Minimized Extracorporeal Circulation Is Associated with Reduced Plasma Levels of Free-Circulating Mitochondrial DNA Compared to Conventional Cardiopulmonary Bypass: A Secondary Analysis of an Exploratory, Prospective, Interventional Study
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Management of Cardiopulmonary Bypass
2.3. Sample Processing
2.4. Quantitative Polymerase Chain Reaction
2.5. Statistical Analysis
3. Results
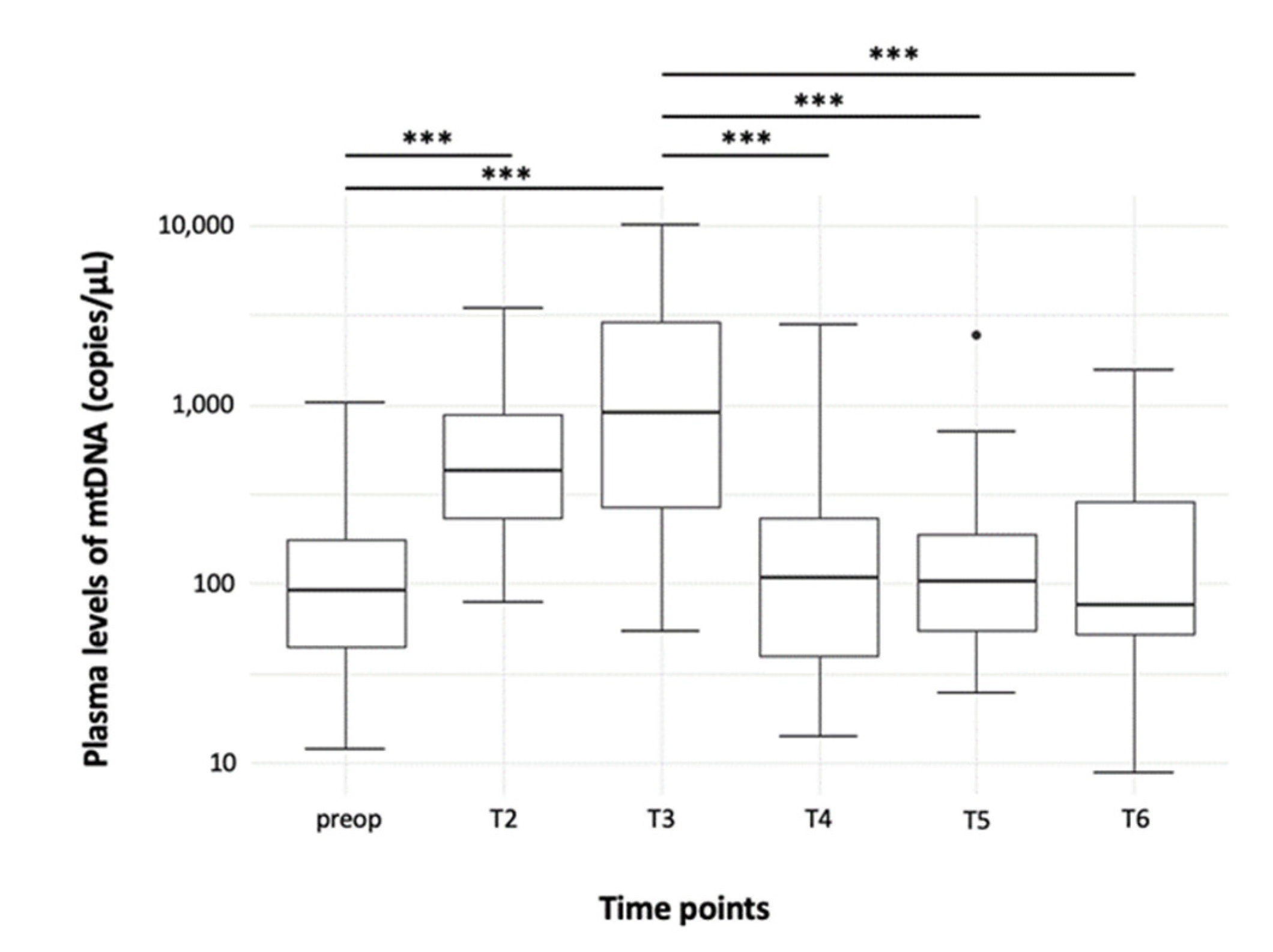
3.1. Quantification of Free-Circulating ND1 mtDNA Plasma Levels
3.1.1. Time Course
3.1.2. Comparison of mtDNA Levels between MiECC and cCPB
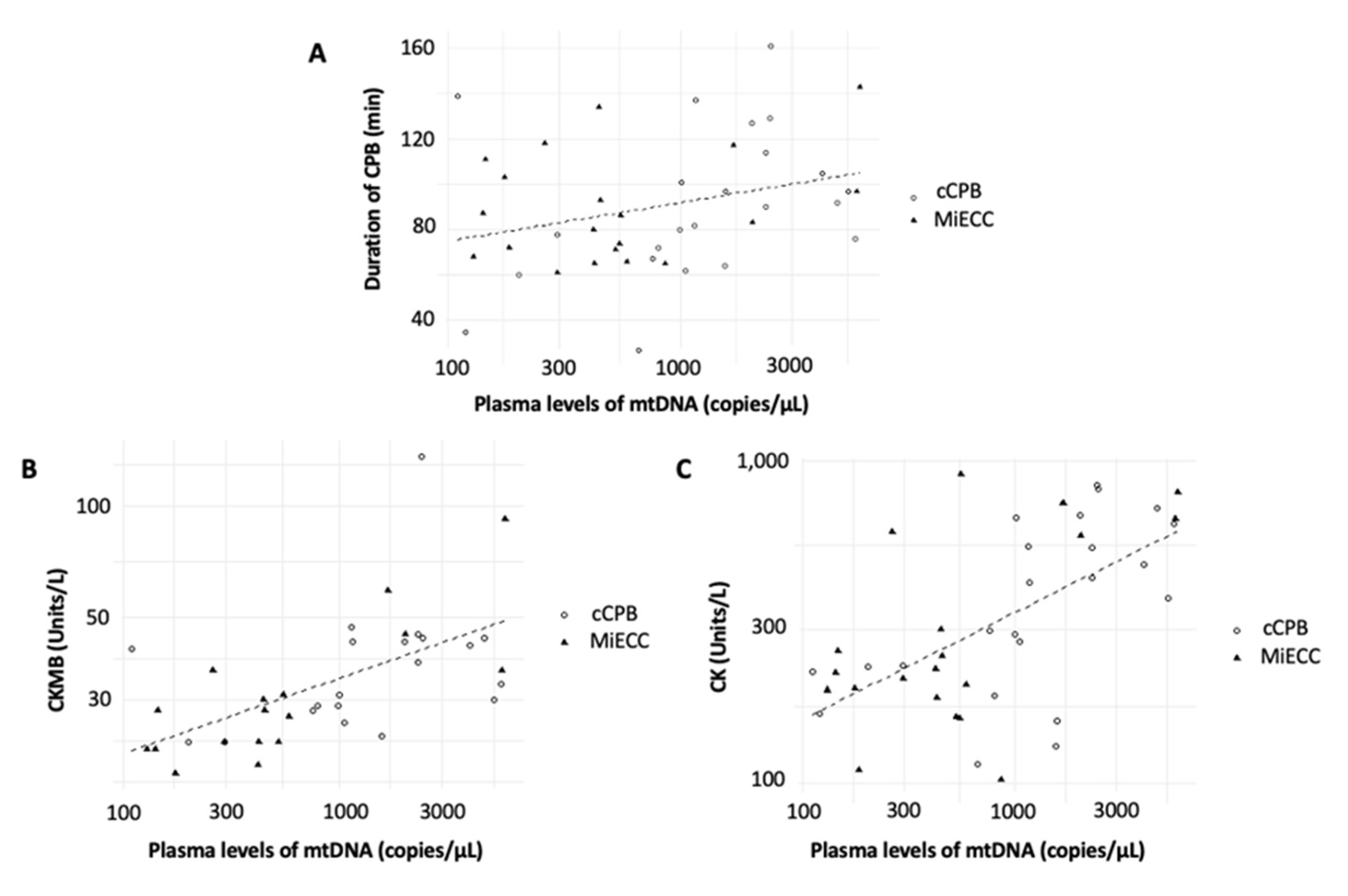
3.2. Correlations between Plasma Free-Circulating ND1 mtDNA Levels and Inflammatory and Ischemic Parameters
3.3. Correlations between Plasma Free-Circulating ND1 mtDNA Levels and Outcome Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anastasiadis, K.; Murkin, J.; Antonitsis, P.; Bauer, A.; Ranucci, M.; Gygax, E.; Schaarschmidt, J.; Fromes, Y.; Philipp, A.; Eberle, B.; et al. Use of minimal invasive extracorporeal circulation in cardiac surgery: Principles, definitions and potential benefits. A position paper from the Minimal Invasive Extra-Corporeal Technologies international Society (MiECTiS). Interact. Cardiovasc. Thorac. Surg. 2016, 22, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadis, K.; Antonitsis, P.; Asteriou, C.; Deliopoulos, A.; Argiriadou, H. Modular minimally invasive extracorporeal circulation ensures perfusion safety and technical feasibility in cardiac surgery: A systematic review of the literature. Perfusion 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Diez, C.; Haneya, A.; Brünger, F.; Philipp, A.; Hirt, S.; Ruppecht, L.; Kobuch, R.; Keyser, A.; Hilker, M.; Puehler, T.; et al. Minimized extracorporeal circulation cannot prevent acute kidney injury but attenuates early renal dysfunction after coronary bypass grafting. ASAIO J. 2009, 55, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Zangrillo, A.; Garozzo, F.A.; Biondi-Zoccai, G.; Pappalardo, F.; Monaco, F.; Crivellari, M.; Bignami, E.; Nuzzi, M.; Landoni, G. Miniaturized cardiopulmonary bypass improves short-term outcome in cardiac surgery: A meta-analysis of randomized controlled studies. J. Thorac. Cardiovasc. Surg. 2010, 139, 1162–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anastasiadis, K.; Antonitsis, P.; Haidich, A.-B.; Argiriadou, H.; Deliopoulos, A.; Papakonstantinou, C. Use of minimal extracorporeal circulation improves outcome after heart surgery: A systematic review and meta-analysis of randomized controlled trials. Int. J. Cardiol. 2013, 164, 158–169. [Google Scholar] [CrossRef]
- Ranucci, M.; Baryshnikova, E. Inflammation and coagulation following minimally invasive extracorporeal circulation technologies. J. Thorac. Dis. 2019, 11, S1480–S1488. [Google Scholar] [CrossRef]
- Mazzei, V.; Nasso, G.; Salamone, G.; Castorino, F.; Tommasini, A.; Anselmi, A. Prospective randomized comparison of coronary bypass grafting with minimal extracorporeal circulation system (MECC) versus off-pump coronary surgery. Circulation 2007, 116, 1761–1767. [Google Scholar] [CrossRef] [Green Version]
- Formica, F.; Mariani, S.; Broccolo, F.; Caruso, R.; Corti, F.; D’Alessandro, S.; Amigoni, P.; Sangalli, F.; Paolini, G. Systemic and myocardial inflammatory response in coronary artery bypass graft surgery with miniaturized extracorporeal circulation: Differences with a standard circuit and off-pump technique in a randomized clinical trial. ASAIO J. 2013, 59, 600–606. [Google Scholar] [CrossRef] [Green Version]
- Winkler, B.; Heinisch, P.P.; Gahl, B.; Aghlmandi, S.; Jenni, H.J.; Carrel, T.P. Minimally invasive extracorporeal circulation circuit is not inferior to off-pump coronary artery bypass grafting: Meta-analysis using the Bayesian method. Ann. Thorac. Surg. 2017, 103, 342–350. [Google Scholar] [CrossRef] [Green Version]
- Denning, N.L.; Aziz, M.; Gurien, S.D.; Wang, P. DAMPs and NETs in Sepsis. Front. Immunol. 2019, 10, 2536. [Google Scholar] [CrossRef]
- Supinski, G.S.; Schroder, E.A.; Callahan, L.A. Mitochondria and critical illness. Chest 2020, 157, 310–322. [Google Scholar] [CrossRef]
- Naase, H.; Harling, L.; Kidher, E.; Sepehripour, A.; Nguyen, B.; Kapelouzou, A.; Cokkinos, D.; Stavridis, G.; Angelini, G.; Evans, P.C.; et al. Toll-like receptor 9 and the inflammatory response to surgical trauma and cardiopulmonary bypass. J. Cardiothorac. Surg. 2020, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Sandler, N.; Kaczmarek, E.; Itagaki, K.; Zheng, Y.; Otterbein, L.; Khabbaz, K.; Liu, D.; Senthilnathan, V.; Gruen, R.L.; Hauser, C.J. Mitochondrial DAMPs are released during cardiopulmonary bypass surgery and are associated with postoperative atrial fibrillation. Heart. Lung Circ. 2017, 29, 697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallavia, B.; Liu, F.; Lefrançais, E.; Cleary, S.J.; Kwaan, N.; Tian, J.J.; Magnen, M.; Sayah, D.M.; Soong, A.; Chen, J.; et al. Mitochondrial DNA stimulates TLR9-dependent neutrophil extracellular trap formation in primary graft dysfunction. Am. J. Respir. Cell Mol. Biol. 2020, 62, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.P.B.; Pulskens, W.P.; Butter, L.M.; Florquin, S.; Juffermans, N.P.; Roelofs, J.J.T.H.; Leemans, J.C. Mitochondrial DNA is released in urine of SIRS patients with acute kidney injury and correlates with severity of renal dysfunction. Shock 2018, 49, 301–310. [Google Scholar] [CrossRef]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Qin, C.; Liu, R.; Gu, J.; Li, Y.; Qian, H.; Shi, Y.; Meng, W. Variation of perioperative plasma mitochondrial DNA correlate with peak inflammatory cytokines caused by cardiac surgery with cardiopulmonary bypass. J Cardiothorac Surg. 2015, 10, 85. [Google Scholar] [CrossRef] [Green Version]
- Qin, C.; Gu, J.; Hu, J.; Qian, H.; Fei, X.; Li, Y.; Liu, R.; Meng, W.; Kirklin, J.; Dushane, J.; et al. Platelets activation is associated with elevated plasma mitochondrial DNA during cardiopulmonary bypass. J. Cardiothorac. Surg. 2016, 11, 90. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; Eckardt, K.-U.; Dorman, N.M.; Christiansen, S.L.; Hoorn, E.J.; Ingelfinger, J.R.; Inker, L.A.; Levin, A.; Mehrotra, R.; Palevsky, P.M.; et al. Nomenclature for kidney function and disease: Report of a kidney disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020, 97, 1117–1129. [Google Scholar] [CrossRef]
- McGeachie, M.J.; Johansson, P.I.; Christopher, K.B.; Fredenburgh, L.E.; Choi, A.M.K.; Nakahira, K.; Baron, R.M.; Rogers, A.J.; Harrington, J. Plasma mitochondrial DNA and metabolomic alterations in severe critical illness. Crit. Care 2018, 22, 360. [Google Scholar]
- Kraft, B.D.; Chen, L.; Suliman, H.B.; Piantadosi, C.A.; Welty-Wolf, K.E. Peripheral blood mononuclear cells demonstrate mitochondrial damage clearance during sepsis. Crit. Care Med. 2019, 47, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xu, W.; Wang, F.; Lv, T.; Yin, Z.; Song, Y. Plasma mtDNA analysis aids in predicting pancreatic necrosis in acute pancreatitis patients: A pilot study. Dig. Dis. Sci. 2018, 63, 2975–2982. [Google Scholar] [CrossRef] [PubMed]
- Schneck, E.; Edinger, F.; Hecker, M.; Sommer, N.; Pak, O.; Weissmann, N.; Hecker, A.; Reichert, M.; Markmann, M.; Sander, M.; et al. Blood levels of free-circulating mitochondrial DNA in septic shock and postsurgical systemic inflammation and its influence on coagulation : A secondary analysis of a prospective observational study. J. Clin. Med. 2020, 9, 2056. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, K.; Kyung, S.Y.; Rogers, A.J.; Gazourian, L.; Youn, S.; Massaro, A.F.; Quintana, C.; Osorio, J.C.; Wang, Z.; Zhao, Y.; et al. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: Derivation and validation. PLoS Med. 2013, 10, e1001577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, R.W.K.; Chan, L.Y.S.; Lam, N.Y.L.; Tsui, N.B.Y.; Ng, E.K.O.; Rainer, T.H.; Lo, Y.M.D. Quantitative analysis of circulating mitochondrial DNA in plasma. Clin. Chem. 2003, 49, 719–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Q.; Ren, J.; Wu, J.; Li, G.; Wu, X.; Liu, S.; Wang, G.; Gu, G.; Li, J. Elevated levels of plasma mitochondrial DNA Are associated with clinical outcome in intra-abdominal infections caused by severe trauma. Surg. Infect. 2017, 18, 610–618. [Google Scholar] [CrossRef]
- Qin, C.; Gu, J.; Qian, H.; Meng, W. Analysis of circulatory mitochondrial DNA level after cardiac surgery with cardiopulmonary bypass and potential prognostic implications. Indian Heart J. 2016, 68, 389–390. [Google Scholar] [CrossRef] [Green Version]
- Baysa, A.; Fedorov, A.; Kondratov, K.; Ruusalepp, A.; Minasian, S.; Galagudza, M.; Popov, M.; Kurapeev, D.; Yakovlev, A.; Valen, G.; et al. Release of mitochondrial and nuclear DNA during on-pump heart surgery: Kinetics and relation to extracellular vesicles. J. Cardiovasc. Transl. Res. 2019, 12, 184–192. [Google Scholar] [CrossRef]
- Trumpff, C.; Michelson, J.; Lagranha, C.J.; Taleon, V.; Karan, K.R.; Sturm, G.; Lindqvist, D.; Fernström, J.; Moser, D.; Kaufman, B.A.; et al. Stress and circulating cell-free mitochondrial DNA: A systematic review of human studies, physiological considerations, and technical recommendations. Mitochondrion 2021, 59, 225–245. [Google Scholar] [CrossRef]
- Zhao, L. Mitochondrial DNA degradation: A quality control measure for mitochondrial genome maintenance and stress response. Enzymes 2019, 45, 311–341. [Google Scholar]
- He, J.; Lu, Y.; Xia, H.; Liang, Y.; Wang, X.; Bao, W.; Yun, S.; Ye, Y.; Zheng, C.; Liu, Z.; et al. Circulating mitochondrial DAMPs are not effective inducers of proteinuria and kidney injury in rodents. PLoS ONE 2015, 10, e0124469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, J.-U.; Bouadma, L. Why biomarkers failed in sepsis. Intensive Care Med. 2016, 42, 2049–2051. [Google Scholar] [CrossRef] [PubMed]
- Pierrakos, C.; Velissaris, D.; Bisdorff, M.; Marshall, J.C.; Vincent, J.L. Biomarkers of sepsis: Time for a reappraisal. Crit. Care 2020, 24, 287. [Google Scholar] [CrossRef] [PubMed]
- Gygax, E.; Kaeser, H.-U.; Stalder, M.; Gahl, B.; Rieben, R.; Carrel, T.; Erdoes, G. Type II minimal-invasive extracorporeal circuit for aortic valve replacement: A randomized controlled trial. Artif. Organs 2018, 42, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, A.H.; Keller, H.; Moritz, A. prospective, randomized un-blinded three arm controlled study in coronary artery revascularization with minimal invasive extracorporeal circulation systems (MiECC): Surrogate parameter analysis of biocompatibility. Heart Surg. Forum 2018, 21, E179–E186. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, S.; Xu, Y.; Liu, Y.; Li, Z.; Zhang, Y.; Jin, Y.; Xue, X.; Wang, H. Relation of mitochondrial DNA copy number in peripheral blood to postoperative atrial fibrillation after isolated off-pump coronary artery bypass grafting. Am. J. Cardiol. 2017, 119, 473–477. [Google Scholar] [CrossRef]
- Manghelli, J.L.; Kelly, M.O.; Carter, D.I.; Gauthier, J.M.; Scozzi, D.; Lancaster, T.S.; MacGregor, R.M.; Khiabani, A.J.; Schuessler, R.B.; Gelman, A.E.; et al. Pericardial mitochondrial DNA levels are associated with atrial fibrillation after cardiac surgery. Ann. Thorac. Surg. 2021, 111, 1593–1600. [Google Scholar] [CrossRef]
- Wiersma, M.; van Marion, D.M.S.; Bouman, E.J.; Li, J.; Zhang, D.; Ramos, K.S.; Lanters, E.A.H.; de Groot, N.M.S.; Brundel, B.J.J.M. Cell-free circulating mitochondrial DNA: A potential blood-based marker for atrial fibrillation. Cells 2020, 9, 1159. [Google Scholar] [CrossRef]
- Wiersma, M.; van Marion, D.; Wüst, R.C.; Houtkooper, R.H.; Zhang, D.; de Groot, N.; Henning, R.H.; Brundel, B.J. Mitochondrial dysfunction underlies cardiomyocyte remodeling in experimental and clinical atrial fibrillation. Cells 2019, 8, 1202. [Google Scholar] [CrossRef] [Green Version]
- Shemiakova, T.; Ivanova, E.; Wu, W.-K.; Kirichenko, T.V.; Starodubova, A.V.; Orekhov, A.N. Atherosclerosis as mitochondriopathy: Repositioning the disease to help finding new therapies. Front. Cardiovasc. Med. 2021, 8, 374. [Google Scholar] [CrossRef]


| Parameters | MiECC | cCPB | All Patients |
|---|---|---|---|
| Age (Years) | 62 (56–70) | 67 (59–73) | 65 (57–73) |
| Male Sex | 21/22 (95.5) | 17/23 (73.9) | 38/45 (84.4) |
| BMI (kg/m2) | 29 (28–31) | 28 (24–32) | 29 (25–32) |
| EuroSCORE | 0.88 (0.72–1.25) | 0.99 (0.74–1.2) | 0.96 (0.73–1.24) |
| Pre-existing Diseases | |||
| Angina Pectoris | 15/22 (68.1) | 15/23 (65.2) | 30/45 (66.7) |
| Arterial Hypertension | 21/22 (95.5) | 19/23 (82.2) | 40/45 (88.9) |
| Acute Myocardial Infarction | 3/22 (13.6) | 5/23 (21.7) | 8/45 (17.8) |
| Myocardial Infarction Within the Last 90 Days Prior to Surgery | 3/22 (13.6) | 8/23 (34.8) | 11/45 (24.4) |
| Concurrent Valvular Disease | 6/22 (27.2) | 1/23 (4.3) | 7/45 (15.6) |
| Stroke | 2/22 (9.1) | 3/23 (13.0) | 5/45 (11.1) |
| Chronic Kidney Disease | 1/22 (4.5) | 3/23 (13.0) | 4/45 (8.9) |
| Diabetes | 9/22 (40.9) | 6/23 (26.1) | 15/45 (33.3) |
| Chronic Obstructive Pulmonary Disease | 2/20 (10.0) | 2/23 (8.7) | 4/45 (8.9) |
| Smoker | 12/22 (54.5) | 14/23 (60.9) | 26/45 (57.8) |
| Alcohol Abuse | 6/22 (27.3) | 8/23 (34.8) | 14/45 (31.3) |
| Process Times | |||
| Duration of Anesthesia (min) | 275 (251–322.0) | 279.8 (248.5–320.3) | 277.4 (249.5–321.6) |
| Duration of CPB (min) | 83 (68–103) | 90 (70–110) | 85 (68–107) |
| Duration of Invasive Ventilation (h) | 13.8 (10–16.7) | 11.2 (9.4–15.3) | 12.5 (9.6–16.5) |
| Plasma Levels of mtDNA (copies/µL) | |||
|---|---|---|---|
| Time Points | MiECC | cCPB | All patients |
| Preoperative (T1) | 68.2 (26.5–104.9) | 152.5 (80.9–207.6) | 91.1 (44.8–175.1) |
| 15 Minutes after Start of CPB (T2) | 316.4 (184.2–683.8) | 673.0 (334.9–1111.2) | 431.7 (235.5–882.4) |
| 60 Minutes after Start of CPB (T3) | 536.5 (215.7–919.6) | 1818.0 (844.2–3932.2) | 904.8 (266.2–2909.3) |
| 15 Minutes after End of CPB (T4) | 59.2 (33.2–151.7) | 155.0 (79.0–361.8) | 108.3 (40.0–232.1) |
| 120 Minutes after End of CPB (T5) | 74.6 (52.9–173.0) | 124.0 (66.9–182.4) | 104.1 (55.6–191.9) |
| First Postoperative Day (T6) | 122.6 (52.6–305.9) | 72.9 (56.1–196.6) | 75.9 (52.6–287.4) |
| Outcome Parameter | Incidence of Outcome Parameter per Study Group | mtDNA > 650 Copies/µL | ||
|---|---|---|---|---|
| 2 × 2 Contingency Table | Odd’s Ratio [CI] | p-Value | ||
| Acute Kidney Injury | MiECC 3/22 cCPB 1/23 | yes 3/4 (75%) no 15/40 (38%) | 4.82 (0.35–272.3) | 0.29 |
| ICU Readmission | MiECC 2/22 cCPB 1/23 | yes 0/3 (0%) no 18/41 (44%) | 0 (0–3.46) | 0.26 |
| POAF | MiECC 3/22 cCPB 5/23 | yes 6/8 (75%) no 12/36 (33%) | 5.74 (0.86–66.6) | 0.048 |
| Postoperative Infections | MiECC 2/22 cCPB 3/23 | yes 2/5 (40%) no 16/39 (41%) | 0.96 (0.07–9.41) | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zajonz, T.; Koch, C.; Schwiddessen, J.; Markmann, M.; Hecker, M.; Edinger, F.; Schmidt, G.; Boening, A.; Sander, M.; Schneck, E. Minimized Extracorporeal Circulation Is Associated with Reduced Plasma Levels of Free-Circulating Mitochondrial DNA Compared to Conventional Cardiopulmonary Bypass: A Secondary Analysis of an Exploratory, Prospective, Interventional Study. J. Clin. Med. 2022, 11, 2994. https://doi.org/10.3390/jcm11112994
Zajonz T, Koch C, Schwiddessen J, Markmann M, Hecker M, Edinger F, Schmidt G, Boening A, Sander M, Schneck E. Minimized Extracorporeal Circulation Is Associated with Reduced Plasma Levels of Free-Circulating Mitochondrial DNA Compared to Conventional Cardiopulmonary Bypass: A Secondary Analysis of an Exploratory, Prospective, Interventional Study. Journal of Clinical Medicine. 2022; 11(11):2994. https://doi.org/10.3390/jcm11112994
Chicago/Turabian StyleZajonz, Thomas, Christian Koch, Jan Schwiddessen, Melanie Markmann, Matthias Hecker, Fabian Edinger, Götz Schmidt, Andreas Boening, Michael Sander, and Emmanuel Schneck. 2022. "Minimized Extracorporeal Circulation Is Associated with Reduced Plasma Levels of Free-Circulating Mitochondrial DNA Compared to Conventional Cardiopulmonary Bypass: A Secondary Analysis of an Exploratory, Prospective, Interventional Study" Journal of Clinical Medicine 11, no. 11: 2994. https://doi.org/10.3390/jcm11112994
APA StyleZajonz, T., Koch, C., Schwiddessen, J., Markmann, M., Hecker, M., Edinger, F., Schmidt, G., Boening, A., Sander, M., & Schneck, E. (2022). Minimized Extracorporeal Circulation Is Associated with Reduced Plasma Levels of Free-Circulating Mitochondrial DNA Compared to Conventional Cardiopulmonary Bypass: A Secondary Analysis of an Exploratory, Prospective, Interventional Study. Journal of Clinical Medicine, 11(11), 2994. https://doi.org/10.3390/jcm11112994








