Sarcopenia Is an Independent Risk Factor for Severe Diabetic Nephropathy in Type 2 Diabetes: A Long-Term Follow-Up Propensity Score–Matched Diabetes Cohort Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Data Sources and Study Cohort
2.2. Participant Selection
2.3. Propensity Score Matching and Covariates
2.4. Hazard Ratios of Severe Diabetic Nephropathy
2.5. Statistical Analysis
3. Results
3.1. PSM and Study Cohort
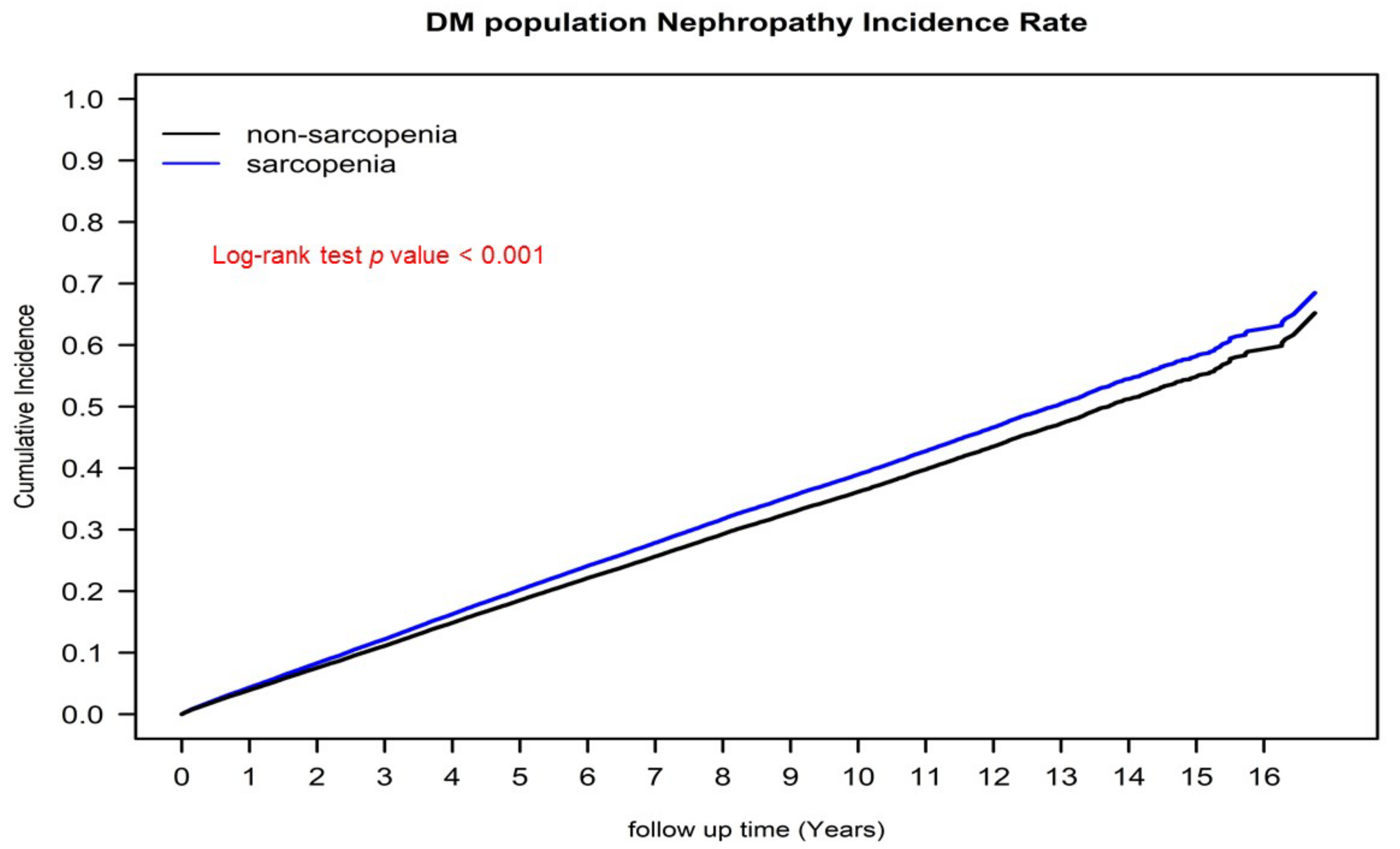
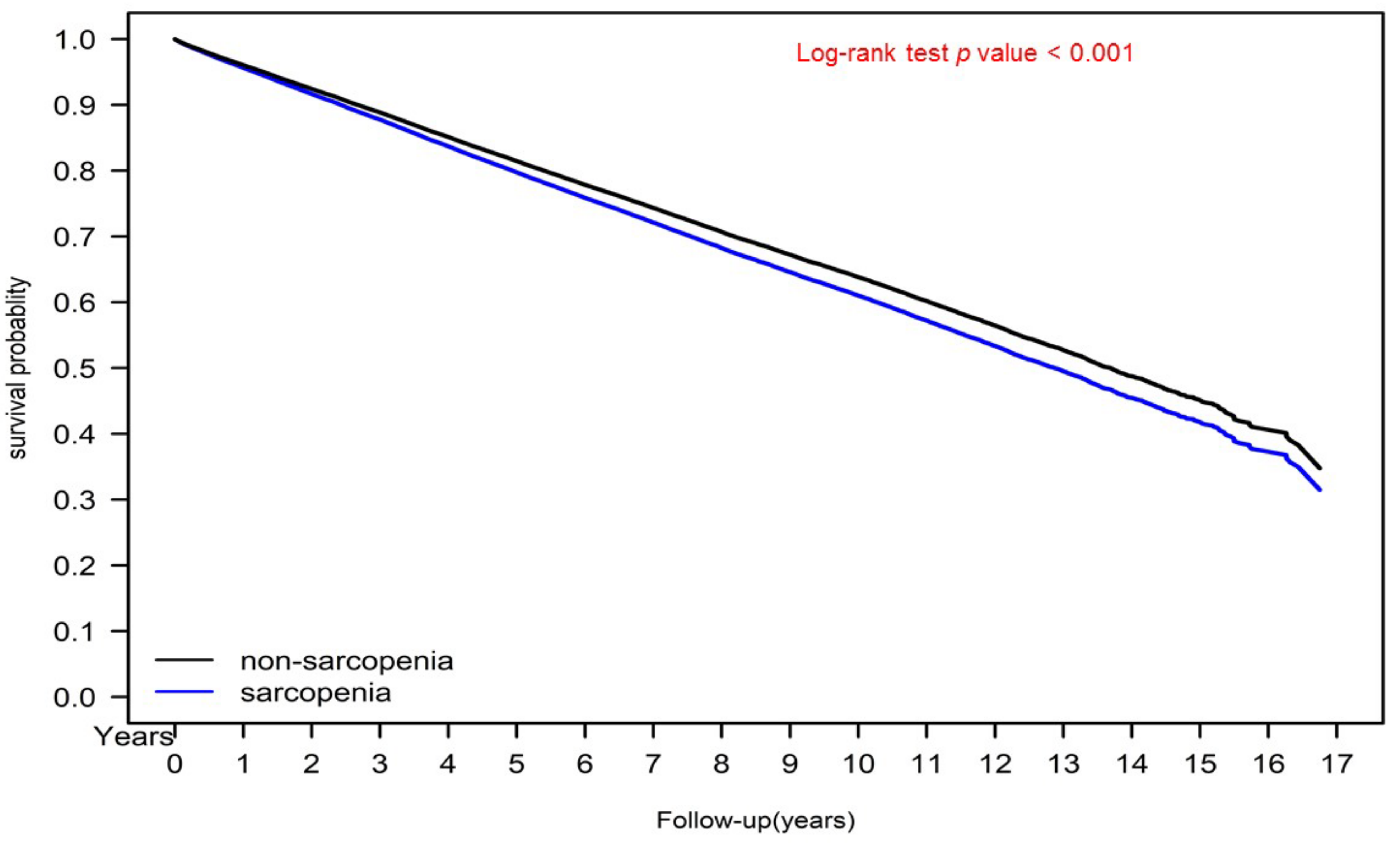
3.2. Kaplan–Meier Cumulative Incidence of Severe Diabetic Nephropathy and Survival Curves of the Sarcopenia and Nonsarcopenia Groups
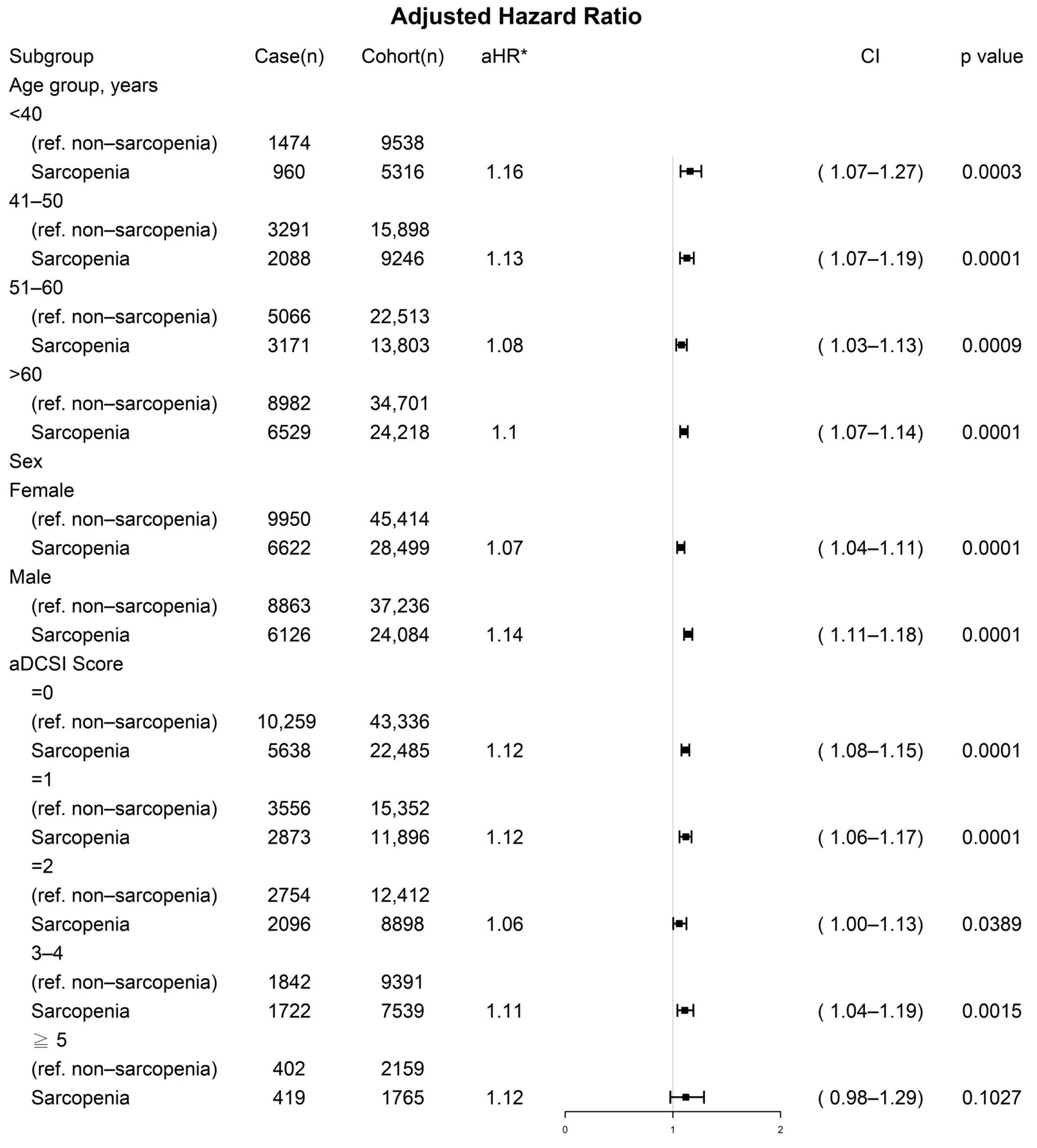
3.3. Prognostic Factors for Severe Diabetic Nephropathy in Multivariate Cox Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- National Institutes of Health USRDS 2017 annual data report: Atlas of chronic kidney disease and end-stage renal disease in the United States. Am. J. Kidney Dis. 2017, 1, 60–65.
- Hostetter, T.H. Hyperfiltration and glomerulosclerosis. Semin. Nephrol. 2003, 23, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Tonneijck, L.; Muskiet, M.H.; Smits, M.M.; van Bommel, E.J.; Heerspink, H.J.; van Raalte, D.H.; Joles, J.A. Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment. J. Am. Soc. Nephrol. 2017, 28, 1023–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helal, I.; Fick-Brosnahan, G.M.; Reed-Gitomer, B.; Schrier, R.W. Glomerular hyperfiltration: Definitions, mechanisms and clinical implications. Nat. Rev. Nephrol. 2012, 8, 293–300. [Google Scholar] [CrossRef]
- Charpentier, G.; Riveline, J.P.; Varroud-Vial, M. Management of drugs affecting blood glucose in diabetic patients with renal failure. Diabetes Metab. 2000, 26, 73–85. [Google Scholar]
- Snyder, R.W.; Berns, J.S. Use of insulin and oral hypoglycemic medications in patients with diabetes mellitus and advanced kidney disease. Semin. Dial. 2004, 17, 365–370. [Google Scholar] [CrossRef]
- Pichler, R.; Afkarian, M.; Dieter, B.P.; Tuttle, K.R. Immunity and inflammation in diabetic kidney disease: Translating mechanisms to biomarkers and treatment targets. Am. J. Physiol. Renal. Physiol. 2017, 312, F716–F731. [Google Scholar] [CrossRef] [Green Version]
- Hojs, R.; Ekart, R.; Bevc, S.; Hojs, N. Markers of Inflammation and Oxidative Stress in the Development and Progression of Renal Disease in Diabetic Patients. Nephron 2016, 133, 159–162. [Google Scholar] [CrossRef]
- An, Y.; Xu, F.; Le, W.; Ge, Y.; Zhou, M.; Chen, H.; Zeng, C.; Zhang, H.; Liu, Z. Renal histologic changes and the outcome in patients with diabetic nephropathy. Nephrol. Dial. Transplant. 2015, 30, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Nilwik, R.; Snijders, T.; Leenders, M.; Groen, B.B.; van Kranenburg, J.; Verdijk, L.B.; van Loon, L.J. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp. Gerontol. 2013, 48, 492–498. [Google Scholar] [CrossRef]
- Cleasby, M.E.; Jamieson, P.M.; Atherton, P.J. Insulin resistance and sarcopenia: Mechanistic links between common co-morbidities. J. Endocrinol. 2016, 229, R67–R81. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, P.; Hevener, A.L.; Karlamangla, A.S. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: Findings from the National Health and Nutrition Examination Survey III. PLoS ONE 2010, 5, e10805. [Google Scholar] [CrossRef] [PubMed]
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017, 96, 10–15. [Google Scholar] [CrossRef]
- Stanford, K.I.; Goodyear, L.J. Exercise and type 2 diabetes: Molecular mechanisms regulating glucose uptake in skeletal muscle. Adv. Physiol. Educ. 2014, 38, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [Green Version]
- Houmard, J.A.; Tanner, C.J.; Slentz, C.A.; Duscha, B.D.; McCartney, J.S.; Kraus, W.E. Effect of the volume and intensity of exercise training on insulin sensitivity. J. Appl. Physiol. 1985, 2004, 96–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koopman, R.; Manders, R.J.; Zorenc, A.H.; Hul, G.B.; Kuipers, H.; Keizer, H.A.; van Loon, L.J. A single session of resistance exercise enhances insulin sensitivity for at least 24 h in healthy men. Eur. J. Appl. Physiol. 2005, 94, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Janssen, I.; Dawson, J.; Kungl, A.M.; Kuk, J.L.; Wong, S.L.; Nguyen-Duy, T.B.; Lee, S.; Kilpatrick, K.; Hudson, R. Exercise-induced reduction in obesity and insulin resistance in women: A randomized controlled trial. Obes. Res. 2004, 12, 789–798. [Google Scholar] [CrossRef]
- Albright, A.; Franz, M.; Hornsby, G.; Kriska, A.; Marrero, D.; Ullrich, I.; Verity, L.S. American College of Sports Medicine position stand. Exercise and type 2 diabetes. Med. Sci. Sports Exerc. 2000, 32, 1345–1360. [Google Scholar] [CrossRef] [Green Version]
- Umpierre, D.; Ribeiro, P.A.; Kramer, C.K.; Leitao, C.B.; Zucatti, A.T.; Azevedo, M.J.; Gross, J.L.; Ribeiro, J.P.; Schaan, B.D. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: A systematic review and meta-analysis. JAMA 2011, 305, 1790–1799. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Scott, C.A.; Mao, C.; Tang, J.; Farmer, A.J. Resistance exercise versus aerobic exercise for type 2 diabetes: A systematic review and meta-analysis. Sports Med. 2014, 44, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Norat, T.; Leitzmann, M.; Tonstad, S.; Vatten, L.J. Physical activity and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis. Eur. J. Epidemiol. 2015, 30, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.Z.; No, M.H.; Heo, J.W.; Park, D.H.; Kang, J.H.; Kim, S.H.; Kwak, H.B. Role of exercise in age-related sarcopenia. J. Exerc Rehabil. 2018, 14, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.Y.; Chang, C.L.; Lu, C.Y.; Wu, S.Y.; Zhang, J.Q. Sarcopenia as an Independent Risk Factor for Specific Cancers: A Propensity Score-Matched Asian Population-Based Cohort Study. Nutrients 2022, 14, 1910. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.Y.; Huang, T.Y.; Wu, Y.T. Prevalence of sarcopenia estimated using a bioelectrical impedance analysis prediction equation in community-dwelling elderly people in Taiwan. J. Am. Geriatr. Soc. 2008, 56, 1710–1715. [Google Scholar] [CrossRef]
- Chang, H.Y.; Weiner, J.P.; Richards, T.M.; Bleich, S.N.; Segal, J.B. Validating the adapted Diabetes Complications Severity Index in claims data. Am. J. Manag. Care 2012, 18, 721–726. [Google Scholar]
- Austin, P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011, 10, 150–161. [Google Scholar] [CrossRef] [Green Version]
- Hung, S.C.; Kuo, K.L.; Tarng, D.C.; Hsu, C.C.; Wu, M.S.; Huang, T.P. Anaemia management in patients with chronic kidney disease: Taiwan practice guidelines. Nephrology 2014, 19, 735–739. [Google Scholar] [CrossRef]
- Hung, S.C.; Chang, Y.K.; Liu, J.S.; Kuo, K.L.; Chen, Y.H.; Hsu, C.C.; Tarng, D.C. Metformin use and mortality in patients with advanced chronic kidney disease: National, retrospective, observational, cohort study. Lancet Diabetes Endocrinol. 2015, 3, 605–614. [Google Scholar] [CrossRef]
- Hsu, T.W.; Liu, J.S.; Hung, S.C.; Kuo, K.L.; Chang, Y.K.; Chen, Y.C.; Hsu, C.C.; Tarng, D.C. Renoprotective effect of renin-angiotensin-aldosterone system blockade in patients with predialysis advanced chronic kidney disease, hypertension, and anemia. JAMA Intern. Med. 2014, 174, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Austin, P.C. The performance of different propensity score methods for estimating marginal hazard ratios. Stat. Med. 2013, 32, 2837–2849. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.L.; Collins, G.S.; Spence, J.; Daures, J.P.; Devereaux, P.J.; Landais, P.; Le Manach, Y. Double-adjustment in propensity score matching analysis: Choosing a threshold for considering residual imbalance. BMC Med. Res. Methodol. 2017, 17, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Kim, H.J.; Lonjon, G.; Zhu, Y.; written on behalf of AME Big-Data Clinical Trial Collaborative Group. Balance diagnostics after propensity score matching. Ann. Transl. Med. 2019, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yung, Y.-F.; Stokes, M. Propensity Score Methods for Causal Inference with the PSMATCH Procedure. In Proceedings of the SAS Global Forum 2017 Conference, Orlando, FL, USA, 2–5 April 2017. [Google Scholar]
- Austin, P.C. The use of propensity score methods with survival or time-to-event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat. Med. 2014, 33, 1242–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollock, C.; Stefansson, B.; Reyner, D.; Rossing, P.; Sjostrom, C.D.; Wheeler, D.C.; Langkilde, A.M.; Heerspink, H.J.L. Albuminuria-lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 429–441. [Google Scholar] [CrossRef]
- Mogensen, C.E.; Christensen, C.K.; Vittinghus, E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes 1983, 32, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Afkarian, M.; Zelnick, L.R.; Hall, Y.N.; Heagerty, P.J.; Tuttle, K.; Weiss, N.S.; de Boer, I.H. Clinical Manifestations of Kidney Disease Among US Adults with Diabetes, 1988–2014. JAMA 2016, 316, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Burrows, N.R.; Li, Y.; Geiss, L.S. Incidence of treatment for end-stage renal disease among individuals with diabetes in the U.S. continues to decline. Diabetes Care 2010, 33, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Ku, E.; McCulloch, C.E.; Mauer, M.; Gitelman, S.E.; Grimes, B.A.; Hsu, C.Y. Association Between Blood Pressure and Adverse Renal Events in Type 1 Diabetes. Diabetes Care 2016, 39, 2218–2224. [Google Scholar] [CrossRef] [Green Version]
- Rossing, K.; Christensen, P.K.; Hovind, P.; Tarnow, L.; Rossing, P.; Parving, H.H. Progression of nephropathy in type 2 diabetic patients. Kidney Int. 2004, 66, 1596–1605. [Google Scholar] [CrossRef] [Green Version]
- Kharroubi, A.T.; Darwish, H.M. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850–867. [Google Scholar] [CrossRef]
- Narres, M.; Claessen, H.; Droste, S.; Kvitkina, T.; Koch, M.; Kuss, O.; Icks, A. The Incidence of End-Stage Renal Disease in the Diabetic (Compared to the Non-Diabetic) Population: A Systematic Review. PLoS ONE 2016, 11, e0147329. [Google Scholar] [CrossRef]
- Volkova, N.; McClellan, W.; Klein, M.; Flanders, D.; Kleinbaum, D.; Soucie, J.M.; Presley, R. Neighborhood poverty and racial differences in ESRD incidence. J. Am. Soc. Nephrol. 2008, 19, 356–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messner, B.; Bernhard, D. Smoking and cardiovascular disease: Mechanisms of endothelial dysfunction and early atherogenesis. Arter. Thromb. Vasc. Biol. 2014, 34, 509–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C.; Diabetes Control and Complications Trial Research Group. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. New Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef]
- Deaton, A.; Cartwright, N. Understanding and misunderstanding randomized controlled trials. Soc. Sci. Med. 2018, 210, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, W.C.; Wu, H.Y.; Peng, Y.S.; Ko, M.J.; Wu, M.S.; Hung, K.Y.; Wu, K.D.; Chu, T.S.; Chien, K.L. Risk Factors for Development and Progression of Chronic Kidney Disease: A Systematic Review and Exploratory Meta-Analysis. Medicine 2016, 95, e3013. [Google Scholar] [CrossRef]
- Chronic Kidney Disease Surveillance System-United States 2018. Available online: http://www.cdc.gov/ckd (accessed on 19 June 2018).
- Park, H.C.; Lee, Y.K.; Cho, A.; Han, C.H.; Noh, J.W.; Shin, Y.J.; Bae, S.H.; Kim, H. Diabetic retinopathy is a prognostic factor for progression of chronic kidney disease in the patients with type 2 diabetes mellitus. PLoS ONE 2019, 14, e0220506. [Google Scholar] [CrossRef] [Green Version]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32, S157–S163. [Google Scholar] [CrossRef] [Green Version]
- Dekkers, C.C.J.; Gansevoort, R.T.; Heerspink, H.J.L. New Diabetes Therapies and Diabetic Kidney Disease Progression: The Role of SGLT-2 Inhibitors. Curr. Diabetes Rep. 2018, 18, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fioretto, P.; Zambon, A.; Rossato, M.; Busetto, L.; Vettor, R. SGLT2 Inhibitors and the Diabetic Kidney. Diabetes Care 2016, 39, S165–S171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Nonsarcopenia | Sarcopenia | SMD | |||
|---|---|---|---|---|---|
| N = 52,583 | N = 52,583 | ||||
| N | % | N | % | ||
| Age (mean ± SD) | 59.06 ± 15.26 | 58.96 ± 14.56 | 0.0070 | ||
| 59.00 (49.00, 70.00) | 59.00 (49.00, 70.00) | ||||
| Age (years) | 52,583 | 52,583 | 0.0000 | ||
| Age ≤ 40 | 5316 | 10.11% | 5316 | 10.11% | |
| 40 ≤ Age ≤ 50 | 9246 | 17.58% | 9246 | 17.58% | |
| 50 ≤ Age ≤ 60 | 13, 803 | 26.25% | 13,803 | 26.25% | |
| Age > 60 | 24,218 | 46.06% | 24,218 | 46.06% | |
| Sex | 52,583 | 52,583 | 0.0000 | ||
| Female | 28,499 | 54.20% | 28,499 | 54.20% | |
| Male | 24,084 | 45.80% | 24,084 | 45.80% | |
| Income Level (NTD) | 52,583 | 52,583 | 0.0690 | ||
| Low-Income | 668 | 1.27% | 775 | 1.47% | |
| ≤20,000 | 34,181 | 65.00% | 32,633 | 62.06% | |
| 20,001–30,000 | 10,052 | 19.12% | 11,343 | 21.57% | |
| 30,001–45,000 | 5023 | 9.55% | 5224 | 9.93% | |
| >45,000 | 2659 | 5.06% | 2608 | 4.96% | |
| Urbanization Level | 52,583 | 52,583 | 0.1000 | ||
| Rural | 15,494 | 29.47% | 17,947 | 34.13% | |
| Urban | 37,089 | 70.53% | 34,636 | 65.87% | |
| aDCSI Score (mean ± SD) | 1.06 ± 1.40 | 1.24 ± 1.45 | 0.1210 | ||
| aDCSI Score | 52,583 | 52,583 | 0.1640 | ||
| 0 | 26,681 | 50.74% | 22,485 | 42.76% | |
| 1 | 9950 | 18.92% | 11,896 | 22.62% | |
| 2 | 8247 | 15.68% | 8898 | 16.92% | |
| 3–4 | 6252 | 11.89% | 7539 | 14.34% | |
| ≥5 | 1453 | 2.76% | 1765 | 3.36% | |
| CCI Score (mean ± SD) | 1.02 ± 1.36 | 1.36 ± 1.98 | 0.1990 | ||
| 0.00 (0.00, 2.00) | 0.00 (0.00, 2.00) | ||||
| CCI Score | 52,583 | 52,583 | 0.0000 | ||
| 0 | 27,195 | 51.72% | 27,195 | 51.72% | |
| ≥ 1 | 25,388 | 48.28% | 25,388 | 48.28% | |
| Comorbidities | |||||
| Congestive Heart Failure | 3017 | 5.74% | 2651 | 5.04% | 0.031 |
| Dementia | 1209 | 2.30% | 1296 | 2.46% | 0.011 |
| Chronic Pulmonary Disease | 10,121 | 19.25% | 9710 | 18.47% | 0.020 |
| Rheumatic Disease | 1174 | 2.23% | 1478 | 2.81% | 0.037 |
| Liver Disease | 10,249 | 19.49% | 10,037 | 19.09% | 0.021 |
| DM With Complications | 2201 | 4.19% | 2197 | 4.18% | 0.000 |
| Hemiplegia and Paraplegia | 879 | 1.67% | 1225 | 2.33% | 0.047 |
| Renal Disease | 60 | 0.11% | 71 | 0.14% | 0.006 |
| AIDS | 22 | 0.04% | 17 | 0.03% | 0.002 |
| Cancer | 5266 | 10.01% | 7124 | 13.55% | 0.1331 |
| Gum and Periodontal Disease | 22,873 | 43.50% | 27,061 | 51.46% | 0.1600 |
| Peptic Ulcer | 15,567 | 29.60% | 20,094 | 38.21% | 0.1830 |
| Sleep Disorder | 26,231 | 49.88% | 28,981 | 55.11% | 0.1400 |
| Conjunctival Disease | 18,788 | 35.73% | 23,459 | 44.61% | 0.1820 |
| Proteinuria | 816 | 1.55% | 1053 | 2.00% | 0.0340 |
| Hyperuricemia | 2347 | 4.46% | 2785 | 5.30% | 0.0390 |
| Alcohol-Related Disease | 2252 | 4.28% | 2674 | 5.09% | 0.038 |
| Obesity | 1271 | 2.42% | 1616 | 3.07% | 0.0400 |
| Coronary Arterial Disease | 12,107 | 23.02% | 13,825 | 26.29% | 0.0760 |
| Anemia | 4468 | 8.50% | 5687 | 10.82% | 0.0790 |
| Asthma | 609 | 1.16% | 608 | 1.16% | 0.0000 |
| Hypertension | 25,721 | 48.92% | 27,787 | 52.84% | 0.0790 |
| Hyperlipidemia | 17,397 | 33.08% | 20,623 | 39.22% | 0.1280 |
| Current Smoking Habits | 12,123 | 23.05% | 13,388 | 25.46% | 0.0560 |
| Former Smoking Habits | 728 | 1.38% | 1011 | 1.92% | 0.0420 |
| Drug Use | |||||
| Metformin | 21,117 | 40.16% | 21,724 | 41.31% | 0.0230 |
| Insulin | 3410 | 6.48% | 3419 | 6.50% | 0.0003 |
| ACEIs or ARBs | 14,048 | 26.72% | 10,612 | 20.18% | 0.1550 |
| Statins | 16,468 | 31.32% | 19,091 | 36.31% | 0.1060 |
| p Value | |||||
| Follow-Up (years; mean ± SD) | 7.94 ± 4.18 | 7.43 ± 4.10 | <0.0001 | ||
| Follow-Up (years; median [IQR, Q1,Q3]) | 7.46 (2.36, 9.15) | 7.79 (1.75, 8.47) | <0.0001 | ||
| Outcomes | |||||
| Severe Diabetic Nephropathy | 7169 | 13.63% | 10,723 | 20.39% | <0.0001 |
| Diabetic Chronic Kidney Disease | 4302 | 8.18% | 6434 | 12.24% | <0.0001 |
| Diabetic End-Stage Kidney Disease | 2867 | 5.45% | 4289 | 8.16% | <0.0001 |
| Crude HR (95% CI) | p Value | Adjusted HR * (95% CI) | p Value | |||
|---|---|---|---|---|---|---|
| Sarcopenia (ref. no) | ||||||
| Yes | 1.17 | (1.14, 1.2) | <0.0001 | 1.106 | (1.08, 1.13) | <0.0001 |
| Sex (ref. female) | ||||||
| Male | 1.216 | (1.19, 1.24) | <0.0001 | 1.292 | (1.26, 1.32) | <0.0001 |
| Age (years; ref. Age ≤ 40) | ||||||
| 40 < Age ≤ 50 | 1.4 | (1.33, 1.47) | <0.0001 | 1.321 | (1.26, 1.39) | <0.0001 |
| 50 < Age ≤ 60 | 1.765 | (1.69, 1.85) | <0.0001 | 1.553 | (1.48, 1.63) | <0.0001 |
| Age > 60 | 2.699 | (2.59, 2.82) | <0.0001 | 2.141 | (2.04, 2.24) | <0.0001 |
| Income Levels (NTD; ref. Low-Income) | ||||||
| ≤ 20,000 | 0.848 | (0.77, 1.24) | 0.2311 | 0.896 | (0.81, 1.19) | 0.2301 |
| 20,001–30,000 | 0.758 | (0.68, 1.14) | 0.4525 | 0.822 | (0.74, 1.11) | 0.5426 |
| 30,001–45,000 | 0.596 | (0.54, 1.16) | 0.2972 | 0.76 | (0.68, 1.14) | 0.3482 |
| >45,000 | 0.544 | (0.49, 1.26) | 0.6452 | 0.704 | (0.63, 1.17) | 0.3287 |
| Urbanization (ref. rural) | ||||||
| Urban | 0.876 | (0.76, 1.29) | 0.2352 | 0.972 | (0.95, 1.13) | 0.4234 |
| aDCSI Score | ||||||
| 1 | 1.305 | (1.27, 1.34) | <0.0001 | 1.011 | (1.07, 1.14) | 0.0012 |
| 2 | 1.572 | (1.52, 1.62) | <0.0001 | 1.073 | (1.03, 1.11) | 0.0002 |
| 3–4 | 1.821 | (1.76, 1.89) | <0.0001 | 1.095 | (1.05, 1.15) | <0.0001 |
| ≥ 5 | 2.539 | (2.37, 2.73) | <0.0001 | 1.36 | (1.26, 1.47) | <0.0001 |
| CCI ≥ 1 (ref. 0) | 1.313 | (0.88, 1.34) | 0.1409 | 1.076 | (0.95, 1.1) | 0.1247 |
| Comorbidities (ref. no) | ||||||
| Congestive Heart Failure | 1.193 | (0.55, 1.63) | 0.3405 | 1.117 | (0.68, 1.15) | 0.2591 |
| Dementia | 1.215 | (0.58, 1.25) | 0.5016 | 0.948 | (0.91, 1.18) | 0.1434 |
| Chronic Pulmonary Disease | 1.066 | (0.43, 1.51) | 0.3942 | 1.216 | (0.88, 1.26) | 0.3863 |
| Rheumatic Disease | 1.164 | (0.61, 1.72) | 0.4309 | 1.16 | (0.82, 1.2) | 0.2752 |
| Liver Disease | 1.314 | (0.78, 1.35) | 0.3680 | 1.055 | (0.82, 1.09) | 0.4233 |
| DM With Complications | 0.967 | (0.94, 1.19) | 0.2181 | 0.907 | (0.88, 1.03) | 0.2483 |
| Hemiplegia and Paraplegia | 1.293 | (0.76, 1.33) | 0.4391 | 1.044 | (0.91, 1.07) | 0.4236 |
| Renal Disease | 1.289 | (0.86, 1.33) | 0.5925 | 1.021 | (0.99, 1.05) | 0.1395 |
| AIDS | 1.206 | (0.87, 1.24) | 0.6320 | 0.971 | (0.94, 1.04) | 0.2375 |
| Cancer | 1.356 | (0.42, 1.23) | 0.4051 | 1.001 | (0.97, 1.03) | 0.9730 |
| Anemia | 1.31 | (0.86, 1.37) | 0.4827 | 1.186 | (0.94, 1.24) | 0.4028 |
| Asthma | 1.294 | (0.85, 1.46) | 0.7921 | 1.005 | (0.89, 1.13) | 0.9297 |
| Proteinuria | 1.115 | (0.58, 1.86) | 0.7201 | 1.194 | (0.88, 1.62) | 0.5017 |
| Hyperuricemia | 1.399 | (0.73, 1.47) | 0.3294 | 1.131 | (0.87, 1.19) | 0.5302 |
| Obesity | 0.963 | (0.89, 1.04) | 0.3465 | 1.028 | (0.95, 1.11) | 0.5025 |
| Alcohol-Related Disease | 1.222 | (0.75, 1.30) | 0.4804 | 1.099 | (0.93, 1.16) | 0.6553 |
| Coronary Arterial Disease | 1.105 | (0.57, 1.54) | 0.6402 | 1.028 | (0.99, 1.06) | 0.0985 |
| Gum and Periodontal Disease | 0.973 | (0.95, 1.03) | 0.1184 | 0.911 | (0.89, 1.03) | 0.2116 |
| Peptic Ulcer | 1.297 | (0.87, 1.33) | 0.4781 | 1.038 | (0.91, 1.07) | 0.2251 |
| Sleep Disorder | 1.313 | (0.58, 1.34) | 0.5420 | 1.024 | (0.89, 1.05) | 0.2674 |
| Conjunctival Disease | 1.222 | (0.79, 1.25) | 0.2508 | 0.973 | (0.95, 1.04) | 0.3337 |
| Hypertension | 1.181 | (0.58, 1.65) | 0.2853 | 1.115 | (0.68, 1.15) | 0.4492 |
| Hyperlipidemia | 1.236 | (0.71, 1.27) | 0.4903 | 0.951 | (0.92, 1.18) | 0.1324 |
| Current Smoking Habits (ref. no) | 1.374 | (0.94, 1.41) | 0.3772 | 1.01 | (0.98, 1.04) | 0.4883 |
| Former Smoking Habits (ref. no) | 1.282 | (0.95, 1.43) | 0.7421 | 1.01 | (0.91, 1.13) | 0.8532 |
| Drug Use (ref. no) | ||||||
| Metformin | 1.086 | (0.75, 1.52) | 0.7704 | 1.021 | (0.91, 1.25) | 0.4502 |
| ACEIs or ARBs | 1.087 | (0.94, 1.73) | 0.6713 | 1.069 | (0.93, 1.21) | 0.6710 |
| Statins | 1.036 | (0.60, 1.37) | 0.5621 | 1.049 | (0.92, 1.08) | 0.2235 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-M.; Chen, W.-M.; Chen, M.; Shia, B.-C.; Wu, S.-Y. Sarcopenia Is an Independent Risk Factor for Severe Diabetic Nephropathy in Type 2 Diabetes: A Long-Term Follow-Up Propensity Score–Matched Diabetes Cohort Study. J. Clin. Med. 2022, 11, 2992. https://doi.org/10.3390/jcm11112992
Huang Y-M, Chen W-M, Chen M, Shia B-C, Wu S-Y. Sarcopenia Is an Independent Risk Factor for Severe Diabetic Nephropathy in Type 2 Diabetes: A Long-Term Follow-Up Propensity Score–Matched Diabetes Cohort Study. Journal of Clinical Medicine. 2022; 11(11):2992. https://doi.org/10.3390/jcm11112992
Chicago/Turabian StyleHuang, Yen-Min, Wan-Ming Chen, Mingchih Chen, Ben-Chang Shia, and Szu-Yuan Wu. 2022. "Sarcopenia Is an Independent Risk Factor for Severe Diabetic Nephropathy in Type 2 Diabetes: A Long-Term Follow-Up Propensity Score–Matched Diabetes Cohort Study" Journal of Clinical Medicine 11, no. 11: 2992. https://doi.org/10.3390/jcm11112992
APA StyleHuang, Y.-M., Chen, W.-M., Chen, M., Shia, B.-C., & Wu, S.-Y. (2022). Sarcopenia Is an Independent Risk Factor for Severe Diabetic Nephropathy in Type 2 Diabetes: A Long-Term Follow-Up Propensity Score–Matched Diabetes Cohort Study. Journal of Clinical Medicine, 11(11), 2992. https://doi.org/10.3390/jcm11112992








