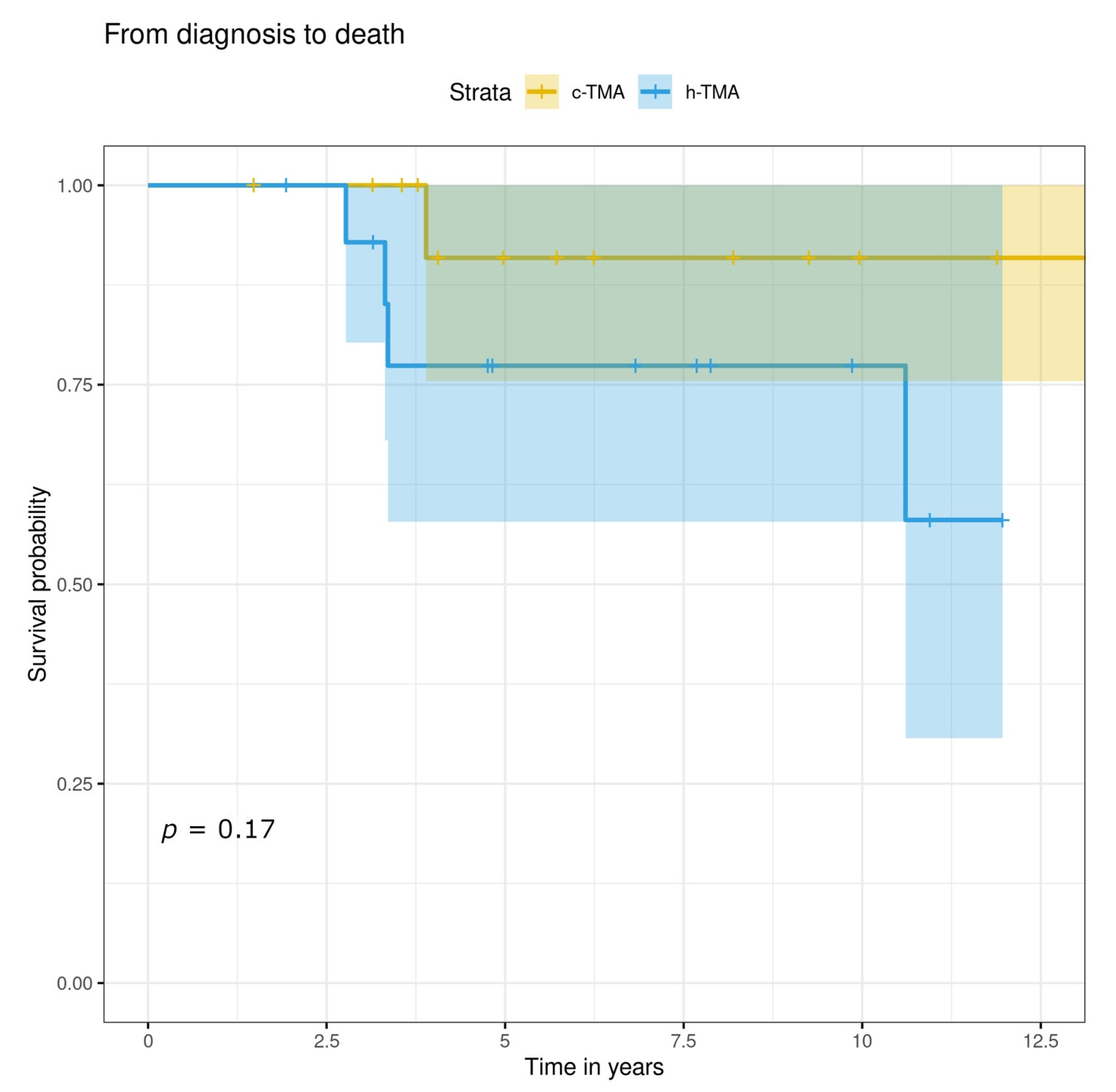
3.5. Treatment, Renal and Overall Prognosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katsuno, T.; Ito, Y.; Kagami, S.; Kitamura, H.; Maruyama, S.; Shimizu, A.; Sugiyama, H.; Sato, H.; Yokoyama, H.; Kashihara, N. A nationwide cross-sectional analysis of thrombotic microangiopathy in the Japan Renal Biopsy Registry (J-RBR). Clin. Exp. Nephrol. 2020, 24, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Goodship, T.H.; Cook, H.T.; Fakhouri, F.; Fervenza, F.C.; Fremeaux-Bacchi, V.; Kavanagh, D.; Nester, C.M.; Noris, M.; Pickering, M.C.; Rodriguez de Cordoba, S.; et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 2017, 91, 539–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Serres, S.A.; Isenring, P. Athrombocytopenic thrombotic microangiopathy, a condition that could be overlooked based on current diagnostic criteria. Nephrol. Dial. Transplant. 2008, 24, 1048–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toriu, N.; Sekine, A.; Mizuno, H.; Hasegawa, E.; Yamanouchi, M.; Hiramatsu, R.; Hayami, N.; Hoshino, J.; Kawada, M.; Suwabe, T.; et al. Renal-Limited thrombotic microangiopathy due to bevacizumab therapy for metastatic colorectal cancer: A case report. Case Rep. Oncol. 2019, 12, 391–400. [Google Scholar] [CrossRef]
- Cook, H.T.; Pickering, M.C. Histopathology of MPGN and C3 glomerulopathies. Nat. Rev. Nephrol 2015, 11, 14–22. [Google Scholar] [CrossRef]
- Ankawi, G.A.; Clark, W.F. Atypical haemolytic uremic syndrome (aHUS) and membranoproliferative glomerulonephritis (MPGN), different diseases or a spectrum of complement-mediated glomerular diseases? BMJ Case Rep. 2017, 10, bcr-2017-220974. [Google Scholar] [CrossRef]
- Kavanagh, D.; Goodship, T.H.; Richards, A. Atypical hemolytic uremic syndrome. Semin. Nephrol. 2013, 33, 508–530. [Google Scholar] [CrossRef] [Green Version]
- Sethi, S.; Fervenza, F.C. Membranoproliferative glomerulonephritis—A new look at an old entity. N. Engl. J. Med. 2012, 366, 1119–1131. [Google Scholar] [CrossRef] [Green Version]
- Colvin, R.B.; Chang, A. Diagnostic Pathology: Kidney Diseases, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Brocklebank, V.; Wood, K.M.; Kavanagh, D. Thrombotic microangiopathy and the kidney. Clin. J. Am. Soc. Nephrol. 2018, 13, 300–317. [Google Scholar] [CrossRef] [Green Version]
- Zipfel, P.F.; Skerka, C.; Chen, Q.; Wiech, T.; Goodship, T.; Johnson, S.; Fremeaux-Bacchi, V.; Nester, C.; Córdoba, S.R.d.; Noris, M.; et al. The role of complement in C3 glomerulopathy. Mol. Immunol. 2015, 67, 21–30. [Google Scholar] [CrossRef]
- Ernandes Neto, M.; Soler, L.d.M.; Gallindo Vasconcelos, H.V.; dos Santos, D.C.; Viero, R.M.; Modelli de Andrade, L.G. Histopathological features of thrombotic microangiopathies in renal biopsies. J. Nephropathol. 2019, 8, 27. [Google Scholar] [CrossRef]
- Yu, X.-J.; Yu, F.; Song, D.; Wang, S.-X.; Song, Y.; Liu, G.; Zhao, M.-H. Clinical and renal biopsy findings predicting outcome in renal thrombotic microangiopathy: A large cohort study from a single institute in China. Sci. World J. 2014, 2014, 680502. [Google Scholar] [CrossRef]
- Sreedharanunni, S.; Joshi, K.; Duggal, R.; Nada, R.; Minz, M.; Sakhuja, V. An analysis of transplant glomerulopathy and thrombotic microangiopathy in kidney transplant biopsies. Transpl. Int. 2014, 27, 784–792. [Google Scholar] [CrossRef]
- Merrill, J.T.; Erkan, D.; Winakur, J.; James, J.A. Emerging evidence of a COVID-19 thrombotic syndrome has treatment implications. Nat. Rev. Rheumatol. 2020, 16, 581–589. [Google Scholar] [CrossRef]
- Moake, J.L. Thrombotic microangiopathies. N. Engl. J. Med. 2002, 347, 589–600. [Google Scholar] [CrossRef]
- Coppo, P.; Schwarzinger, M.; Buffet, M.; Wynckel, A.; Clabault, K.; Presne, C.; Poullin, P.; Malot, S.; Vanhille, P.; Azoulay, E.; et al. Predictive features of severe acquired adamts13 deficiency in idiopathic thrombotic microangiopathies: The french tma reference center experience. PLoS ONE 2010, 5, e10208. [Google Scholar] [CrossRef]
- Bayer, G.; von Tokarski, F.; Thoreau, B.; Bauvois, A.; Barbet, C.; Cloarec, S.; Mérieau, E.; Lachot, S.; Garot, D.; Bernard, L.; et al. Etiology and outcomes of thrombotic microangiopathies. Clin. J. Am. Soc. Nephrol. 2019, 14, 557–566. [Google Scholar] [CrossRef]
- Watson, R.; Lindner, S.; Bordereau, P.; Hunze, E.-M.; Tak, F.; Ngo, S.; Zipfel, P.F.; Skerka, C.; Dragon-Durey, M.-A.; Marchbank, K.J. Standardisation of the factor H autoantibody assay. Immunobiology 2014, 219, 9–16. [Google Scholar] [CrossRef]
- Haas, M.; Seshan, S.V.; Barisoni, L.; Amann, K.; Bajema, I.M.; Becker, J.U.; Kensuke, J.; Ljubanovic, D.; Roberts, I.S.D.; Roelofs, J.J.; et al. Consensus definitions for glomerular lesions by light and electron microscopy: Recommendations from a working group of the Renal Pathology Society. Kidney Int. 2020, 98, 1120–1134. [Google Scholar] [CrossRef]
- Roufosse, C.; Simmonds, N.; Clahsen-van Groningen, M.; Haas, M.; Henriksen, K.J.; Horsfield, C.; Loupy, A.; Mengel, M.; Perkowska-Ptasińska, A.; Rabant, M.; et al. A 2018 reference guide to the banff classification of renal allograft pathology. Transplantation 2018, 102, 1795–1814. [Google Scholar] [CrossRef]
- Messias, N.C.; Walker, P.D.; Larsen, C.P. Paraffin immunofluorescence in the renal pathology laboratory: More than a salvage technique. Modern Pathology 2015, 28, 854–860. [Google Scholar] [CrossRef] [Green Version]
- Le Clech, A.; Simon-Tillaux, N.; Provôt, F.; Delmas, Y.; Vieira-Martins, P.; Limou, S.; Halimi, J.M.; Le Quintrec, M.; Lebourg, L.; Grangé, S.; et al. Atypical and secondary hemolytic uremic syndromes have a distinct presentation and no common genetic risk factors. Kidney Int. 2019, 95, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Manickam, N.; Agrawal, V.; Prasad, P.; Jain, M.; Prasad, N. Clinico-Histological features of thrombotic microangiopathy in renal biopsies: A retrospective study. Turk. Patoloji Derg. 2022, 38, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, S.; Işık Gönül, İ.; Canpolat, N.; Gökçe, İ.; Özlü, S.G.; Özçakar, Z.B.; Ozaltin, F.; Söylemezoğlu, O. Renal biopsy prognostic findings in children with atypical hemolytic uremic syndrome. Pediatr. Dev. Pathol. 2020, 23, 362–371. [Google Scholar] [CrossRef]
- Leung, N.; Bridoux, F.; Batuman, V.; Chaidos, A.; Cockwell, P.; D’Agati, V.D.; Dispenzieri, A.; Fervenza, F.C.; Fermand, J.P.; Gibbs, S.; et al. The evaluation of monoclonal gammopathy of renal significance: A consensus report of the International Kidney and Monoclonal Gammopathy Research Group. Nat. Rev. Nephrol. 2019, 15, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, A.; Go, R.S.; Fervenza, F.C.; Sethi, S. Thrombotic microangiopathy associated with monoclonal gammopathy. Kidney Int. 2017, 91, 691–698. [Google Scholar] [CrossRef] [PubMed]
| Baseline Variable | h-TMA n = 15 | c-TMA n = 15 | p-Value |
|---|---|---|---|
| Age (y, range) 1 | 56.7 (29–78) | 48.9 (24–73) | 0.144 |
| Male gender, n (%) | 8 (53.3) | 10 (66.7) | 0.71 |
| Race | |||
| Caucasian, n (%) | 15 (100) | 13 (86.7) | 0.483 |
| African, n (%) | 0 (0) | 1 (6.7) | 1.0 |
| Asian, n (%) | 0 (0) | 1 (6.7) | 1.0 |
| Estimated duration of renal findings (months) | 25.2 (0.5–228) | 2.8 (0–18) | 0.188 |
| S-creatinine µmol/L, (≤100 male, ≤90 female) 1 | 183 (49–464) | 246 (85–1065) | 0.126 |
| eGFR mL/min/1.73 m2, (>60) | 50.3 (9–117) | 28.3 (4–62) | 0.067 |
| eGFR < 60 mL/min/1.73 m2, n (%) | 10 (66.7) | 14 (93.3) | 0.169 |
| Proteinuria detected (any method), n (%) | 13/14 * (92.9) | 14/14 * (100) | 1.0 |
| Hematuria detected (any method), n (%) | 10/14 * (71.4) | 9/14 * (64.3) | 1.0 |
| Family history of | |||
| HUS or thrombotic microangiopathy, n (%) | 0 (0) | 1 (6.7) | 1.0 |
| kidney disease of unknown origin, n (%) | 1 (6.7) | 3 (20) | 0.598 |
| Presented with extrarenal manifestation, n (%) 2 | 2 (13.3) | 5 (33.3) | 0.39 |
| Biopsy indication, n (%) | |||
| Acute kidney injury and proteinuria, n (%) | 1 (6.7) | 4 (26.7) | 0.33 |
| Acute kidney injury, proteinuria and hematuria, n (%) | 9 (60.0) | 9 (60.0) | 1.0 |
| Kidney failure and proteinuria, n (%) | 2 (13.3) | 0 (0) | 0.483 |
| Hematuria and proteinuria, n (%) | 2 (13.3) | 0 (0) | 0.483 |
| Other, n (%) 3 | 1 (6.7) | 2 (13.3) | 1.0 |
| Clinical diagnosis by study investigators | |||
| aHUS, n (%) | 5 (33.3) | 4 (26.7) | 1.0 |
| secondary HUS, n (%) | 10 (66.7) | 11 (73.3) | 1.0 |
| Clinical diagnosis by a treating physician | |||
| aHUS, n (%) | 2 (13.3) | 4 (26.7) | 0.651 |
| secondary HUS, n (%) | 5 (33.3) | 7 (46.7) | 0.71 |
| TMA not otherwise specified, n (%) | 2 (13.3) | 1 (6.7) | 1.0 |
| Other, n (%) 4 | 6 (40.0) | 3 (20.0) | 0.427 |
| Variable | h-TMA n = 15 | c-TMA n = 15 |
|---|---|---|
| Predisposing factors identified in aHUS | ||
| History of recent surgery, n (%) | 0 (0) | 1 (6.7) |
| Histology of recent infection, n (%) | 0 (0) | 2 (13.3) |
| Multiple factors, n (%) 1 | 0 (0) | 1 (6.7) |
| Secondary HUS caused by | ||
| hypertension, n (%) | 2 (13.3) | 3 (20) |
| History of autoimmune disease, n (%) | 1 (6.7) | 0 (0) |
| Other, n (%) 2 | 1 (6.7) | 4 (26.7) |
| Multiple conditions present, n (%) 3 | 6 (40) | 4 (26.7) |
| Hemolytic Laboratory Variables at Onset (Reference) | h-TMA n = 15 | c-TMA n = 15 | p-Value |
|---|---|---|---|
| Hemoglobin (134–167 M, 117–155 F, g/L) | 119.5 (±14) | 103.5 (±17.6) | 0.01 |
| Platelets (150–360 E9/L) 1 | 171 (111–503) | 187 (72–353) | 0.852 |
| LDH (115–235 U/L) | 206.9 (±40.4) (n = 7 2) | 348.4 (±156.2) | 0.004 |
| Haptoglobin (0.29–2 g/L) 1 | 1.4 (0.68–1.91) (n = 8 2) | 0.6 (0–3.42) | 0.129 |
| E-Schistocytes (%) (<1) | 0.1 (±0.2) (n = 6 2) | 2 (±1.5) (n = 11 2) | 0.002 |
| Baseline Variable (Reference) | h-TMA n = 15 | c-TMA n = 15 |
|---|---|---|
| Complement proteins | ||
| S-C3 (0.5–1.5 g/L) | 1.0 (±0.3) (n = 11 *) | 1.0 (±0.3) (n = 14 *) |
| Below normal, n (%) | 0/11 * (0) | 1/14 * (7.1) |
| S-C4 (0.12–0.42 g/L) | 0.2 (±0.1) (n = 11 *) | 0.2 (±0.1) (n = 14 *) |
| Below normal, n (%) | 1/11 * (9.1) | 2/14 * (14.3) |
| Functional complement analyses | ||
| S-CH100Al (>39%) | 97.7 (±48.1) (n = 3 *) | 101 (±18.5) (n = 9 *) |
| Below normal, n (%) | 0/3 * (0) | 0/9 * (0) |
| S-CH100C1 (>74%) | 104.3 (±29.9) (n = 3 *) | 96 (±22.5) (n = 9 *) |
| Below normal, n (%) | 1/3 * (33.3) | 2/9 * (22.2) |
| S-CH100L (>10%) | 109.7 (±94.9) (n = 3 *) | 69 (±53.7) (n = 9 *) |
| Below, n (%) | 1/3 * (33.3) | 2/9 * (22.2) |
| Complement autoantibodies and mutations | ||
| C3nef positivity, n (%) | 0/3 * (0) | 3/6 * (50) |
| Factor H antibody positivity, n (%) | 1/3 * (33.3) | 0/5 * (0) |
| No mutation found, n (%) | 2/3 * (66.7) | 2/5 * (40) |
| Disease causing mutation found, n (%) | 0 (0) | 1/5 (20) |
| Only risk haplotype found, n (%) | 1/3 * (33.3) | 0 (0) |
| Mutation + only risk haplotype found, n (%) | 0 (0) | 1/5 * (20) |
| Mutation + variant of unknown significance, n (%) | 0 (0) | 1/5 * (20) |
| Paraprotein finding | ||
| Only in blood, n (%) | 1/4 * (25) | 2/3 * (66.7) |
| Only in urine, n (%) | 0/4 * (0) | 0/3 * (0) |
| Both in blood and urine, n (%) | 2/4 * (50) | 0/3 * (0) |
| Type of paraprotein in serum | ||
| IgG kappa, n (%) | 1/3 * (33.3) | 1/2 * (50) |
| IgG lambda, n (%) | 1/3 * (33.3) | 1/2 * (50) |
| Multiple types, n (%) | 1/3 * (33.3) | 0/2 * (0) |
| Hematologic disease when paraprotein observed | ||
| MGRS or MGUS, n (%) | 2/3 * (66.7) | 2/2 * (100) |
| Overt malignancy, n (%) | 1/3 * (33.3) | 0/2 * (0) |
| Variable | h-TMA n = 15 | c-TMA n = 15 | p-Value |
|---|---|---|---|
| Glomerular changes | |||
| Capillary wrinkling, n (%) | 8/14 1 (57.1) | 11 (73.3) | 0.450 |
| Mesangiolysis, n (%) | 4 (26.7) | 2 (13.3) | 0.651 |
| Microaneurysm, n (%) | 1 (6.7) | 1 (6.7) | 1.000 |
| Thrombi, n (%) | 4 (26.7) | 4 (26.7) | 0.651 |
| Biopsies with crescents, n (%) | 3 (20) | 1 (6.7) | 0.598 |
| Sclerotic glomeruli, n (%) (SD) | 19.5 (±21.6) | 13.6 (±15.8) | 0.408 |
| Mesangial matrix expansion | |||
| None, n (%) | 5 (33.3) | 9 (60) | 0.272 |
| Mild, n (%) | 6 (40) | 3 (20) | 0.427 |
| Moderate, n (%) | 1 (6.7) | 0 (0) | 1.000 |
| Severe, n (%) | 3 (20) | 3 (20) | 1.000 |
| Double basement membrane | |||
| None, n (%) | 3 (20) | 5 (33.3) | 0.682 |
| Mild, n (%) | 5 (33.3) | 1 (6.7) | 0.169 |
| Moderate, n (%) | 2 (13.3) | 4 (26.7) | 0.651 |
| Severe, n (%) | 5 (33.3) | 5 (33.3) | 1.000 |
| Lobulated glomeruli, n (%) | 6 (40) | 2 (13.3) | 0.215 |
| Tubulointerstitial changes | |||
| Interstitial fibrosis | |||
| None, n (%) | 6 (40) | 11 (73.3) | 0.139 |
| Mild, n (%) | 6 (40) | 3 (20) | 0.427 |
| Moderate, n (%) | 2 (13.3) | 1 (6.7) | 1.000 |
| Severe, n (%) | 1 (6.7) | 0 (0) | 1.000 |
| Tubular atrophy | |||
| None, n (%) | 6 (40) | 4 (26.7) | 0.700 |
| Mild, n (%) | 7 (46.7) | 9 (60) | 0.715 |
| Moderate, n (%) | 0 (0) | 2 (13.3) | 0.483 |
| Severe, n (%) | 2 (13.3) | 0 (0) | 0.483 |
| Total interstitial inflammation | |||
| None, n (%) | 7 (46.7) | 7 (46.7) | 1.000 |
| Mild, n (%) | 5 (33.3) | 7 (46.7) | 0.710 |
| Moderate, n (%) | 3 (20) | 1 (6.7) | 0.598 |
| Severe, n (%) | 0 (0) | 0 (0) | 1.000 |
| Vascular changes | |||
| Myxoid swelling, n (%) | 1 (6.7) | 9 (60) | 0.005 |
| Intramural fibrin, n (%) | 0 (0) | 3 (20) | 0.224 |
| Thrombi, n (%) | 0 (0) | 3 (20) | 0.224 |
| Onion-skinning, n (%) | 0 (0) | 6 (40) | 0.017 |
| Arterial sclerosis | |||
| None, n (%) | 9 (60) | 3/14 1 (21.4) | 0.060 |
| Mild, n (%) | 3 (20) | 7/14 1 (50) | 0.128 |
| Moderate, n (%) | 3 (20) | 2/14 1 (14.3) | 1.000 |
| Severe, n (%) | 0 (0) | 2/14 1 (14.3) | 0.224 |
| Electron microscopy changes | |||
| Glomerular subendothelial flocculent material, n (%) | 9/11 1 (81.8) | 12/13 1 (92.3) | 0.576 |
| Wrinkled glomerular basement membrane, n (%) | 5/12 1 (41.7) | 7/13 1 (53.8) | 0.695 |
| Loss of endothelial fenestration, n (%) | 8/11 1 (72.7) | 13/13 1 (100) | 0.082 |
| Degree of podocyte effacement,% (SD) | 21.4 (±17.0) (n = 11 1) | 32.7 (±33.1), (n = 13 1) | 0.295 |
| Doubled glomerular basement membrane, n (%) | 7/11 1 (63.6) | 9/13 1 (69.2) | 1.000 |
| Thin basement membrane (<264 nm), n (%) | 2/10 1 (20.0) | 2/13 1 (15.4) | 1.000 |
| Variable | h-TMA n = 15 | c-TMA n = 15 |
|---|---|---|
| Mean follow-up time since biopsy (years) (range) | 6.9 (2.8–12) | 5.6 (1.5–13) |
| On chronic dialysis, n (%) | 4 (26.7) | 5 (33.3) |
| Kidney transplantation, n (%) | 2 (13.3) | 1 (6.7) |
| Kidney failure * not necessitating chronic dialysis or transplant, n (%) | 6 (40.0) | 6 (40.0) |
| Death during follow-up, n (%) | 4 (26.7) | 1 (6.7) |
| Multiple outbreaks during follow-up, n (%) | 1 (6.7) | 2 (13.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovala, M.; Seppälä, M.; Kaartinen, K.; Meri, S.; Honkanen, E.; Räisänen-Sokolowski, A. Vascular Occlusion in Kidney Biopsy Is Characteristic of Clinically Manifesting Thrombotic Microangiopathy. J. Clin. Med. 2022, 11, 3124. https://doi.org/10.3390/jcm11113124
Kovala M, Seppälä M, Kaartinen K, Meri S, Honkanen E, Räisänen-Sokolowski A. Vascular Occlusion in Kidney Biopsy Is Characteristic of Clinically Manifesting Thrombotic Microangiopathy. Journal of Clinical Medicine. 2022; 11(11):3124. https://doi.org/10.3390/jcm11113124
Chicago/Turabian StyleKovala, Marja, Minna Seppälä, Kati Kaartinen, Seppo Meri, Eero Honkanen, and Anne Räisänen-Sokolowski. 2022. "Vascular Occlusion in Kidney Biopsy Is Characteristic of Clinically Manifesting Thrombotic Microangiopathy" Journal of Clinical Medicine 11, no. 11: 3124. https://doi.org/10.3390/jcm11113124
APA StyleKovala, M., Seppälä, M., Kaartinen, K., Meri, S., Honkanen, E., & Räisänen-Sokolowski, A. (2022). Vascular Occlusion in Kidney Biopsy Is Characteristic of Clinically Manifesting Thrombotic Microangiopathy. Journal of Clinical Medicine, 11(11), 3124. https://doi.org/10.3390/jcm11113124








