A Case-Control Study on the Changes in Natural Killer Cell Activity following Administration of Polyvalent Mechanical Bacterial Lysate in Korean Adults with Recurrent Respiratory Tract Infection
Abstract
:1. Introduction
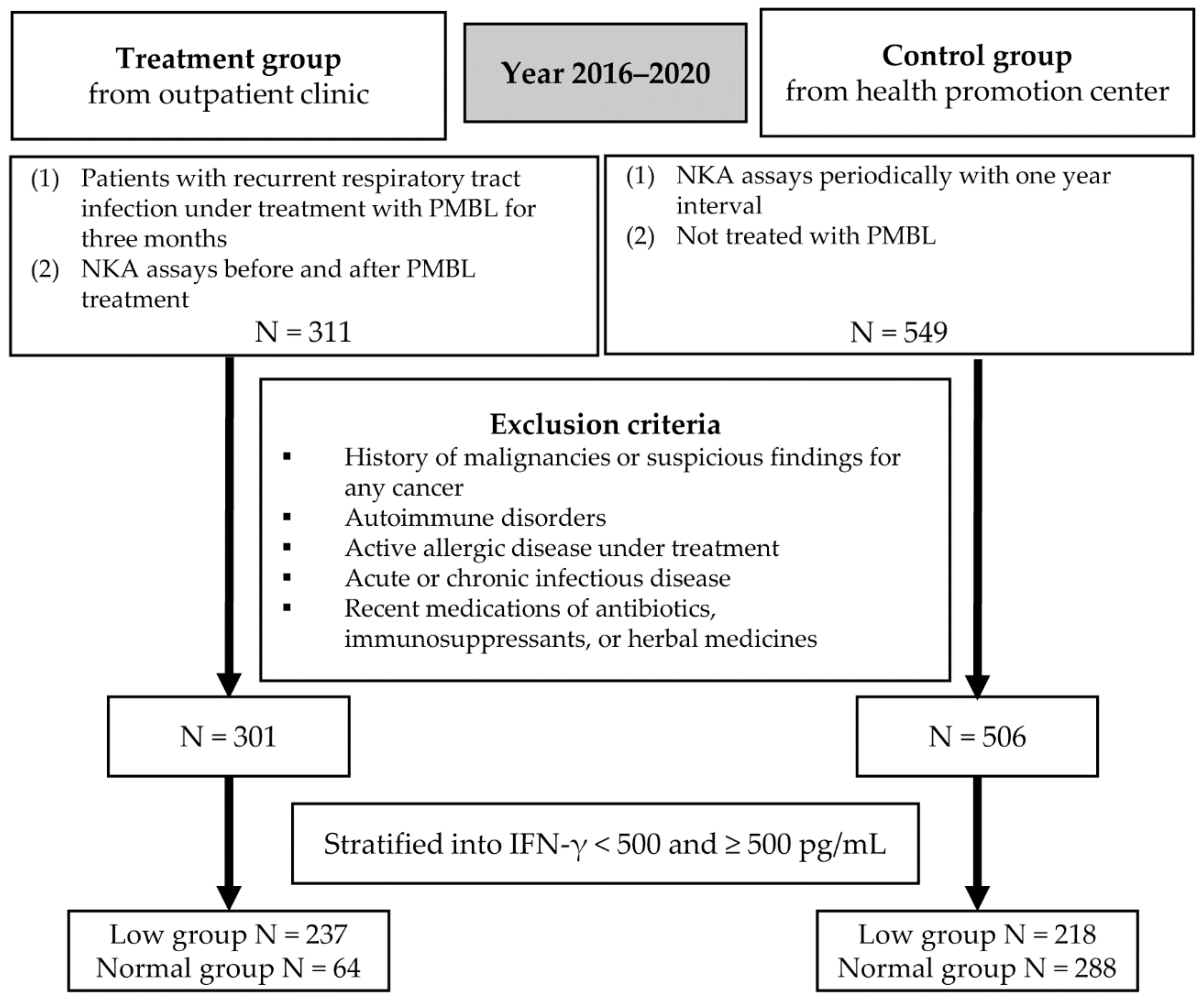
2. Subjects and Methods
2.1. Study Subjects
2.2. PMBL
2.3. Medical History, Measurements, and Blood Sampling
2.4. IFN-γ Measurement for NKA
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Subjects
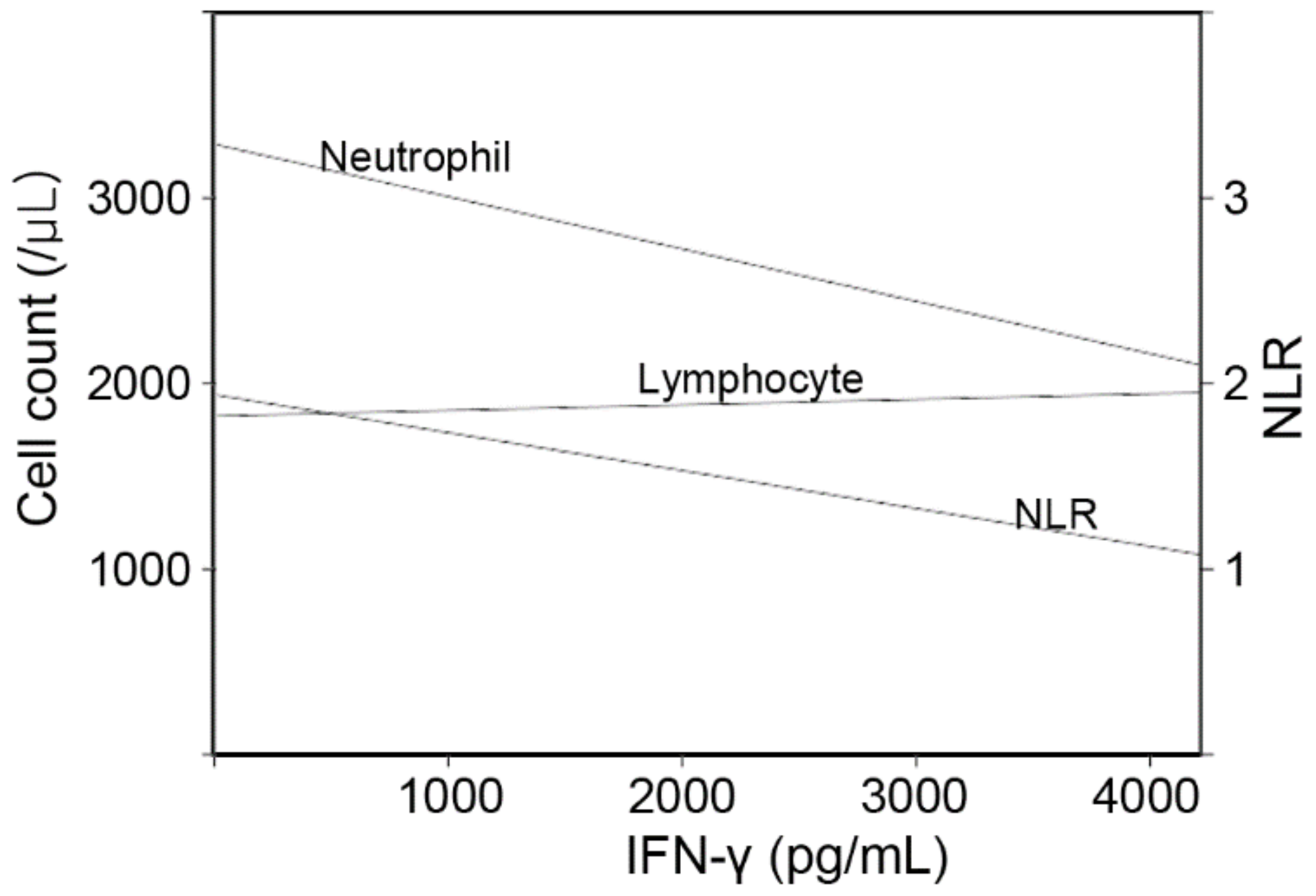
3.2. Factors That Influence Baseline IFN-γ Levels in the Whole Subjects
3.3. Changes in NKA from Baseline to Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bryceson, Y.T.; Chiang, S.C.; Darmanin, S.; Fauriat, C.; Schlums, H.; Theorell, J.; Wood, S.M. Molecular mechanisms of natural killer cell activation. J. Innate Immun. 2011, 3, 216–226. [Google Scholar] [CrossRef]
- Mody, C.H.; Ogbomo, H.; Xiang, R.F.; Kyei, S.K.; Feehan, D.; Islam, A.; Li, S.S. Microbial killing by NK cells. J. Leukoc. Biol. 2019, 105, 1285–1296. [Google Scholar] [CrossRef]
- Culley, F.J. Natural killer cells in infection and inflammation of the lung. Immunology 2009, 128, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.E.; Marranci, S.; De Marco, A.; Lega, L.; Minutello, M.A.; Azzari, C.; Resti, M.; Vierucci, A. Reduced natural killer function in children with recurrent respiratory tract infections. Pediatr. Med. Chir. 1993, 15, 1–4. [Google Scholar] [PubMed]
- Van Eeden, C.; Khan, L.; Osman, M.S.; Cohen Tervaert, J.W. Natural Killer Cell Dysfunction and Its Role in COVID-19. Int. J. Mol. Sci. 2020, 21, 6351. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.; Faridi, R.M.; Sligl, W.; Shabani-Rad, M.T.; Dharmani-Khan, P.; Parker, A.; Kalra, A.; Tripathi, M.B.; Storek, J.; Cohen Tervaert, J.W.; et al. Impaired natural killer cell counts and cytolytic activity in patients with severe COVID-19. Blood Adv. 2020, 4, 5035–5039. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Anapurapu, S.; Page, C.P. Polyvalent mechanical bacterial lysate for the prevention of recurrent respiratory infections: A meta-analysis. Pulm. Pharmacol. Ther. 2012, 25, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Noschese, P.; Di Perna, F. Value of adding a polyvalent mechanical bacterial lysate to therapy of COPD patients under regular treatment with salmeterol/fluticasone. Ther. Adv. Respir. Dis. 2009, 3, 59–63. [Google Scholar] [CrossRef]
- Suárez, N.; Ferrara, F.; Rial, A.; Dee, V.; Chabalgoity, J.A. Bacterial lysates as immunotherapies for respiratory infections: Methods of preparation. Front. Bioeng. Biotechnol. 2020, 8, 545. [Google Scholar] [CrossRef]
- Lanzilli, G.; Traggiai, E.; Braido, F.; Garelli, V.; Folli, C.; Chiappori, A.; Riccio, A.M.; Bazurro, G.; Agazzi, A.; Magnani, A.; et al. Administration of a polyvalent mechanical bacterial lysate to elderly patients with COPD: Effects on circulating T, B and NK cells. Immunol. Lett. 2013, 149, 62–67. [Google Scholar] [CrossRef]
- Lanzilli, G.; Falchetti, R.; Cottarelli, A.; Macchi, A.; Ungheri, D.; Fuggetta, M.P. In vivo effect of an immunostimulating bacterial lysate on human B lymphocytes. Int. J. Immunopathol. Pharmacol. 2006, 19, 551–559. [Google Scholar] [CrossRef] [Green Version]
- Lanzilli, G.; Falchetti, R.; Tricarico, M.; Ungheri, D.; Fuggetta, M.P. In vitro effects of an immunostimulating bacterial lysate on human lymphocyte function. Int. J. Immunopathol. Pharmacol. 2005, 18, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Jurkiewicz, D.; Zielnik-Jurkiewicz, B. Bacterial lysates in the prevention of respiratory tract infections. Otolaryngol. Pol. 2018, 72, 1–8. [Google Scholar] [CrossRef]
- Nogusa, S.; Ritz, B.W.; Kassim, S.H.; Jennings, S.R.; Gardner, E.M. Characterization of age-related changes in natural killer cells during primary influenza infection in mice. Mech. Ageing Dev. 2008, 129, 223–230. [Google Scholar] [CrossRef]
- Guo, H.; Kumar, P.; Moran, T.M.; Garcia-Sastre, A.; Zhou, Y.; Malarkannan, S. The functional impairment of natural killer cells during influenza virus infection. Immunol. Cell Biol. 2009, 87, 579–589. [Google Scholar] [CrossRef]
- Du, N.; Zhou, J.; Lin, X.; Zhang, Y.; Yang, X.; Wang, Y.; Shu, Y. Differential activation of NK cells by influenza A pseudotype H5N1 and 1918 and 2009 pandemic H1N1 viruses. J. Virol. 2010, 84, 7822–7831. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.B.; Cha, J.; Kim, I.K.; Yoon, J.C.; Lee, H.J.; Park, S.W.; Cho, S.; Youn, D.Y.; Lee, H.; Lee, C.H.; et al. A high-throughput assay of NK cell activity in whole blood and its clinical application. Biochem. Biophys. Res. Commun. 2014, 445, 584–590. [Google Scholar] [CrossRef]
- Oh, S.; Chun, S.; Hwang, S.; Kim, J.; Cho, Y.; Lee, J.; Kwack, K.; Choi, S.W. Vitamin D and Exercise Are Major Determinants of Natural Killer Cell Activity, Which Is Age- and Gender-Specific. Front. Immunol. 2021, 12, 594356. [Google Scholar] [CrossRef]
- Lee, Y.K.; Haam, J.H.; Cho, S.H.; Kim, Y.S. Cross-Sectional and Time-Dependent Analyses on Inflammatory Markers following Natural Killer Cell Activity. Diagnostics 2022, 12, 448. [Google Scholar] [CrossRef]
- Koo, K.C.; Shim, D.H.; Yang, C.M.; Lee, S.B.; Kim, S.M.; Shin, T.Y.; Kim, K.H.; Yoon, H.G.; Rha, K.H.; Lee, J.M.; et al. Reduction of the CD16− CD56bright NK cell subset precedes NK cell dysfunction in prostate cancer. PLoS ONE 2013, 8, e78049. [Google Scholar] [CrossRef] [Green Version]
- Nederby, L.; Jakobsen, A.; Hokland, M.; Hansen, T.F. Quantification of NK cell activity using whole blood: Methodological aspects of a new test. J. Immunol. Methods 2018, 458, 21–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Park, K.H.; Ryu, J.H.; Bae, H.J.; Choi, A.; Lee, H.; Lim, J.; Han, K.; Park, C.H.; Jung, E.S.; et al. Natural killer cell activity for IFN-gamma production as a supportive diagnostic marker for gastric cancer. Oncotarget 2017, 8, 70431. [Google Scholar] [CrossRef]
- Braido, F.; Schenone, G.; Pallestrini, E.; Reggiardo, G.; Cangemi, G.; Canonica, G.W.; Melioli, G. The relationship between mucosal immunoresponse and clinical outcome in patients with recurrent upper respiratory tract infections treated with a mechanical bacterial lysate. J. Biol. Regul. Homeost. Agents 2011, 25, 477–485. [Google Scholar] [PubMed]
- Morandi, B.; Agazzi, A.; D’Agostino, A.; Antonini, F.; Costa, G.; Sabatini, F.; Ferlazzo, G.; Melioli, G. A mixture of bacterial mechanical lysates is more efficient than single strain lysate and of bacterial-derived soluble products for the induction of an activating phenotype in human dendritic cells. Immunol. Lett. 2011, 138, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Banche, G.; Allizond, V.; Mandras, N.; Garzaro, M.; Cavallo, G.P.; Baldi, C.; Scutera, S.; Musso, T.; Roana, J.; Tullio, V.; et al. Improvement of clinical response in allergic rhinitis patients treated with an oral immunostimulating bacterial lysate: In vivo immunological effects. Int. J. Immunopathol. Pharmacol. 2007, 20, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Janeczek, K.; Emeryk, A.; Rachel, M.; Duma, D.; Zimmer, L.; Poleszak, E. Polyvalent Mechanical Bacterial Lysate Administration Improves the Clinical Course of Grass Pollen-Induced Allergic Rhinitis in Children: A Randomized Controlled Trial. J. Allergy Clin. Immunol. Pract. 2021, 9, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Bartkowiak-Emeryk, M.; Emeryk, A.; Rolinski, J.; Wawryk-Gawda, E.; Markut-Miotla, E. Impact of Polyvalent Mechanical Bacterial Lysate on lymphocyte number and activity in asthmatic children: A randomized controlled trial. Allergy Asthma Clin. Immunol. 2021, 17, 10. [Google Scholar] [CrossRef]
- Freeman, B.E.; Raue, H.P.; Hill, A.B.; Slifka, M.K. Cytokine-Mediated Activation of NK Cells during Viral Infection. J. Virol. 2015, 89, 7922–7931. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. How Do Vaccines Work? Available online: https://www.who.int/news-room/feature-stories/detail/how-do-vaccines-work (accessed on 27 March 2022).
- Schapiro, J.M.; Segev, Y.; Rannon, L.; Alkan, M.; Rager-Zisman, B. Natural killer (NK) cell response after vaccination of volunteers with killed influenza vaccine. J. Med. Virol. 1990, 30, 196–200. [Google Scholar] [CrossRef]
- Wagstaffe, H.R.; Susannini, G.; Thiebaut, R.; Richert, L.; Levy, Y.; Bockstal, V.; Stoop, J.N.; Luhn, K.; Douoguih, M.; Riley, E.M.; et al. Durable natural killer cell responses after heterologous two-dose Ebola vaccination. NPJ Vaccines 2021, 6, 19. [Google Scholar] [CrossRef]
- Coviello, S.; Wimmenauer, V.; Polack, F.P.; Irusta, P.M. Bacterial lysates improve the protective antibody response against respiratory viruses through Toll-like receptor 4. Hum. Vaccines Immunother. 2014, 10, 2896–2902. [Google Scholar] [CrossRef]
- Dons’koi, B.V.; Chernyshov, V.P.; Osypchuk, D.V. Measurement of NK activity in whole blood by the CD69 up-regulation after co-incubation with K562, comparison with NK cytotoxicity assays and CD107a degranulation assay. J. Immunol. Methods 2011, 372, 187–195. [Google Scholar] [CrossRef]

| Whole Subjects | Baseline IFN-γ < 500 pg/mL | Baseline IFN-γ ≥ 500 pg/mL | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment Group | Control Group | p | Treatment Group | Control Group | p | Treatment Group | Control Group | p | |
| (N = 301) | (N = 506) | (N = 237) | (N = 218) | (N = 64) | (N = 288) | ||||
| Age (years) | 55.0 ± 13.6 | 53.3 ± 10.6 | 0.052 | 53.7 ± 13.6 | 52.4 ± 10.2 | 0.248 | 60.0 ± 12.3 | 54.1 ± 10.8 | <0.001 |
| Sex (men) | 118 (39.2%) | 255 (50.4%) | 0.003 | 89 (37.6%) | 112 (51.4%) | 0.004 | 29 (45.3%) | 143 (49.7%) | 0.624 |
| Hypertension | 40 (13.3%) | 95 (18.8%) | 0.055 | 29 (12.2%) | 43 (19.7%) | 0.040 | 11 (17.2%) | 52 (18.1%) | 1.000 |
| Dyslipidemia | 73 (24.3%) | 121 (23.9%) | 0.981 | 57 (24.1%) | 44 (20.2%) | 0.380 | 16 (25.0%) | 77 (26.7%) | 0.898 |
| Diabetes | 22 (7.3%) | 25 (4.9%) | 0.217 | 17 (7.2%) | 9 (4.1%) | 0.232 | 5 (7.8%) | 16 (5.6%) | 0.691 |
| Body mass index (kg/m2) | 22.7 ± 3.1 | 23.5 ± 3.3 | 0.002 | 22.7 ± 3.2 | 23.5 ± 3.4 | 0.012 | 22.9 ± 2.7 | 23.4 ± 3.2 | 0.183 |
| Glucose (mg/dL) | 92.2 ± 15.4 | 91.5 ± 19.3 | 0.570 | 91.7 ± 15.8 | 91.1 ± 20.4 | 0.697 | 93.9 ± 13.8 | 91.8 ± 18.4 | 0.397 |
| WBC (/µL) | 5621 ± 1464 | 5386 ± 1444 | 0.027 | 5627 ± 1433 | 5676 ± 1587 | 0.728 | 5601 ± 1587 | 5167 ± 1286 | 0.020 |
| Neutrophil (/µL) | 3205 ± 1173 | 2989 ± 1092 | 0.010 | 3222 ± 1166 | 3290 ± 1219 | 0.540 | 3145 ± 1206 | 2761 ± 923 | 0.005 |
| Proportion (%) | 56.1 ± 8.4 | 54.8 ± 8.7 | 0.027 | 56.4 ± 8.5 | 57.3 ± 8.9 | 0.291 | 55.2 ± 8.0 | 52.9 ± 8.1 | 0.039 |
| Lymphocyte (/µL) | 1845 ± 533 | 1849 ± 563 | 0.919 | 1837 ± 528 | 1834 ± 610 | 0.953 | 1875 ± 557 | 1861 ± 525 | 0.843 |
| Proportion (%) | 33.6 ± 8.1 | 35.0 ± 8.0 | 0.018 | 33.4 ± 8.1 | 32.9 ± 8.1 | 0.504 | 34.3 ± 8.2 | 36.6 ± 7.6 | 0.039 |
| NLR | 1.85 ± 0.81 | 1.74 ± 0.84 | 0.055 | 1.87 ± 0.80 | 1.97 ± 1.04 | 0.242 | 1.79 ± 0.86 | 1.56 ± 0.60 | 0.043 |
| NKA (pg/mL) | 463 ± 662 | 962 ± 942 | <0.001 | 213 ± 139 | 235 ± 135 | 0.096 | 1387 ± 955 | 1512 ± 917 | 0.329 |
| Beta (SE) | p | |
|---|---|---|
| Model 1 | ||
| Age | 0.085 (0.034) | 0.013 |
| Neutrophil | −0.234 (0.035) | <0.001 |
| Lymphocyte | 0.102 (0.035) | 0.004 |
| Model 2 | ||
| Age | 0.094 (0.034) | 0.006 |
| NLR | −0.215 (0.034) | <0.001 |
| Treatment Group | Control Group | p | |
|---|---|---|---|
| Whole subjects | 287 ± 822 | 58 ± 809 | <0.001 |
| Low NKA | 384 ± 638 | 283 ± 615 | 0.087 |
| Normal NKA | −72 ± 1237 | −112 ± 893 | 0.809 |
| Whole Subjects | Baseline IFN-γ < 500 pg/mL | Baseline IFN-γ ≥ 500 pg/mL | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficients | SE | p | Coefficients | SE | p | Coefficients | SE | p | |
| Baseline to follow-up | 0.533 | 0.084 | <0.001 | 1.030 | 0.075 | <0.001 | −0.024 | 0.124 | 0.848 |
| Treatment group (vs. control) | −0.245 | 0.083 | 0.003 | 0.137 | 0.111 | 0.218 | 0.008 | 0.125 | 0.948 |
| Interaction between visit and group | <0.001 | 0.030 | 0.618 | ||||||
| Age (years) | 0.010 | 0.003 | 0.002 | 0.001 | 0.003 | 0.738 | −0.005 | 0.003 | 0.128 |
| NLR | −0.247 | 0.043 | <0.001 | −0.049 | 0.041 | 0.230 | 0.232 | 0.051 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.K.; Haam, J.-H.; Suh, E.; Cho, S.H.; Kim, Y.-S. A Case-Control Study on the Changes in Natural Killer Cell Activity following Administration of Polyvalent Mechanical Bacterial Lysate in Korean Adults with Recurrent Respiratory Tract Infection. J. Clin. Med. 2022, 11, 3014. https://doi.org/10.3390/jcm11113014
Lee YK, Haam J-H, Suh E, Cho SH, Kim Y-S. A Case-Control Study on the Changes in Natural Killer Cell Activity following Administration of Polyvalent Mechanical Bacterial Lysate in Korean Adults with Recurrent Respiratory Tract Infection. Journal of Clinical Medicine. 2022; 11(11):3014. https://doi.org/10.3390/jcm11113014
Chicago/Turabian StyleLee, Yun Kyong, Ji-Hee Haam, Eunkyung Suh, Sung Hoon Cho, and Young-Sang Kim. 2022. "A Case-Control Study on the Changes in Natural Killer Cell Activity following Administration of Polyvalent Mechanical Bacterial Lysate in Korean Adults with Recurrent Respiratory Tract Infection" Journal of Clinical Medicine 11, no. 11: 3014. https://doi.org/10.3390/jcm11113014
APA StyleLee, Y. K., Haam, J.-H., Suh, E., Cho, S. H., & Kim, Y.-S. (2022). A Case-Control Study on the Changes in Natural Killer Cell Activity following Administration of Polyvalent Mechanical Bacterial Lysate in Korean Adults with Recurrent Respiratory Tract Infection. Journal of Clinical Medicine, 11(11), 3014. https://doi.org/10.3390/jcm11113014






