Simple Predictors for Cardiac Fibrosis in Patients with Type 2 Diabetes Mellitus: The Role of Circulating Biomarkers and Pulse Wave Velocity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Assays
2.3. Blood Pressure Measurement
2.4. Pulse Wave Analysis
2.5. Reactive Hyperemia Peripheral Arterial Tonometry
2.6. Echocardiography
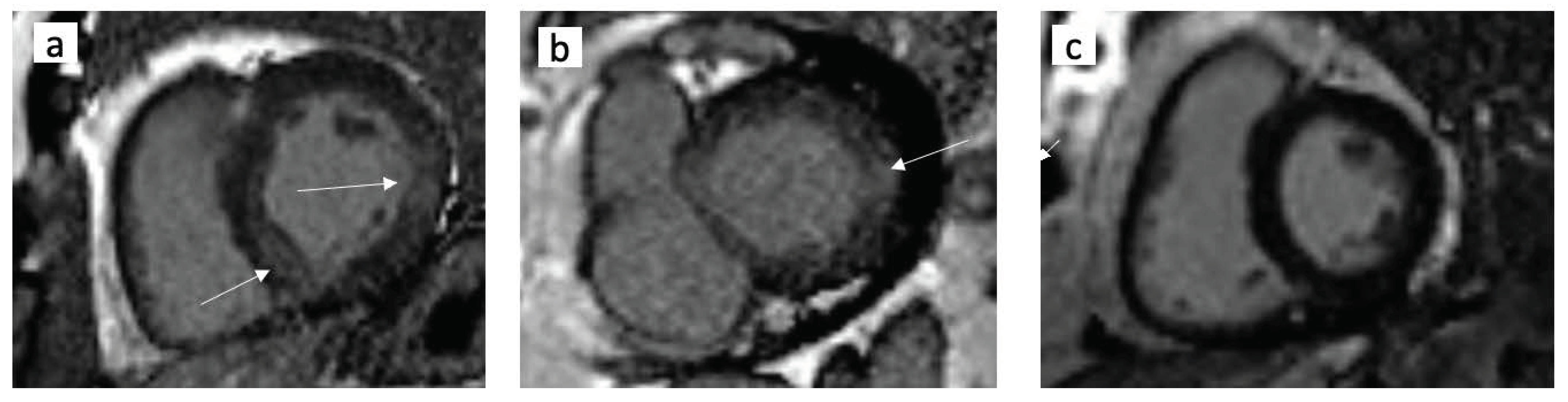
2.7. Cardiac MRI
2.8. Carotid Ultrasonography
2.9. Statistical Analysis
3. Results
3.1. Patient Baseline Characteristics
3.2. Laboratory Measurements
3.3. Echocardiography and Cardiac MRI Analysis
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawshani, A.; Rawshani, A.; Franzén, S.; Sattar, N.; Eliasson, B.; Svensson, A.M.; Zethelius, B.; Miftaraj, M.; McGuire, D.K.; Rosengren, A.; et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2018, 379, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, M.; Bentham, J.; Stevens, G.A.; Zhou, B.; Danaei, G.; Lu, Y.; Bixby, H.; Cowan, M.J.; Riley, L.M.; Hajifathalian, K.; et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef] [Green Version]
- Beckman, J.A.; Creager, M.A.; Libby, P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA 2002, 287, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Kenny, H.C.; Abel, E.D. Heart Failure in Type 2 Diabetes Mellitus. Circ. Res. 2019, 124, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Burlew, B.S.; Weber, K.T. Cardiac fibrosis as a cause of diastolic dysfunction. Herz 2002, 27, 92–98. [Google Scholar] [CrossRef]
- Karamitsos, T.D.; Arvanitaki, A.; Karvounis, H.; Neubauer, S.; Ferreira, V.M. Myocardial tissue characterization and fibrosis by imaging. JACC Cardiovasc. Imaging 2020, 13, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M.; Cuspidi, C.; Calicchio, F.; Grassi, G.; Mancia, G. Diabetic cardiomyopathy: How can cardiac magnetic resonance help? Acta Diabetol. 2020, 57, 1027–1034. [Google Scholar] [CrossRef]
- López, B.; González, A.; Ravassa, S.; Beaumont, J.; Moreno, M.U.; San José, G.; Querejeta, R.; Díez, J. Circulating biomarkers of myocardial fibrosis: The need for a reappraisal. J. Am. Coll. Cardiol. 2015, 65, 2449–2456. [Google Scholar] [CrossRef] [Green Version]
- Richards, A.M. Circulating biomarkers of cardiac fibrosis: Do we have any and what use are they? Circ. Heart Fail. 2017, 10, e003936. [Google Scholar] [CrossRef] [Green Version]
- Shim, C.Y.; Hong, G.R.; Ha, J.W. Ventricular stiffness and ventricular-arterial coupling in heart failure: What is it, how to assess, and why? Heart Fail. Clin. 2019, 15, 267–274. [Google Scholar] [CrossRef]
- Yoshida, Y.; Nakanishi, K.; Daimon, M.; Ishiwata, J.; Sawada, N.; Hirokawa, M.; Kaneko, H.; Nakao, T.; Mizuno, Y.; Morita, H.; et al. Sex-specific difference in the association between arterial stiffness and subclinical left ventricular dysfunction. Eur. Heart J. Cardiovasc. Imaging 2020, 28, jeaa156. [Google Scholar] [CrossRef]
- Ban, C.R.; Twigg, S.M. Fibrosis in diabetes complications: Pathogenic mechanisms and circulating and urinary markers. Vasc. Health Risk Manag. 2008, 4, 575–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Bortel, L.M.; Laurent, S.; Boutouyrie, P.; Chowienczyk, P.; Cruickshank, J.K.; De Backer, T.; Filipovsky, J.; Huybrechts, S.; Mattace-Raso, F.U.; Protogerou, A.D.; et al. Artery Society; European Society of Hypertension working group on vascular structure and function; European Network for Noninvasive Investigation of Large Arteries. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J. Hypertens. 2012, 30, 445–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michelsen, M.M.; Mygind, N.D.; Pena, A.; Aziz, A.; Frestad, D.; Host, N.; Prescott, E. Steering committee of the iPOWER study. Peripheral reactive hyperemia index and coronary microvascular function in women with no obstructive CAD: The iPOWER study. JACC Cardiovasc. Imaging 2016, 9, 411–417. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr 2016, 29, 277–314. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [Green Version]
- Hundley, W.G.; Bluemke, D.A.; Finn, J.P.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Ho, V.B.; Jerosch-Herold, M.; Kramer, C.M.; Manning, W.J.; et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation 2010, 121, 2462–2508. [Google Scholar] [CrossRef]
- Kim, E.K.; Lee, S.C.; Hwang, J.W.; Chang, S.A.; Park, S.J.; On, Y.K.; Park, K.M.; Choe, Y.H.; Kim, S.M.; Park, S.W.; et al. Differences in apical and non-apical types of hypertrophic cardiomyopathy: A prospective analysis of clinical, echocardiographic, and cardiac magnetic resonance findings and outcome from 350 patients. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 678–686. [Google Scholar] [CrossRef]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S.; et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002, 105, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Giusca, S.; Kelle, S.; Nagel, E.; Buss, S.J.; Voss, A.; Puntmann, V.; Fleck, E.; Katus, H.A.; Korosoglou, G. Differences in the prognostic relevance of myocardial ischaemia and scar by cardiac magnetic resonance in patients with and without diabetes mellitus. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 812–820. [Google Scholar] [CrossRef]
- Tuleta, I.; Frangogiannis, N.G. Diabetic fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166044. [Google Scholar] [CrossRef] [PubMed]
- Paolillo, S.; Marsico, F.; Prastaro, M.; Renga, F.; Esposito, L.; De Martino, F.; Di Napoli, P.; Esposito, I.; Ambrosio, A.; Ianniruberto, M.; et al. Diabetic cardiomyopathy: Definition, diagnosis, and therapeutic implications. Heart Fail. Clin. 2019, 15, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Hogg, K.; Swedberg, K.; McMurray, J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J. Am. Coll. Cardiol. 2004, 43, 317–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Bello, V.; Santini, F.; Di Cori, A.; Pucci, A.; Palagi, C.; Delle Donne, M.G.; Fierabracci, P.; Marsili, A.; Talini, E.; Giannetti, M.; et al. Obesity cardiomyopathy: Is it a reality? An ultrasonic tissue characterization study. J. Am. Soc. Echocardiogr. 2006, 19, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Turkbey, E.B.; McClelland, R.L.; Kronmal, R.A.; Burke, G.L.; Bild, D.E.; Tracy, R.P.; Arai, A.E.; Lima, J.A.; Bluemke, D.A. The impact of obesity on the left ventricle: The Multi-Ethnic Study of Atherosclerosis (MESA). JACC Cardiovasc. Imaging 2010, 3, 266–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milan, A.; Zocaro, G.; Leone, D.; Tosello, F.; Buraioli, I.; Schiavone, D.; Veglio, F. Current assessment of pulse wave velocity: Comprehensive review of validation studies. J. Hypertens. 2019, 37, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, T.; Borlaug, B.A.; Pellikka, P.A.; Turner, S.T.; Kullo, I.J. Sex differences in arterial stiffness and ventricular-arterial interactions. J. Am. Coll. Cardiol. 2013, 61, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Smulyan, H.; Lieber, A.; Safar, M.E. Hypertension, diabetes type II, and their association: Role of arterial stiffness. Am. J. Hypertens. 2016, 29, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Mottram, P.M.; Haluska, B.A.; Leano, R.; Carlier, S.; Case, C.; Marwick, T.H. Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease. Heart 2005, 91, 1551–1556. [Google Scholar] [CrossRef] [Green Version]
- Sharif, S.; Visseren, F.L.J.; Spiering, W.; de Jong, P.A.; Bots, M.L.; Westerink, J. SMART study group. Arterial stiffness as a risk factor for cardiovascular events and all-cause mortality in people with Type 2 diabetes. Diabet. Med. 2019, 36, 1125–1132. [Google Scholar] [CrossRef] [Green Version]
- Desamericq, G.; Tissot, C.M.; Akakpo, S.; Tropeano, A.I.; Millasseau, S.; Macquin-Mavier, I. Carotid-femoral pulse wave velocity is not increased in obesity. Am. J. Hypertens. 2015, 28, 546–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso, N.; Lupón, J.; Barallat, J.; de Antonio, M.; Domingo, M.; Zamora, E.; Moliner, P.; Galán, A.; Santesmases, J.; Pastor, C.; et al. Impact of diabetes on the predictive value of heart failure biomarkers. Cardiovasc. Diabetol. 2016, 15, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vora, A.; de Lemos, J.A.; Ayers, C.; Grodin, J.L.; Lingvay, I. Association of galectin-3 with diabetes mellitus in the Dallas Heart Study. J. Clin. Endocrinol. Metab. 2019, 104, 4449–4458. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.N.; Ayyadurai, P.; Saad, M.; Kosmas, C.E.; Dogar, M.U.; Patel, U.; Vittorio, T.J. Galactin-3 and soluble ST2 as complementary tools to cardiac MRI for sudden cardiac death risk stratification in heart failure: A review. JRSM Cardiovasc. Dis. 2020, 9, 2048004020957840. [Google Scholar] [CrossRef] [PubMed]
- Barchetta, I.; Cimini, F.A.; De Gioannis, R.; Ciccarelli, G.; Bertoccini, L.; Lenzi, A.; Baroni, M.G.; Cavallo, M.G. Procollagen-III peptide identifies adipose tissue-associated inflammation in type 2 diabetes with or without nonalcoholic liver disease. Diabetes Metab. Res. Rev. 2018, 34, e2998. [Google Scholar] [CrossRef]
- Agarwal, I.; Arnold, A.; Glazer, N.L.; Barasch, E.; Djousse, L.; Fitzpatrick, A.L.; Gottdiener, J.S.; Ix, J.H.; Jensen, R.A.; Kizer, J.R.; et al. Fibrosis-related biomarkers and large and small vessel disease: The Cardiovascular Health Study. Atherosclerosis 2015, 239, 539–546. [Google Scholar] [CrossRef] [Green Version]
- Russo, I.; Frangogiannis, N.G. Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities. J. Mol. Cell. Cardiol. 2016, 90, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Hermida, N.; Markl, A.; Hamelet, J.; Van Assche, T.; Vanderper, A.; Herijgers, P.; van Bilsen, M.; Hilfiker-Kleiner, D.; Noppe, G.; Beauloye, C.; et al. HMGCoA reductase inhibition reverses myocardial fibrosis and diastolic dysfunction through AMP-activated protein kinase activation in a mouse model of metabolic syndrome. Cardiovasc. Res. 2013, 99, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Sundström, J.; Evans, J.C.; Benjamin, E.J.; Levy, D.; Larson, M.G.; Sawyer, D.B.; Siwik, D.A.; Colucci, W.S.; Wilson, P.W.; Vasan, R.S. Relations of plasma total TIMP-1 levels to cardiovascular risk factors and echocardiographic measures: The Framingham heart study. Eur. Heart J. 2004, 25, 1509–1516. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, J.M.; Pan, A.; Koh, W.P. Tissue inhibitor matrix metalloproteinase 1 and risk of type 2 diabetes in a Chinese population. BMJ Open Diabetes Res. Care 2020, 8, e001051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variables | T2DM Group n = 37 | RF Group n = 27 | HC Group n = 15 | p1,2-Value | p2,3-Value |
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| Age, years | 57.5 ± 8.4 | 54.0 ± 8.9 | 55.6 ± 3.6 | 0.122 | 0.378 |
| Male, n (%) | 17 (46) | 12 (44) | 7 (47) | 0.905 | 0.735 |
| BMI, kg/m | 32.9 ± 6.5 | 35.6 ± 2.7 | 23.8 ± 2.0 | 0.051 | <0.001 |
| Waist circumference, cm | 109.4 ± 14.0 | 113.6 ± 8.9 | 0.186 | ||
| Male | 111.5 ± 14.3 | 118.2 ± 8.7 | 0.166 | ||
| Female | 107.8 ± 14.0 | 109.9 ± 7.4 | 0.598 | ||
| T2DM duration, years | 9.0 [5.0–12.0] | - | |||
| Hypertension, n (%) | 21 (57) | 19 (70) | 0 | 0.058 | |
| Current smoker, n (%) | 12 (32) | 11 (41) | 3 (20) | 0.792 | 0.071 |
| Office systolic BP, mm Hg | 131 ± 17 | 130 ± 17 | 118 ± 9 | 0.673 | 0.002 |
| Office diastolic BP, mm Hg | 77 ± 10 | 81 ± 14 | 75 ± 8 | 0.415 | 0.130 |
| Carotid-femoral PWV, m/s | 9.9 ± 2.2 | 7.9 ± 1.7 | 0.0002 | ||
| Carotid IMT, mm | 0.715 ± 0.374 | 0.618 ± 0.113 | 0.535 ± 0.114 | 0.010 | <0.001 |
| RHI | 1.50 ± 0.35 | 1.70 ± 0.31 | 0.019 | ||
| eGFR, mL/min/1.73 m2 | 88.4 ± 15.8 | 90.1 ± 15.9 | 0.550 | ||
| Echocardiography | |||||
| LA volume index, mL/m2 | 36.7 ± 6.8 | 32.7 ± 6.0 | 0.016 | ||
| LV mass index, g/m2 | 120.8 ± 32.0 | 102.0 ± 23.3 | 90.3 ± 13.4 | 0.008 | 0.002 |
| Male | 131.9 ± 38.6 | 111.8 ± 24.1 | 0.170 | ||
| Female | 111.3 ± 21.9 | 93.6 ± 19.7 | 0.014 | ||
| Relative wall thickness | 0.448 ± 0.050 | 0.434 ± 0.048 | 0.813 | ||
| LV EF, % | 60.6 ± 5.5 | 60.9 ± 3.3 | 0.603 | ||
| E/e′ | 8.2 ± 1.9 | 7.3 ± 1.2 | 0.021 | ||
| GLS, % | −18.0 ± 3.0 | −19.1 ± 2.1 | 0.110 | ||
| Medication | |||||
| Metformin, n (%) | 22(59) | 1(4) | <0.001 | ||
| DPP-4 inhibitors, n (%) | 5(13.5) | _ | _ | ||
| Sulphonylureas, n (%) | 2(5.4) | _ | _ | ||
| Insulin, n (%) | 8 (21.6) | _ | _ | ||
| ACEI or ARB, n (%) | 21 (56.8) | 19 (70) | 0.058 | ||
| Low-dose aspirin, n (%) | 13 (48.1) | 4 (14.8) | 0.002 | ||
| Statins, n (%) | 18 (48.6) | 4 (14.8) | <0.01 | ||
| Variables | T2DM Group n = 37 | RF Group n = 27 | HC Group n = 15 | p1,2-Value | p2,3-Value |
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| Total cholesterol, mmol/L | 4.84 ± 0.97 | 5.40 ± 1,11 | 4.52 ± 1,24 | 0.056 | 0.095 |
| HDL-C, mmol/L | 1.11 ± 0.26 | 1.15 ± 0.28 | 1.16 ± 0.31 | 0.757 | 0.62 |
| LDL-C, mmol/L | 2.67 ± 0.91 | 3.49 ± 0.92 | 2.62 ± 0.98 | 0.002 | 0.017 |
| Triglycerides, mmol/L | 2.58 ± 1.07 | 1.88 ± 0.78 | 1.67 ± 0.93 | 0.007 | 0.22 |
| hsCRP, mg/L | 2.55 [1.21–4.78] | 3.84 [1.99–5.70] | 1.67 [0.73–2.96] | 0.185 | 0.11 |
| HbA1c, % | 8.9 ± 1.4 | 5.74 ± 0.85 | - | <0.001 | - |
| NT-proBNP, pg/mL | 91 [16–148] | 27.5 [15.7–47.6] | - | <0.001 | - |
| PICP, ng/mL | 136.0 [117.2–166.0] | 108.4 [93.2–148.8] | 84.0 [69.0–98.3] | 0.006 | 0.001 |
| PIIINP, ng/mL | 5.74 [4.43–6.77] | 5.09 [4.44–5.96] | 3.99 [3.27–4.27] | 0.265 | 0.002 |
| sST2, ng/mL | 19.1 [14.9–26.7] | 13.2 [10.2–21.8] | 12.6 [10.3–16.2] | 0.016 | 0.912 |
| MMP-9, ng/mL | 794 [497–1015] | 490 [341–911] | 277 [253–319] | 0.084 | 0.002 |
| TIMP-1, ng/mL | 188 [171–237] | 152 [137–185] | 141 [120–164] | 0.004 | 0.023 |
| TGF-β1, ng/mL | 35.7 [24.5–48.6] | 29.6 [15.3–42.2] | 12.8 [11.9–18.6] | 0.067 | <0.001 |
| galectin-3, ng/mL | 9.5 [7.8–12.5] | 7.8 [6.8–9.9] | 6.9 [6.0–7.2] | 0.029 | 0.010 |
| ICTP, ng/mL | 5,25 [3.5–6.8] | 3.49 [3.03–5.89] | 2.98 [2.68–3.97] | 0.046 | 0.030 |
| Estimate | Standard Error | Wald Stat. | Lower CL—95. % | Upper CL—95. % | p | |
|---|---|---|---|---|---|---|
| All subjects (T2DM+RF+HC groups) | ||||||
| T2DM: Yes | 1.101 | 0.32 | 11.807 | 0.473 | 1.728 | <0.001 |
| BMI, kg/m2 | −0.104 | 0.053 | 3.832 | −0.209 | 0.0001 | 0.05 |
| Metformin baseline therapy: Yes | 1.06 | 0.305 | 12.042 | 0.461 | 1.659 | <0.001 |
| Statins: Yes | 0.785 | 0.281 | 7.794 | 0.234 | 1.335 | 0.005 |
| PWV, m/s | 0.327 | 0.135 | 5.898 | 0.063 | 0.591 | 0.015 |
| RHI | −1.398 | 0.8 | 3.053 | −2.966 | 0.17 | 0.081 |
| LV hypertrophy | 0.799 | 0.299 | 7.161 | 0.214 | 1.384 | 0.008 |
| HbA1c, % | 0.52 | 0.167 | 9.682 | 0.192 | 0.848 | 0.002 |
| LDL-C, mM/L | −0.534 | 0.294 | 3.306 | −1.109 | 0.042 | 0.069 |
| TIMP-1, ng/mL | 0.018 | 0.006 | 8.438 | 0.006 | 0.03 | 0.004 |
| Galectin-3, ng/mL | 0.225 | 0.09 | 6.159 | 0.047 | 0.403 | 0.013 |
| T2DM patients only | ||||||
| PWV, m/s | −0.351 | 0.194 | 3.261 | −0.73 | 0.03 | 0.042 |
| TIMP-1, ng/mL | −0.02 | 0.008 | 5.187 | 0.036 | −0.003 | 0.05 |
| Estimate | Standard Error | Wald Stat. | Lower CL—95, % | Upper CL—95, % | p | |
|---|---|---|---|---|---|---|
| All subjects (T2DM+RF+HC groups) | ||||||
| Intercept | −5.596 | 2.189 | 6.533 | −9.887 | −1.304 | 0.01 |
| PWV, m/s | 0.12 | 0.175 | 0.471 | −0.223 | 0.464 | 0.492 |
| TIMP-1, ng/mL | 0.014 | 0.007 | 4.042 | 0.0003 | 0.028 | 0.044 |
| Galectin-3, ng/mL | 0.136 | 0.109 | 1.57 | −0.077 | 0.349 | 0.21 |
| T2DM: Yes | 0.67 | 0.426 | 2.466 | −0.166 | 1.506 | 0.116 |
| LV hypertrophy: Yes | 0.524 | 0.412 | 1.623 | −0.282 | 1.331 | 0.203 |
| T2DM patients only | ||||||
| Intercept | 6.607 | 2.778 | 5.657 | 1.163 | 12.052 | 0.02 |
| PWV, m/s | −0.353 | 0.218 | 2.623 | −0.780 | 0.074 | 0.12 |
| TIMP-1, ng/mL | −0.018 | 0.009 | 4.596 | −0.035 | −0.002 | 0.03 |
| Estimate | Standard Error | Wald Stat. | Lower CL—95, % | Upper CL—95, % | p | |
|---|---|---|---|---|---|---|
| All subjects (T2DM+RF+HC groups) | ||||||
| Intercept | −7.128 | 1.996 | 12.749 | −11.04 | −3.215 | 0.0004 |
| PWV, m/s | 0.208 | 0.155 | 1.796 | −0.096 | 0.512 | 0.18 |
| TIMP-1, ng/mL | 0.017 | 0.007 | 6.265 | 0.004 | 0.029 | 0.01 |
| Galectin-3, ng/mL | 0.189 | 0.099 | 3.648 | −0.005 | 0.384 | 0.049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luneva, E.B.; Vasileva, A.A.; Karelkina, E.V.; Boyarinova, M.A.; Mikhaylov, E.N.; Ryzhkov, A.V.; Babenko, A.Y.; Konradi, A.O.; Moiseeva, O.M. Simple Predictors for Cardiac Fibrosis in Patients with Type 2 Diabetes Mellitus: The Role of Circulating Biomarkers and Pulse Wave Velocity. J. Clin. Med. 2022, 11, 2843. https://doi.org/10.3390/jcm11102843
Luneva EB, Vasileva AA, Karelkina EV, Boyarinova MA, Mikhaylov EN, Ryzhkov AV, Babenko AY, Konradi AO, Moiseeva OM. Simple Predictors for Cardiac Fibrosis in Patients with Type 2 Diabetes Mellitus: The Role of Circulating Biomarkers and Pulse Wave Velocity. Journal of Clinical Medicine. 2022; 11(10):2843. https://doi.org/10.3390/jcm11102843
Chicago/Turabian StyleLuneva, Ekaterina B., Anastasia A. Vasileva, Elena V. Karelkina, Maria A. Boyarinova, Evgeny N. Mikhaylov, Anton V. Ryzhkov, Alina Y. Babenko, Alexandra O. Konradi, and Olga M. Moiseeva. 2022. "Simple Predictors for Cardiac Fibrosis in Patients with Type 2 Diabetes Mellitus: The Role of Circulating Biomarkers and Pulse Wave Velocity" Journal of Clinical Medicine 11, no. 10: 2843. https://doi.org/10.3390/jcm11102843
APA StyleLuneva, E. B., Vasileva, A. A., Karelkina, E. V., Boyarinova, M. A., Mikhaylov, E. N., Ryzhkov, A. V., Babenko, A. Y., Konradi, A. O., & Moiseeva, O. M. (2022). Simple Predictors for Cardiac Fibrosis in Patients with Type 2 Diabetes Mellitus: The Role of Circulating Biomarkers and Pulse Wave Velocity. Journal of Clinical Medicine, 11(10), 2843. https://doi.org/10.3390/jcm11102843






