Comparison of Efficacy and Safety of Brigatinib in First-Line Treatments for Patients with Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Systematic Review and Indirect Treatment Comparison
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Extraction and Quality Assessment
2.3. Outcome Measures and Statistical Methods
3. Results
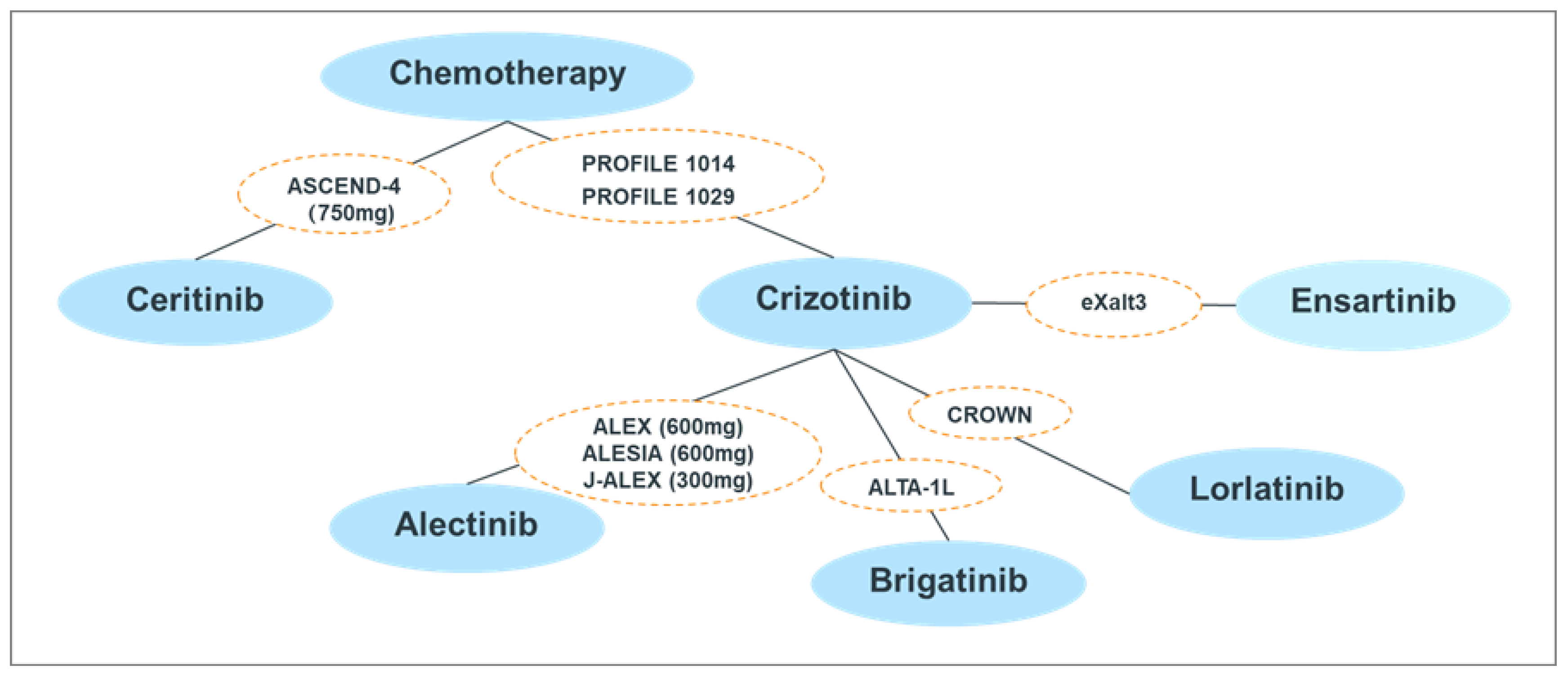
3.1. Studies Included in the ITC
3.2. Risk of Bias
3.3. ITC Results of Efficacy Endpoints
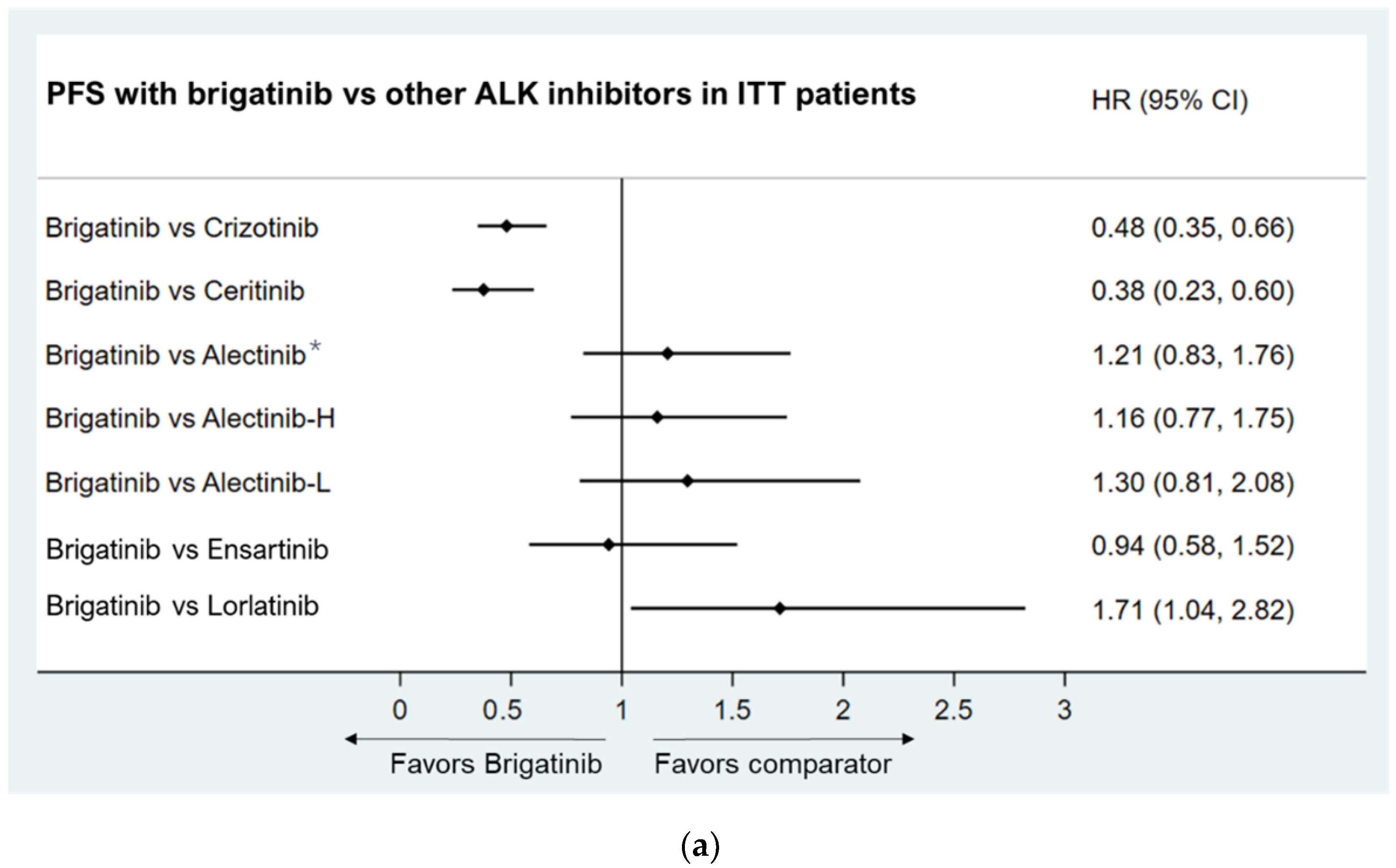
3.3.1. Progression-Free Survival
- Intent-to-treat (ITT) population
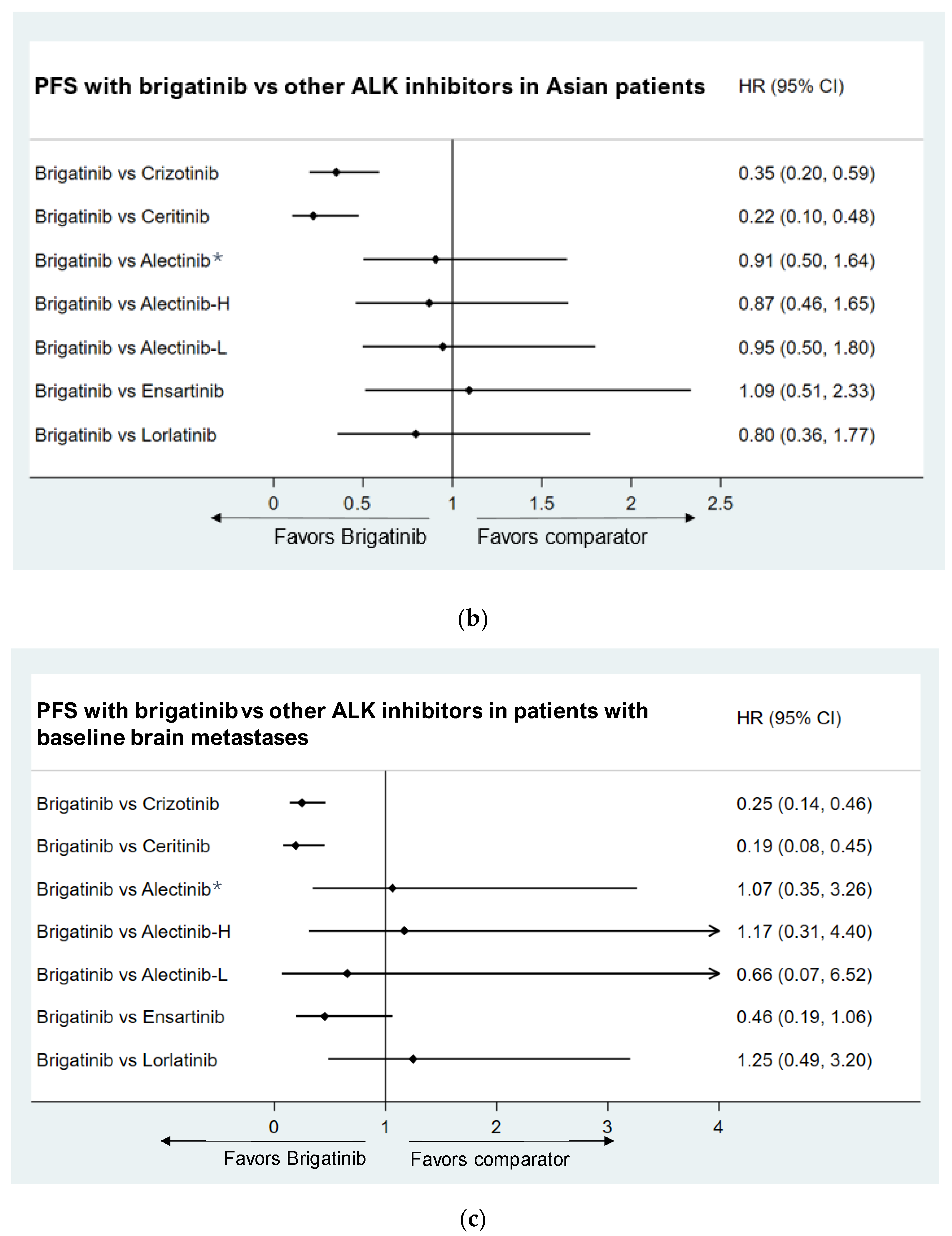
- Asian Population subgroup
- Baseline brain metastases population
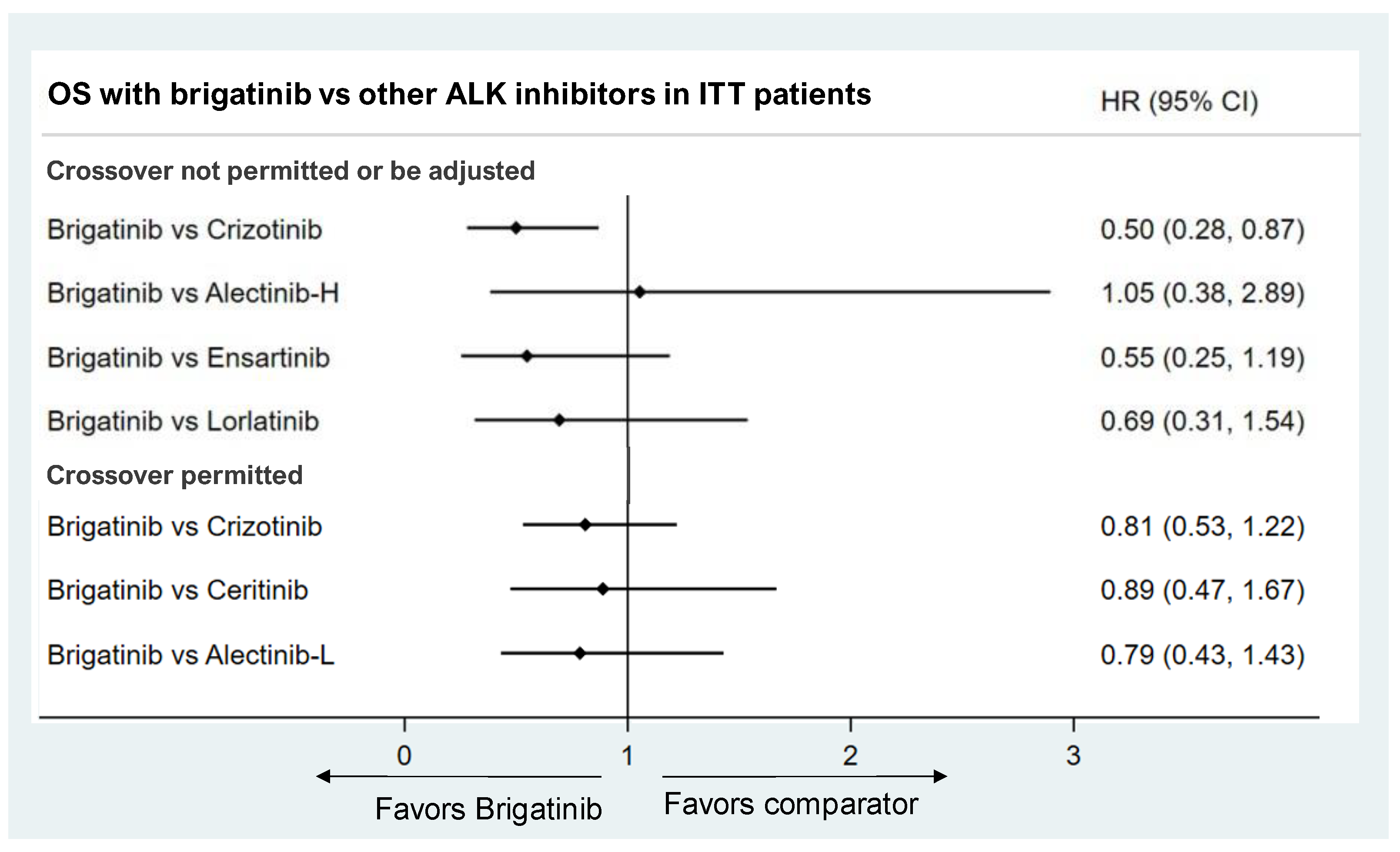
3.3.2. Overall Survival
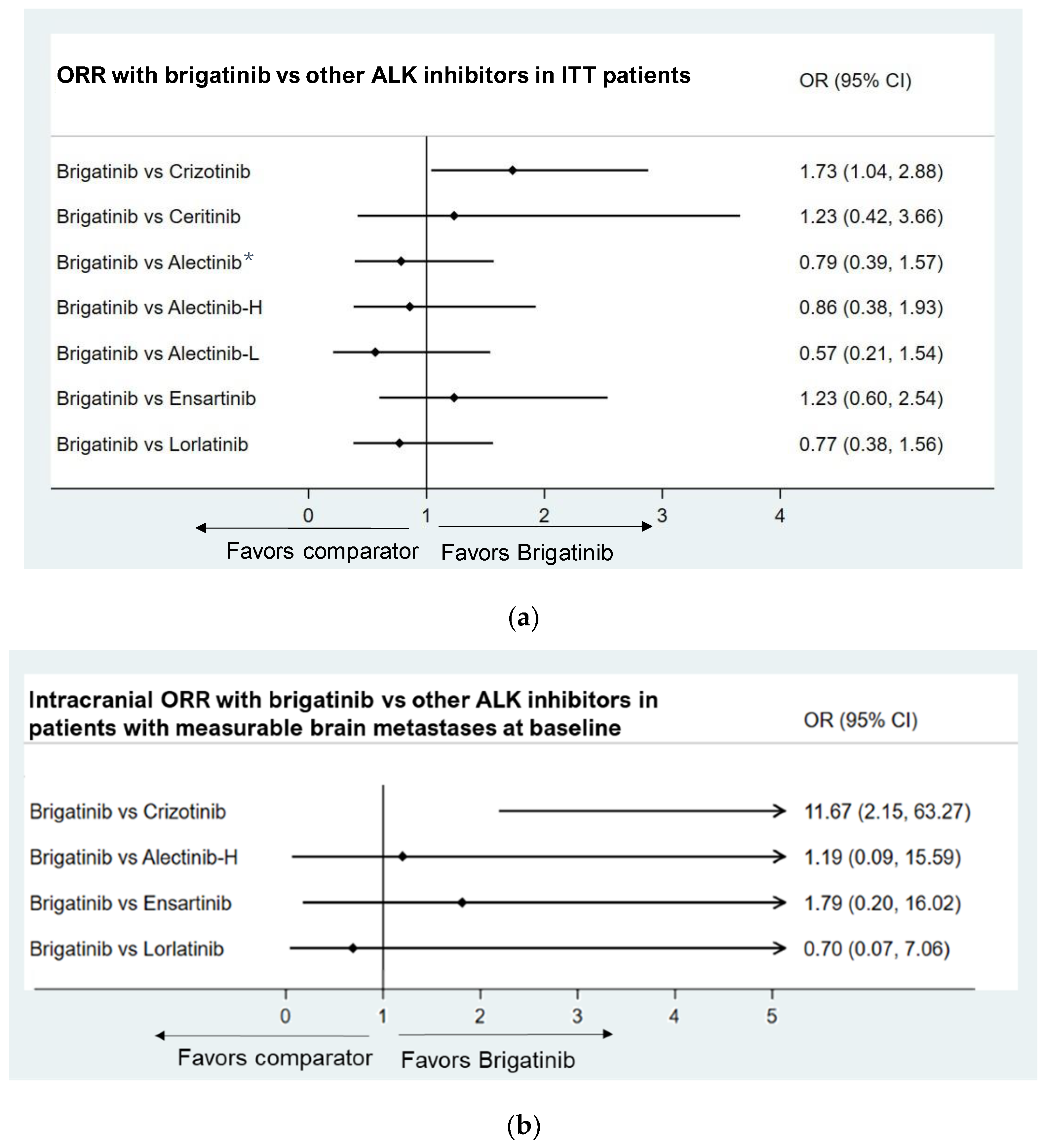
3.3.3. Objective Response Rate
- Patients with measurable brain metastases at baseline
3.3.4. Safety Profile
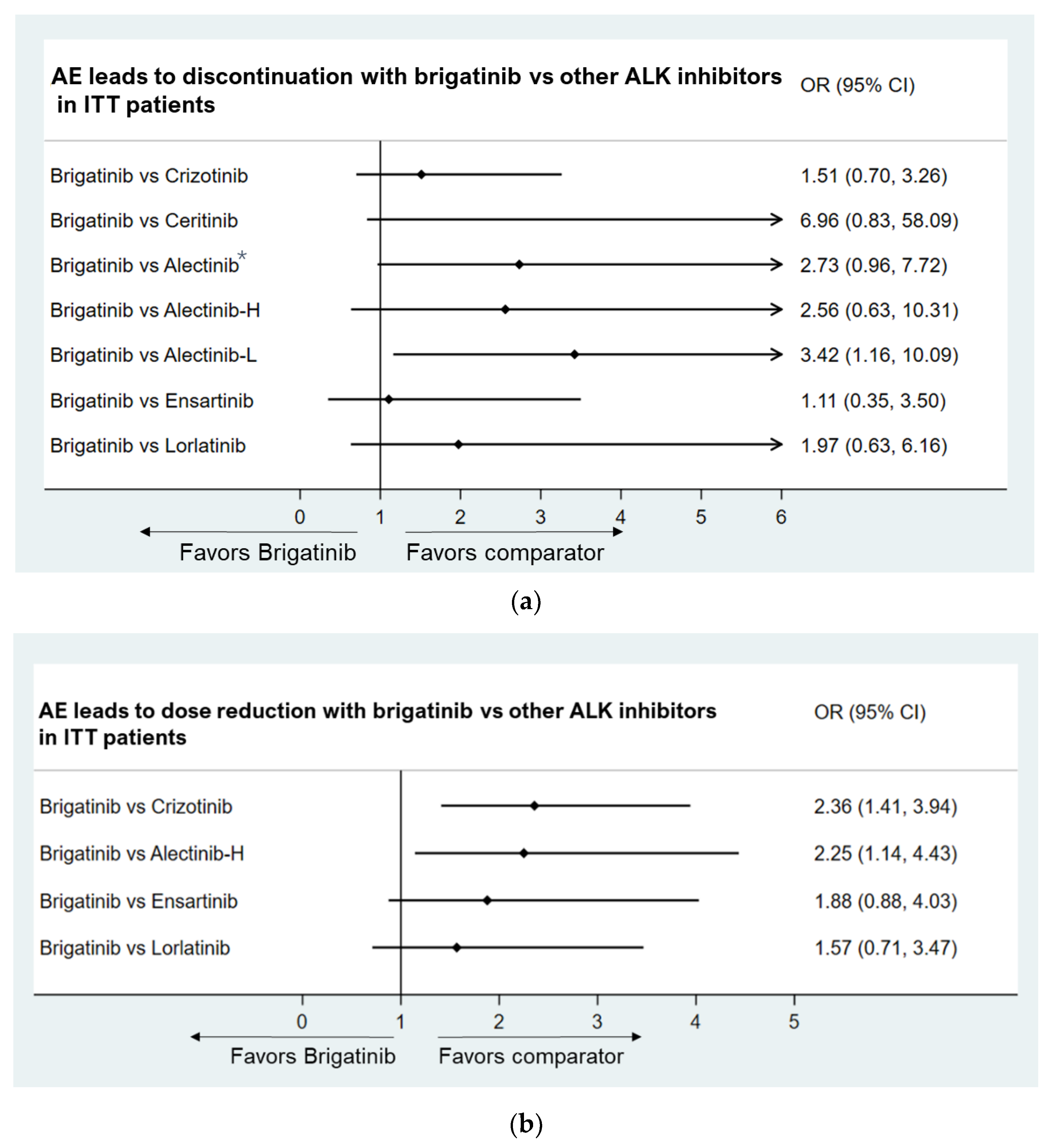
- Grade ≥ 3 AEs
- AEs leading to discontinuation
- AEs leading to dose reduction
- Health-related quality of life (HRQoL)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424, Erratum in CA Cancer J. Clin. 2020, 70, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Gou, L.Y.; Wu, Y.L. Prevalence of driver mutations in non-small-cell lung cancers in the People’s Republic of China. Lung Cancer 2014, 5, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangachari, D.; Yamaguchi, N.; Laan, P.A.V.; Folch, E.; Mahadevan, A.; Floyd, S.R.; Uhlmann, E.J.; Wong, E.T.; Dahlberg, S.E.; Huberman, M.S.; et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015, 88, 108–111. [Google Scholar] [CrossRef] [Green Version]
- Solomon, B.J.; Mok, T.; Kim, D.; Wu, Y.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef] [Green Version]
- Dagogo-Jack, I.; Shaw, A.T. Crizotinib resistance: Implications for therapeutic strategies. Ann. Oncol. 2016, 27 (Suppl. S3), iii42–iii50. [Google Scholar] [CrossRef]
- Soria, J.C.; Tan, D.S.W.; Chiari, R.; Wu, Y.; Paz-Ares, L.; Wolf, J.; Geater, S.L.; Orlov, S.; Cortinovis, D.; Yu, C.; et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): A randomised, open-label, phase 3 study. Lancet 2017, 389, 917–929. [Google Scholar] [CrossRef]
- Hida, T.; Nokihara, H.; Kondo, M.; Kim, Y.H.; Azuma, K.; Seto, T.; Takiguchi, Y.; Nishio, M.; Yoshioka, H.; Imamura, F.; et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): An open-label, randomised phase 3 trial. Lancet 2017, 390, 29–39. [Google Scholar] [CrossRef]
- Zhou, C.; Kim, S.-W.; Reungwetwattana, T.; Zhou, J.; Zhang, Y.; He, J.; Yang, J.-J.; Cheng, Y.; Lee, S.-H.; Bu, L.; et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): A randomised phase 3 study. Lancet Respir. Med. 2019, 7, 437–446. [Google Scholar] [CrossRef]
- Mok, T.; Camidge, D.; Gadgeel, S.; Rosell, R.; Dziadziuszko, R.; Kim, D.-W.; Pérol, M.; Ou, S.-H.; Ahn, J.; Shaw, A.; et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann. Oncol. 2020, 31, 1056–1064. [Google Scholar] [CrossRef]
- Horn, L.; Wang, Z.; Wu, G.; Poddubskaya, E.; Mok, T.; Reck, M.; Wakelee, H.; Chiappori, A.A.; Lee, D.H.; Breder, V.; et al. Ensartinib vs Crizotinib for Patients with Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer: A Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Bauer, T.M.; de Marinis, F.; Felip, E.; Goto, Y.; Liu, G.; Mazieres, J.; Kim, D.-W.; Mok, T.; Polli, A.; et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med. 2020, 383, 2018–2029. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.C.-H.; Han, J.-Y.; Lee, J.-S.; Hochmair, M.J.; Li, J.Y.-C.; Chang, G.-C.; Lee, K.H.; et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.C.H.; Han, J.-Y.; Hochmair, M.J.; Lee, K.H.; Delmonte, A.; Campelo, M.R.G.; Kim, D.-W.; et al. Brigatinib Versus Crizotinib in Advanced ALK Inhibitor-Naive ALK-Positive Non-Small Cell Lung Cancer: Second Interim Analysis of the Phase III ALTA-1L Trial. J. Clin. Oncol. 2020, 38, 3592–3603. [Google Scholar] [CrossRef]
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.C.; Han, J.-Y.; Hochmair, M.J.; Lee, K.H.; Delmonte, A.; Campelo, M.R.G.; Kim, D.-W.; et al. Brigatinib Versus Crizotinib in ALK Inhibitor-Naive Advanced ALK-Positive NSCLC: Final Results of Phase 3 ALTA-1L Trial. J. Thorac. Oncol. 2021, 16, 2091–2108. [Google Scholar] [CrossRef]
- Bucher, H.C.; Guyatt, G.H.; Griffith, L.E.; Walter, S.D. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J. Clin. Epidemiol. 1997, 50, 683–691. [Google Scholar] [CrossRef]
- Hoaglin, D.C.; Hawkins, N.; Jansen, J.P.; Scott, D.A.; Itzler, R.; Cappelleri, J.C.; Boersma, C.; Thompson, D.; Larholt, K.M.; Diaz, M.; et al. Conducting Indirect-Treatment-Comparison and Network-Meta-Analysis Studies: Report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: Part 2. Value Health 2011, 14, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.-L.; Lu, S.; Lu, Y.; Zhou, J.; Shi, Y.-K.; Sriuranpong, V.; Ho, J.C.; Ong, C.K.; Tsai, C.-M.; Chung, C.-H.; et al. Results of PROFILE 1029, a Phase III Comparison of First-Line Crizotinib versus Chemotherapy in East Asian Patients with ALK-Positive Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 1539–1548. [Google Scholar] [CrossRef] [Green Version]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.; et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Doebele, R.C. Treating ALK-positive lung cancer—Early successes and future challenges. Nat. Rev. Clin. Oncol. 2012, 9, 268–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Z.; Gao, Q.; Fu, M.; Ni, N.; Pei, Y.; Ou, W. Anaplastic lymphoma kinase fusions: Roles in cancer and therapeutic perspectives. Oncol. Lett. 2019, 17, 2020–2030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, C.-H.; Chen, H.-L.; Chang, H.-M.; Tsai, Y.-C.; Wu, K.-L.; Chen, I.-H.; Chen, K.-C.; Lee, J.-Y.; Chang, Y.-C.; Chen, C.-L.; et al. Systematic Review and Network Meta-Analysis of Anaplastic Lymphoma Kinase (ALK) Inhibitors for Treatment-Naïve ALK-Positive Lung Cancer. Cancers 2021, 13, 1966. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Akimoto, K.; Sato, H.; Manabe, R.; Kishino, Y.; Homma, T.; Kusumoto, S.; Yamaoka, T.; Tanaka, A.; Ohmori, T.; et al. Brigatinib and Alectinib for ALK Rearrangement-Positive Advanced Non-Small Cell Lung Cancer With or Without Central Nervous System Metastasis: A Systematic Review and Network Meta-Analysis. Cancers 2020, 12, 942. [Google Scholar] [CrossRef]
- Campelo, M.R.G.; Lin, H.M.; Zhu, Y.; Pérol, M.; Jahanzeb, M.; Popat, S.; Zhang, P.; Camidge, D.R. Health-related quality of life in the randomized phase III trial of brigatinib vs crizotinib in advanced ALK inhibitor-naive ALK + non-small cell lung cancer (ALTA-1L). Lung Cancer 2021, 155, 68–77. [Google Scholar] [CrossRef]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.W.; Ou, S.H.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 9, 829–838. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Zhu, F.; Zhang, W.; Lu, S. Comparison of Efficacy and Safety of Brigatinib in First-Line Treatments for Patients with Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Systematic Review and Indirect Treatment Comparison. J. Clin. Med. 2022, 11, 2963. https://doi.org/10.3390/jcm11112963
Yu Y, Zhu F, Zhang W, Lu S. Comparison of Efficacy and Safety of Brigatinib in First-Line Treatments for Patients with Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Systematic Review and Indirect Treatment Comparison. Journal of Clinical Medicine. 2022; 11(11):2963. https://doi.org/10.3390/jcm11112963
Chicago/Turabian StyleYu, Yongfeng, Fanfan Zhu, Wenxin Zhang, and Shun Lu. 2022. "Comparison of Efficacy and Safety of Brigatinib in First-Line Treatments for Patients with Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Systematic Review and Indirect Treatment Comparison" Journal of Clinical Medicine 11, no. 11: 2963. https://doi.org/10.3390/jcm11112963
APA StyleYu, Y., Zhu, F., Zhang, W., & Lu, S. (2022). Comparison of Efficacy and Safety of Brigatinib in First-Line Treatments for Patients with Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Systematic Review and Indirect Treatment Comparison. Journal of Clinical Medicine, 11(11), 2963. https://doi.org/10.3390/jcm11112963







